Abstract
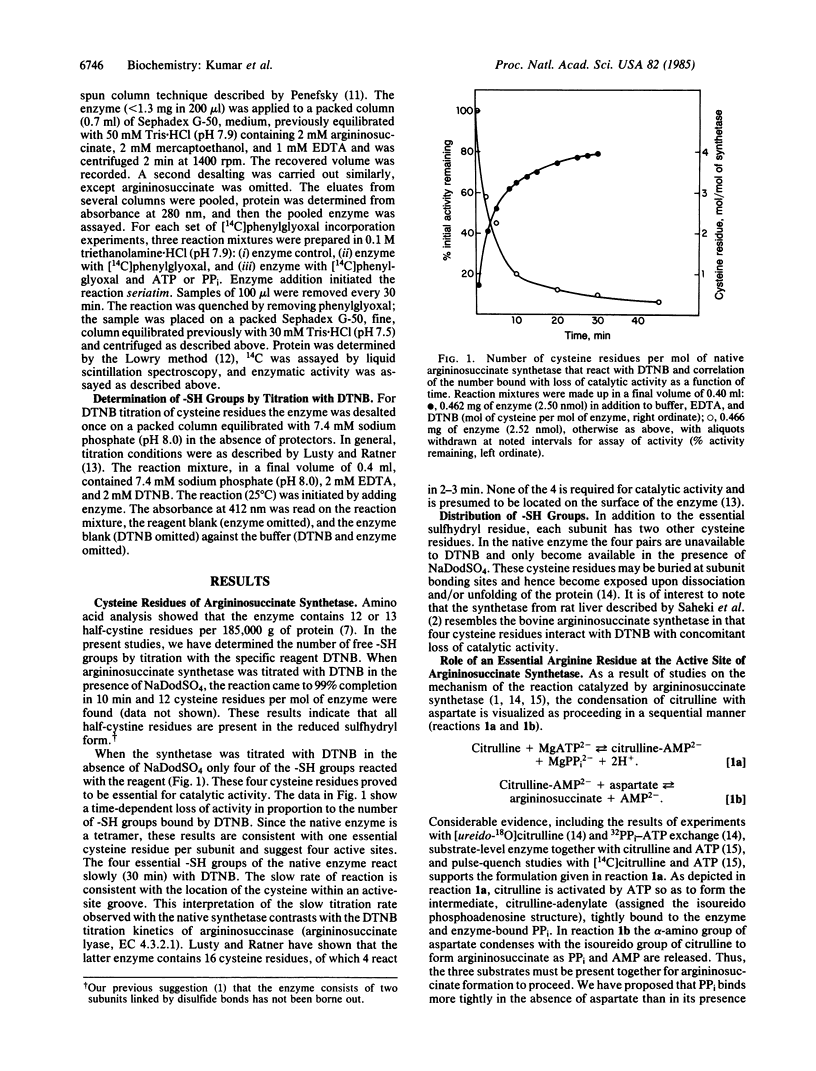
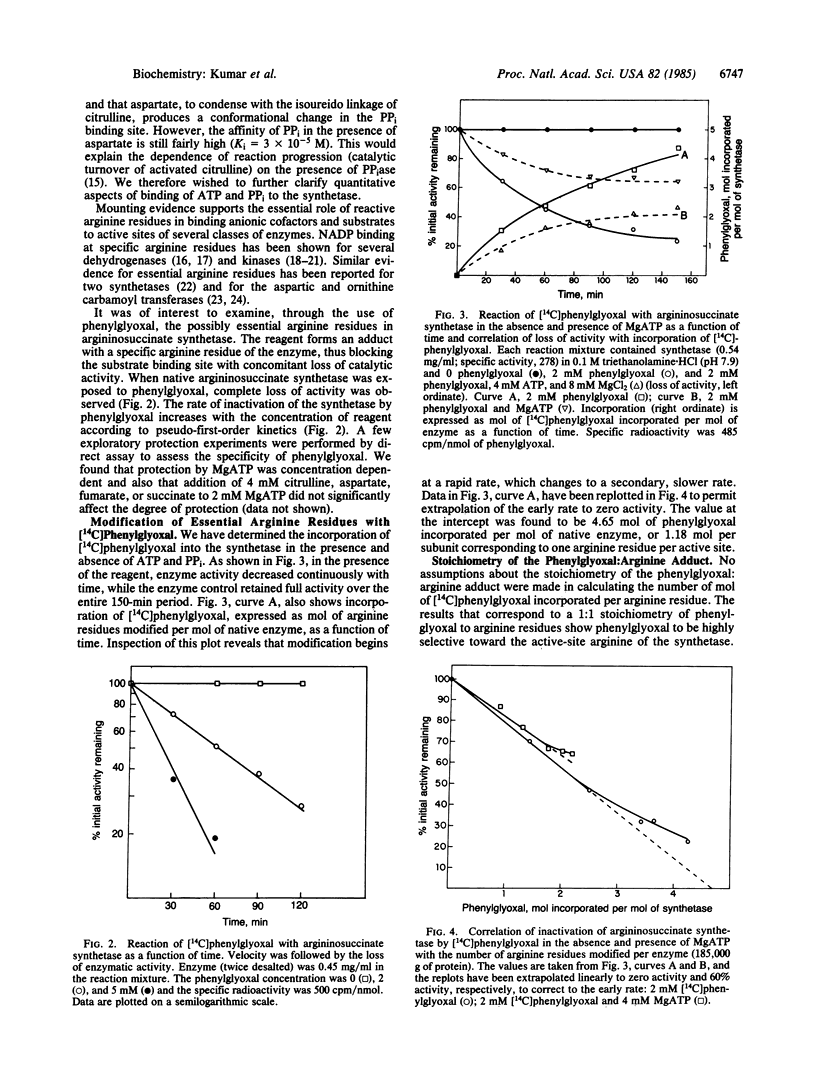
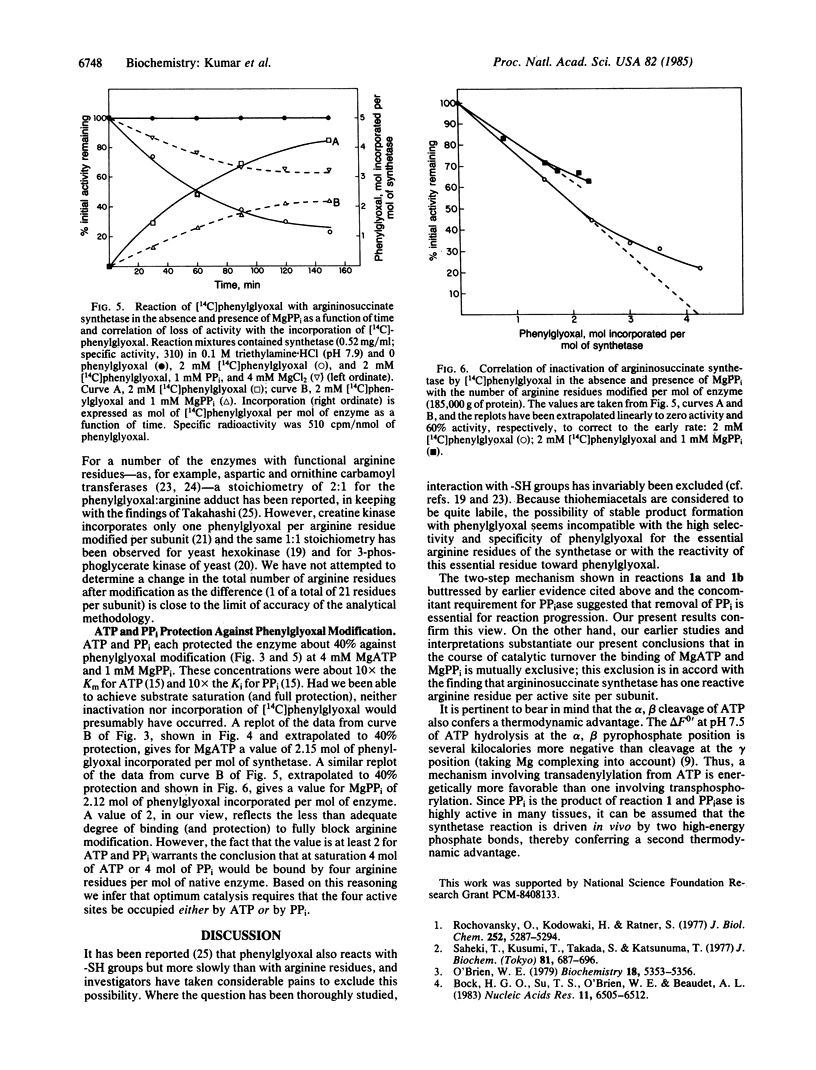
We have undertaken studies to identify amino acid residues that are involved in the catalytic mechanism of argininosuccinate synthetase [L-citrulline:L-aspartate ligase (AMP-forming), EC 6.3.4.5] and have found that a cysteine residue and an arginine residue are required for activity. The reactive cysteine residues are accessible to solvent and available to react with 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB). Four cysteine residues, one per subunit, are shown by enzymatic assay to be required for catalytic activity, suggesting that a reactive cysteine lies within the active site of argininosuccinate synthetase. In the presence of sodium dodecyl sulfate, 12 cysteine residues react with DTNB; consequently, all of the half-cystine residues in the native enzyme are present in the reduced sulfhydryl form. We also present evidence for the participation of arginine groups in the binding of ATP and PPi. Modification of argininosuccinate synthetase with [14C]-phenylglyoxal results in incorporation concomitant with loss of catalytic activity of 4 mol of phenylglyoxal per mol of native enzyme (one arginine per active site). ATP and PPi protect the enzyme from phenylglyoxal incorporation. Based on these results, we propose that the essential arginine in the active site participates in the binding of ATP and PPi. The binding of ATP and PPi at the same site is mutually exclusive; this exclusion is in accord with the finding that argininosuccinate synthetase has one reactive arginine residue per active site per subunit. This is consistent with our previously proposed reaction mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berghäuser J. A reactive arginine in adenylate kinase. Biochim Biophys Acta. 1975 Aug 26;397(2):370–376. doi: 10.1016/0005-2744(75)90126-6. [DOI] [PubMed] [Google Scholar]
- Blumenthal K. M., Smith E. L. Functional arginine residues involved in coenzyme binding by glutamate dehydrogenases. J Biol Chem. 1975 Aug 25;250(16):6555–6559. [PubMed] [Google Scholar]
- Bock H. G., Su T. S., O'Brien W. E., Beaudet A. L. Sequence for human argininosuccinate synthetase cDNA. Nucleic Acids Res. 1983 Sep 24;11(18):6505–6512. doi: 10.1093/nar/11.18.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borders C. L., Jr, Riordan J. F. An essential arginyl residue at the nucleotide binding site of creatine kinase. Biochemistry. 1975 Oct 21;14(21):4699–4704. doi: 10.1021/bi00692a021. [DOI] [PubMed] [Google Scholar]
- Fortin A. F., Hauber J. M., Kantrowitz E. R. Comparison of the essential arginine residue in Escherichia coli ornithine and aspartate transcarbamylases. Biochim Biophys Acta. 1981 Nov 13;662(1):8–14. doi: 10.1016/0005-2744(81)90216-3. [DOI] [PubMed] [Google Scholar]
- HAVIR E. A., TAMIR H., RATNER S., WARNER R. C. BIOSYNTHESIS OF UREA. XI. PREPARATION AND PROPERTIES OF CRYSTALLINE ARGININOSUCCINASE. J Biol Chem. 1965 Jul;240:3079–3088. [PubMed] [Google Scholar]
- Kantrowitz E. R., Lipscomb W. N. An essential residue at the active site of aspartate transcarbamylase. J Biol Chem. 1976 May 10;251(9):2688–2695. [PubMed] [Google Scholar]
- Lange L. G., 3rd, Riordan J. F., Vallee B. L. Functional arginyl residues as NADH binding sites of alcohol dehydrogenases. Biochemistry. 1974 Oct 8;13(21):4361–4370. doi: 10.1021/bi00718a019. [DOI] [PubMed] [Google Scholar]
- Lusty C. J., Ratner S. Biosynthesis of urea. XIV. The quaternary structure of argininosuccinase. J Biol Chem. 1972 Nov 10;247(21):7010–7022. [PubMed] [Google Scholar]
- O'Brien W. E. Isolation and characterization of argininosuccinate synthetase from human liver. Biochemistry. 1979 Nov 27;18(24):5353–5356. doi: 10.1021/bi00591a015. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Philips M., Pho D. B., Pradel L. A. An essential arginyl residue in yeast hexokinase. Biochim Biophys Acta. 1979 Feb 9;566(2):296–304. doi: 10.1016/0005-2744(79)90033-0. [DOI] [PubMed] [Google Scholar]
- Philips M., Roustan C., Fattoum A., Pradel L. A. Yeast 3-phosphoglycerate kinase. Essential arginyl residues at the 3-phosphoglycerate binding site. Biochim Biophys Acta. 1978 Apr 12;523(2):368–376. doi: 10.1016/0005-2744(78)90039-6. [DOI] [PubMed] [Google Scholar]
- Powers S. G., Riordan J. F. Functional arginyl residues as ATP binding sites of glutamine synthetase and carbamyl phosphate synthetase. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2616–2620. doi: 10.1073/pnas.72.7.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROCHOVANSKY O., RATNER S. Biosynthesis of ureas. IX. Further studies on mechanism of argininosuccinate synthetase reaction. J Biol Chem. 1961 Aug;236:2254–2260. [PubMed] [Google Scholar]
- Ratner S. Argininosuccinate synthetase of bovine liver: chemical and physical properties. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5197–5199. doi: 10.1073/pnas.79.17.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner S., Murakami-Murofushi K. A new radiochemical assay for argininosuccinase with purified [14C]argininosuccinate. Anal Biochem. 1980 Jul 15;106(1):134–147. doi: 10.1016/0003-2697(80)90129-3. [DOI] [PubMed] [Google Scholar]
- Rochovansky O., Kodowaki H., Ratner S. Biosynthesis of urea. Molecular and regulatory properties of crystalline argininosuccinate synthetase. J Biol Chem. 1977 Aug 10;252(15):5287–5294. [PubMed] [Google Scholar]
- Rochovansky O., Ratner S. Biosynthesis of urea. XII. Further studies on argininosuccinate synthetase: substrate affinity and mechanism of action. J Biol Chem. 1967 Sep 10;242(17):3839–3849. [PubMed] [Google Scholar]
- SCHUEGRAF A., RATNER S., WARNER R. C. Free energy changes of the argininosuccinate synthetase reaction and of the hydrolysis of the inner pyrophosphate bond of adenosine triphosphate. J Biol Chem. 1960 Dec;235:3597–3602. [PubMed] [Google Scholar]
- Saheki T., Kusumi T., Takada S., Katsunuma T. Studies of rat liver argininosucciante synthetase. I. Physicochemical, catalytic, and immunochemical properties. J Biochem. 1977 Mar;81(3):687–696. doi: 10.1093/oxfordjournals.jbchem.a131505. [DOI] [PubMed] [Google Scholar]
- Takahashi K. The reaction of phenylglyoxal with arginine residues in proteins. J Biol Chem. 1968 Dec 10;243(23):6171–6179. [PubMed] [Google Scholar]


