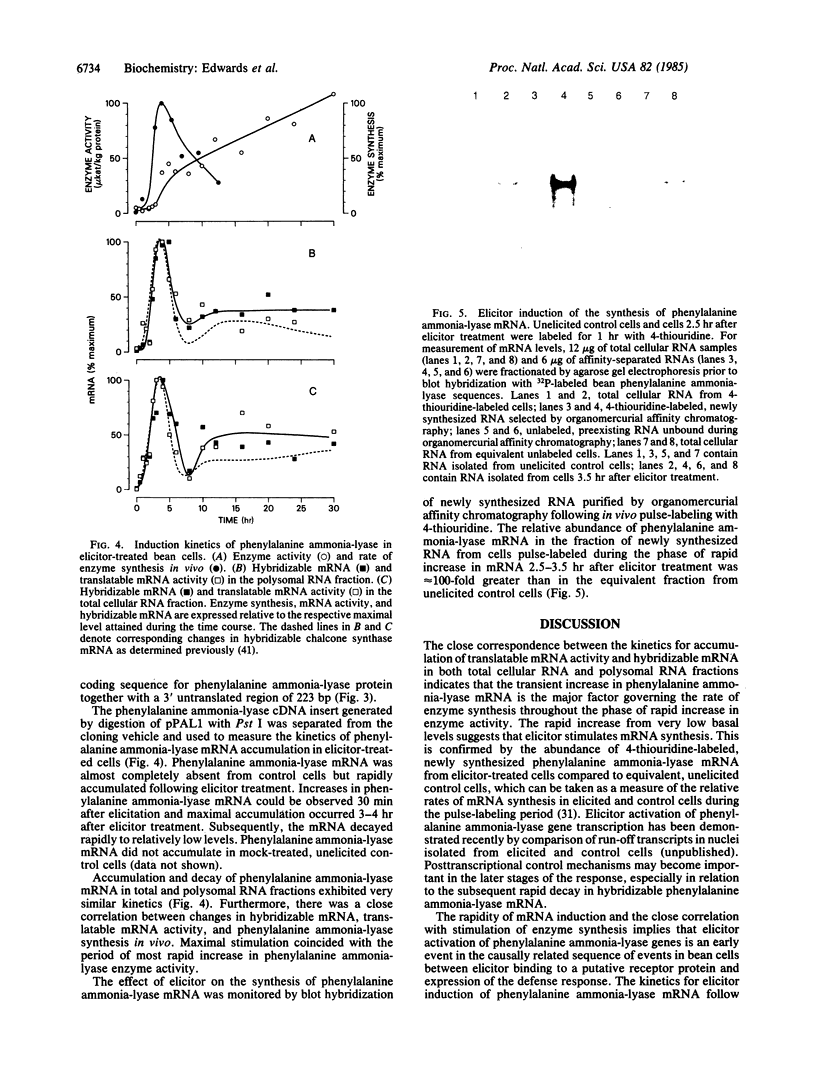
Abstract
DNAs complementary to a size-selected fraction of poly(A)+ RNA present in elicitor-treated cells of bean (Phaseolus vulgaris L.) were inserted into pAT153 and used to transform Escherichia coli strain C600. Five clones were identified by hybrid-selected translation and cross-hybridization that contained sequences complementary to mRNA encoding phenylalanine ammonia-lyase (EC 4.3.1.5), which catalyzes the first reaction of phenylpropanoid biosynthesis. The longest insert contained a single open reading frame of 1520 base pairs together with 223 base pairs of 3′ untranslated sequence. RNA blot hybridization showed that elicitor caused a rapid, marked but transient increase in phenylalanine ammonia-lyase mRNA that was closely correlated with changes in translatable mRNA activity in vitro and enzyme synthesis in vivo. Blot hybridization of newly synthesized mRNA purified by organomercurial affinity chromatography following in vivo pulse-labeling with 4-thiouridine indicates that elicitor caused a rapid stimulation of phenylalanine ammonia-lyase mRNA synthesis as an early in the defense response leading to accumulation of phenylpropanoid-derived phytoalexins.
Keywords: cDNA cloning, cDNA sequence, phytoalexins, plant disease resistance, thiouridine
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson-Prouty A. J., Albersheim P. Host-Pathogen Interactions: VIII. Isolation of a Pathogen-synthesized Fraction Rich in Glucan That Elicits a Defense Response in the Pathogen's Host. Plant Physiol. 1975 Aug;56(2):286–291. doi: 10.1104/pp.56.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels D., Thompson R. D. The characterization of cDNA clones coding for wheat storage proteins. Nucleic Acids Res. 1983 May 25;11(10):2961–2977. doi: 10.1093/nar/11.10.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Bell J. N., Cramer C. L., Schuch W., Lamb C. J., Dixon R. A. L-Phenylalanine ammonia-lyase from Phaseolus vulgaris. Characterisation and differential induction of multiple forms from elicitor-treated cell suspension cultures. Eur J Biochem. 1985 Jun 3;149(2):411–419. doi: 10.1111/j.1432-1033.1985.tb08941.x. [DOI] [PubMed] [Google Scholar]
- Bolwell G. P., Robbins M. P., Dixon R. A. Metabolic changes in elicitor-treated bean cells. Enzymic responses associated with rapid changes in cell wall components. Eur J Biochem. 1985 May 2;148(3):571–578. doi: 10.1111/j.1432-1033.1985.tb08878.x. [DOI] [PubMed] [Google Scholar]
- Börner H., Grisebach H. Enzyme induction in soybean infected by Phytophthora megasperma f.sp. glycinea. Arch Biochem Biophys. 1982 Aug;217(1):65–71. doi: 10.1016/0003-9861(82)90479-9. [DOI] [PubMed] [Google Scholar]
- Cramer C. L., Bell J. N., Ryder T. B., Bailey J. A., Schuch W., Bolwell G. P., Robbins M. P., Dixon R. A., Lamb C. J. Co-ordinated synthesis of phytoalexin biosynthetic enzymes in biologically-stressed cells of bean (Phaseolus vulgaris L.). EMBO J. 1985 Feb;4(2):285–289. doi: 10.1002/j.1460-2075.1985.tb03627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Dey P. M., Lamb C. J. Phytoalexins: enzymology and molecular biology. Adv Enzymol Relat Areas Mol Biol. 1983;55:1–136. doi: 10.1002/9780470123010.ch1. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Lamb C. J. Stimulation of de novo synthesis of L-phenylalanine ammonia-lyase in relation to phytoalexin accumulation in Colletotrichum lindemuthianum elicitor-treated cell suspension cultures of french bean (Phaseolus vulgaris). Biochim Biophys Acta. 1979 Sep 3;586(3):453–463. doi: 10.1016/0304-4165(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Duchesne M., Fritig B., Hirth L. Phenylalanine ammonia-lyase in tobacco mosaic virus-infected hypersensitive tobacco. Density-labelling evidence of de novo synthesis. Biochim Biophys Acta. 1977 Dec 8;485(2):465–481. doi: 10.1016/0005-2744(77)90182-6. [DOI] [PubMed] [Google Scholar]
- Ebel J., Schmidt W. E., Loyal R. Phytoalexin synthesis in soybean cells: elicitor induction of phenylalanine ammonia-lyase and chalcone synthase mRNAs and correlation with phytoalexin accumulation. Arch Biochem Biophys. 1984 Jul;232(1):240–248. doi: 10.1016/0003-9861(84)90540-x. [DOI] [PubMed] [Google Scholar]
- Geiser M., Döring H. P., Wöstemeyer J., Behrens U., Tillmann E., Starlinger P. A cDNA clone from Zea mays endosperm sucrose synthetase mRNA. Nucleic Acids Res. 1980 Dec 20;8(24):6175–6188. doi: 10.1093/nar/8.24.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner M. H., Chin M. B., Lane B. G. Wheat embryo ribonucleates. XII. Formal characterization of terminal and penultimate nucleoside residues at the 5'-ends of "capped" RNA from imbibing wheat embryos. Can J Biochem. 1978 Jul;56(7):729–733. doi: 10.1139/o78-109. [DOI] [PubMed] [Google Scholar]
- Kuhn D. N., Chappell J., Boudet A., Hahlbrock K. Induction of phenylalanine ammonia-lyase and 4-coumarate:CoA ligase mRNAs in cultured plant cells by UV light or fungal elicitor. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1102–1106. doi: 10.1073/pnas.81.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Hahlbrock K., Lamb C. J. Elicitor induction of mRNA activity. Rapid effects of elicitor on phenylalanine ammonia-lyase and chalcone synthase mRNA activities in bean cells. Eur J Biochem. 1983 Jan 17;130(1):131–139. [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Hahlbrock K., Lamb C. Rapid induction of the synthesis of phenylalanine ammonia-lyase and of chalcone synthase in elicitor-treated plant cells. Eur J Biochem. 1983 Jan 1;129(3):593–601. doi: 10.1111/j.1432-1033.1983.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Lamb C. J. Elicitor modulation of the turnover of L-phenylalanine ammonia-lyase in French bean cell suspension cultures. Biochim Biophys Acta. 1980 Dec 1;633(2):162–175. doi: 10.1016/0304-4165(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Loschke D. C., Hadwiger L. A. Effects of Light and of Fusarium solani on Synthesis and Activity of Phenylalanine Ammonia-Lyase in Peas. Plant Physiol. 1981 Sep;68(3):680–685. doi: 10.1104/pp.68.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschke D. C., Hadwiger L. A. Effects of Light and of Fusarium solani on Synthesis and Activity of Phenylalanine Ammonia-Lyase in Peas. Plant Physiol. 1981 Sep;68(3):680–685. doi: 10.1104/pp.68.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rapid switching of plant gene expression induced by fungal elicitor. Science. 1985 Mar 8;227(4691):1240–1243. doi: 10.1126/science.227.4691.1240. [DOI] [PubMed] [Google Scholar]
- Ryder T. B., Cramer C. L., Bell J. N., Robbins M. P., Dixon R. A., Lamb C. J. Elicitor rapidly induces chalcone synthase mRNA in Phaseolus vulgaris cells at the onset of the phytoalexin defense response. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5724–5728. doi: 10.1073/pnas.81.18.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J., Betz B., Hahlbrock K. Light-induced enzyme synthesis in cell suspension cultures of Petroselinum hortense. Demonstration in a heterologous cell-free system of rapid changes in the rate of phenylalanine ammonia-lyase synthesis. Eur J Biochem. 1976 Aug 16;67(2):527–541. doi: 10.1111/j.1432-1033.1976.tb10719.x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietjen K. G., Hunkler D., Matern U. Differential response of cultured parsley cells to elicitors from two non-pathogenic strains of fungi. 1. Identification of induced products as coumarin derivatives. Eur J Biochem. 1983 Mar 15;131(2):401–407. doi: 10.1111/j.1432-1033.1983.tb07277.x. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Wood D., Blanchetot A., Jeffreys A. J. Molecular cloning of seal myoglobin mRNA. Nucleic Acids Res. 1982 Nov 25;10(22):7133–7144. doi: 10.1093/nar/10.22.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]