Abstract
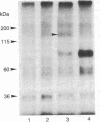
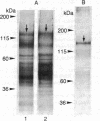
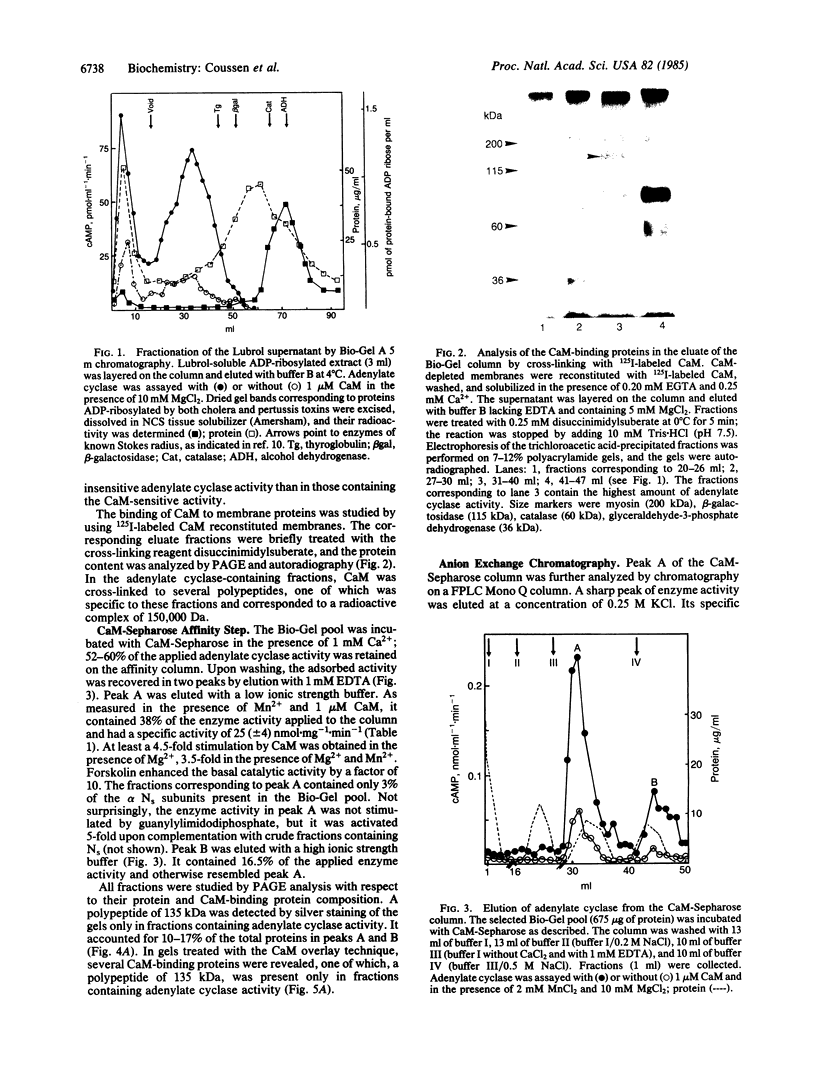
The partial purification of the eukaryote adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] catalytic subunit has been achieved by a procedure based on the calmodulin (CaM) sensitivity of the enzyme. Small amounts of rat brain synaptosomal membranes depleted of CaM were solubilized with Lubrol and subjected to a three-step chromatographic procedure involving gel filtration, a CaM-Sepharose affinity step, and fast protein liquid chromatography. About 20% of the adenylate cyclase activity contained in the membranes was recovered in the final enriched fraction with a specific activity of 200 nmol X mg-1 X min-1. The alpha subunits of the adenylate cyclase stimulatory proteins NS were absent from this final fraction. The addition of CaM, of forskolin, or of preactivated NS-containing fractions to this preparation greatly increased the enzyme activity. A CaM-binding polypeptide of 135,000 Da copurified with the adenylate cyclase activity in each of the three steps. Polyacrylamide gel electrophoresis of the final fraction showed that this polypeptide represented 35% of the total protein. We propose that this polypeptide is likely to be the adenylate cyclase catalytic subunit. This enzyme would represent close to 0.5% of the synaptosomal membrane proteins. Its low turnover number would be due to the absence of the alpha subunits of the NS regulatory proteins and would correspond to the enzymic basal level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen T. J., Heideman W., Rosenberg G. B., Storm D. R. Photoaffinity labeling of brain adenylate cyclase preparations with azido[125 I]iodocalmodulin. Biochemistry. 1983 May 24;22(11):2757–2762. doi: 10.1021/bi00280a025. [DOI] [PubMed] [Google Scholar]
- Autric F., Ferraz C., Kilhoffer M. C., Cavadore J. C., Demaille J. G. Large-scale purification and characterization of calmodulin from ram testis: its metal-ion-dependent conformers. Biochim Biophys Acta. 1980 Aug 1;631(1):139–147. doi: 10.1016/0304-4165(80)90062-8. [DOI] [PubMed] [Google Scholar]
- Berthillier G., Megret F., Alouf J. E., Monneron A. La toxine pertussis stimule l'adényl-cyclase cérébrale et provoque l'ADP-ribosylation d'une protéine membranaire de 40 000 dalton. C R Seances Acad Sci III. 1983;297(12):575–578. [PubMed] [Google Scholar]
- Berthillier G., d'Alayer J., Monneron A. ADP-ribosylation of brain synaptosomal proteins correlates with adenylate cyclase activation. Biochem Biophys Res Commun. 1982 Nov 30;109(2):297–304. doi: 10.1016/0006-291x(82)91720-x. [DOI] [PubMed] [Google Scholar]
- Bradham L. S., Hegazy M. G. Separation of the catalytic and stimulatory regulatory subunits of rat brain adenylate cyclase. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(4-5):331–340. [PubMed] [Google Scholar]
- Brostrom C. O., Huang Y. C., Breckenridge B. M., Wolff D. J. Identification of a calcium-binding protein as a calcium-dependent regulator of brain adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Jan;72(1):64–68. doi: 10.1073/pnas.72.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina J., Hildebrandt J., Sunyer T., Sekura R. D., Manclark C. R., Iyengar R., Birnbaumer L. Mechanisms in the vectorial receptor-adenylate cyclase signal transduction. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:111–125. [PubMed] [Google Scholar]
- Danchin A., Guiso N., Roy A., Ullmann A. Identification of the Escherichia coli cya gene product as authentic adenylate cyclase. J Mol Biol. 1984 May 25;175(3):403–408. doi: 10.1016/0022-2836(84)90356-5. [DOI] [PubMed] [Google Scholar]
- Heideman W., Wierman B. M., Storm D. R. GTP is not required for calmodulin stimulation of bovine brain adenylate cyclase. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1462–1465. doi: 10.1073/pnas.79.5.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Sutherland E. W. Detergent-dispersed adenylate cyclase from rat brain. Effects of fluoride, cations, and chelators. J Biol Chem. 1973 Jul 25;248(14):5114–5121. [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Lynch T. J., Tallant E. A., Cheung W. Y. Ca++-dependent formation of brain adenylate cyclase-protein activator complex. Biochem Biophys Res Commun. 1976 Jan 26;68(2):616–625. doi: 10.1016/0006-291x(76)91190-6. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Gel protein stains: silver stain. Methods Enzymol. 1984;104:441–447. doi: 10.1016/s0076-6879(84)04111-2. [DOI] [PubMed] [Google Scholar]
- Pfeuffer E., Dreher R. M., Metzger H., Pfeuffer T. Catalytic unit of adenlyate cyclase: purification and identification by affinity crosslinking. Proc Natl Acad Sci U S A. 1985 May;82(10):3086–3090. doi: 10.1073/pnas.82.10.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer T., Gaugler B., Metzger H. Isolation of homologous and heterologous complexes between catalytic and regulatory components of adenylate cyclase by forskolin-Sepharose. FEBS Lett. 1983 Nov 28;164(1):154–160. doi: 10.1016/0014-5793(83)80040-4. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Salter R. S., Krinks M. H., Klee C. B., Neer E. J. Calmodulin activates the isolated catalytic unit of brain adenylate cyclase. J Biol Chem. 1981 Oct 10;256(19):9830–9833. [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Calmodulin stimulation of adenylate cyclase in rat brain membranes does not require GTP. Life Sci. 1982 Apr 26;30(17):1457–1464. doi: 10.1016/0024-3205(82)90559-8. [DOI] [PubMed] [Google Scholar]
- Smigel M., Katada T., Northup J. K., Bokoch G. M., Ui M., Gilman A. G. Mechanisms of guanine nucleotide-mediated regulation of adenylate cyclase activity. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:1–18. [PubMed] [Google Scholar]
- Teshima Y., Kakiuchi S. Membrane-bound forms of Ca2+-dependent protein modulator: Ca2+-dependent and independent binding of modulator protein to the particulate fraction from brain. J Cyclic Nucleotide Res. 1978 Jun;4(3):219–231. [PubMed] [Google Scholar]
- Yeager R. E., Heideman W., Rosenberg G. B., Storm D. R. Purification of the calmodulin-sensitive adenylate cyclase from bovine cerebral cortex. Biochemistry. 1985 Jul 2;24(14):3776–3783. doi: 10.1021/bi00335a054. [DOI] [PubMed] [Google Scholar]
- d'Alayer J., Berthillier G., Monneron A. Structure of brain adenylate cyclase: proteolysis-dependent modifications. Biochemistry. 1983 Aug 2;22(16):3948–3953. doi: 10.1021/bi00285a034. [DOI] [PubMed] [Google Scholar]