Abstract
Adolescents and young adults have the highest rates of sexually transmitted infections (STIs) in the USA despite national priority goals targeting their reduction. Research on the role of neighborhoods in shaping STI risk among youth has increased in recent years, but few studies have explored the longitudinal effects of neighborhoods on STI acquisition during the adolescent to young adult transition. The aims of this study were to examine: (1) the longitudinal relationships between the neighborhood context (poverty, residential instability, and racial/ethnic concentration) of exposure during adolescence and young adults’ acquisition of chlamydia, and (2) the extent to which sexual risk behaviors and depression over the transition from adolescence to young adulthood mediate the relationship between the neighborhood context of exposure during adolescence and young adults’ acquisition of chlamydia. A longitudinal observational design was employed using data from the National Longitudinal Study of Adolescent Health (Add Health), waves 1–3 (1994–2002). The sample was composed of 11,460 young adults aged 18 to 27 years. Neighborhood measures during adolescence were derived from the 1990 US Census appended to adolescents’ interview data. Chlamydia infection was measured via urine assay at wave 3 and 4.6 % of the young adults in the sample tested positive for chlamydia. Multilevel logistic regression analyses were conducted adjusting for numerous neighborhood and individual risk factors. Multivariate findings indicated exposure to neighborhood poverty during adolescence increased the likelihood of a positive urine test for chlamydia during young adulthood (AOR = 1.23, 95 % CI = 1.06, 1.42), and the association was not mediated by sexual risk behaviors or depression. Further research is needed to better understand the pathways through which exposure to neighborhood poverty contributes to chlamydia over the life course as are comprehensive STI prevention strategies addressing neighborhood poverty.
Keywords: Neighborhood, Chlamydia, Sexually transmitted infection, Life course
Introduction
Adolescents and young adults have the highest rates of sexually transmitted infections (STIs) in the United States1,2 despite national priority goals targeting their reduction.3 Thus, calls to address the contribution of the social determinants of health to STIs among this vulnerable population have increased.3 One growing body of research has focused on neighborhood context—primarily contemporary neighborhood racial and ethnic composition and poverty concentration and their associations with STI outcomes. These studies are predominantly rooted in social disorganization theory,4–6 which posits neighborhoods characterized by racial and ethnic heterogeneity, concentrated poverty, and residential instability are more likely to experience a wide range of problematic outcomes due to weakened social ties and informal social control capacities and reduced access to institutional resources (e.g., quality schools and health care). Study findings generally support the basic claims of social disorganization theory with respect to associations between community poverty and STI across a range of pathogens. Specifically, ecological studies found higher community rates of gonorrhea,7–10 chlamydia,7 syphilis,7 and HIV11 in communities characterized by concentrated poverty, highlighting the clustering of STIs in vulnerable geographic contexts. In addition, two recent studies, one using cross-sectional multilevel analyses12 and the other a generalized estimating equation approach13 found young adults living in neighborhoods with higher levels of concentrated poverty were more likely to have trichomoniasis or repeated chlamydia infections compared to their peers in more advantaged neighborhoods. Both adjusted for a host of potential confounding relationships strengthening the evidence that neighborhood poverty is associated with individual acquisition of STI above and beyond differences in levels of risk.
Social disorganization theory also posits that racially and ethnically diverse neighborhoods weaken social ties between residents due to distrust of dissimilar “others” whereas homogeneity is thought to enhance community cohesion and facilitate recognition of shared norms and goals.4–6 However, segregated African American communities do not appear to benefit from racial homogeneity with respect to STI outcomes, perhaps due to the negative effects of protracted poverty and social isolation in these communities.6 For example, prior multilevel research found that young adults who lived in neighborhoods with higher concentrations of Black residents were more likely to have trichomoniasis compared to their peers in less segregated neighborhoods, and this relationship was mediated by neighborhood poverty.12 Thus, the neighborhood racial disparity in trichomoniasis was hypothesized to be due in part to higher rates of poverty more commonly experienced in racially segregated African American neighborhoods.12 Furthermore, racial discrimination is linked to the formation of racially segregated sexual networks, and to the disproportionate incarceration of African American males.14 Combined, the subsequent reduction in the male-to-female sex ratio due to incarceration, the segregation of sexual networks, and the high levels of concentrated poverty that characterize many segregated African American communities lead to the development of high-risk environments primed for STI epidemics. In contrast to segregated African American communities, immigrant enclaves are hypothesized to be protective against STI due to traditional norms regarding sexual activity.15 Research examining these relationships is limited though, and the findings mixed. For example, an ecological study found lower incidence rates of gonorrhea and chlamydia in neighborhoods in which 60 % or more of the residents were Hispanic compared to those with similar proportions of Black residents.15 However, no significant association between immigrant concentration and trichomoniasis was found in the aforementioned multilevel study,12 although the direction of the relationship was negative. Differences in the measurement of what constitutes an immigrant enclave, sampling design (local vs national), or analytic strategy (ecological vs multilevel analyses) could account for the disparate findings.
Residentially unstable communities also are hypothesized to experience weakened social ties, trust, and social control capacities due to higher proportions of renters and the greater frequency of in- and out-migration of community members.4–6 High-risk behaviors may then be more likely to occur in residentially unstable communities due to perceptions of greater anonymity and lower informal social control capacities. However, few studies have focused on the effects of residential instability on STI outcomes compared to other community characteristics, and the findings have been contradictory to the disorganization hypothesis. For example, one study found residential instability was associated with fewer self-reported STIs,16 whereas another found no significant relationship between residential instability and trichomoniasis, but the direction of the relationship was consistent with the former study.12 These counter-intuitive findings suggest that rather than increasing STIs due to disrupted social ties, residential instability may reduce STI risk perhaps through the prevention of closed sexual networks12 that are known to increase STI transmission.17
Although previous research has elucidated linkages between neighborhood social disorganization and acquisition of STI, particularly with respect to neighborhood poverty, limitations exist. First, most studies on neighborhoods and STIs have employed ecological analyses, in which the STI outcome was measured at the community level (e.g., community STI rate) via administrative data.7–9,11 Although ecological analyses provide important information about the community context, all data are at the community-level precluding investigation of the variation in individual outcomes and adjustment for potential confounding relationships between the community and individual-level data.18 Consequently, mixed findings between ecological and multilevel analyses on the relationships between neighborhood characteristics and STI outcomes may be explained, in part, by the adjustment of confounding individual and family risk factors in the multilevel model. Second, the majority of studies have focused on contemporaneous relationships.7–9,11 However, life course theory posits that social processes across contexts and over time shape human development and that their effects on outcomes may be more salient during transitions,19 such as the transition from adolescence to young adulthood. Thus, the neighborhood conditions to which individuals are exposed during adolescence may influence their subsequent STI risk during young adulthood. Third, research exploring the neighborhood and individual mechanisms through which the neighborhood context may contribute to individual STI outcomes is limited, but needed to advance our understanding of how neighborhoods operate. For example, the prior research discussed previously found neighborhood poverty mediated the relationship between neighborhood racial concentration of African Americans and young adults’ acquisition of trichomoniasis. Furthermore, others have found neighborhood poverty and neighborhood physical and social disorder were associated with an increased engagement in sexual risk behaviors20–23 and depression24–26 among adolescents—outcomes also linked to adolescents’ STI acquisition1,27 as well as to one another.27,28 However, the majority of prior research has been either cross-sectional or focused on only one phase of the life course, primarily adolescence.
Therefore, the aims of this study were to examine: (1) the longitudinal relationships between the neighborhood context (poverty, residential instability, and racial/ethnic concentration) of exposure during adolescence and young adults’ acquisition of chlamydia, including the potential mediating effects of concentrated poverty and residential instability on the relationship between racial and ethnic concentration and chlamydia infection, and (2) the extent to which sexual risk behaviors and depression over the transition from adolescence to young adulthood mediate the relationship between the neighborhood context of exposure during adolescence and young adults’ acquisition of chlamydia. This study builds on previous research in three significant ways: (1) employment of multilevel analysis to simultaneously examine individual and neighborhood factors that may affect the likelihood of chlamydia; (2) employment of a longitudinal design that included a host of neighborhood and individual measures collected at two-time points during the transition from adolescence to young adulthood; and (3) examination of potential neighborhood and individual-level mechanisms through which the broader neighborhood context may contribute to STI during the transition from adolescence to young adulthood.
Methods
Study Design and Sample
Data for this study were from the National Longitudinal Study of Adolescent Health (Add Health), waves 1 (1994–1995) and 3 (2001–2002).29,30 Add Health is a school-based, longitudinal study whose participants were in the 7th–12th grade at the first wave of data collection. Currently, four waves of data have been collected from multiple sources spanning adolescence to young adulthood. The sample for this study was comprised of those respondents from wave 1 who were relocated and interviewed during the wave 3 data collection time frame (N = 15,170) with a weighted response rate of 75.6 %.31 Add Health provides sampling weights for 14,322 young adults, and of these 12,545 respondents had urine assay results for chlamydia. Participants missing data on model covariates were excluded from the analysis (n = 1,085 or 8.6 %) for a final sample size of 11,460 young adults aged 18 to 27 years. Analysis indicated those missing from the sample were more likely to experience individual and neighborhood poverty at both waves, live in racially concentrated neighborhoods at both waves, and live in residentially unstable neighborhoods at wave 1. Although participants who were excluded were from more vulnerable populations known to be at higher risk for the outcome, we found no statistically significant differences in the odds of chlamydia infection between young adults missing from the sample and those included in the final analysis. Sensitivity analyses addressing the possible bias introduced by listwise deletion of item missing respondents resulted in comparable findings to those presented below (available upon request).
Dependent Variable
The dependent variable was infection with chlamydia (yes = 1) at wave 3. Add Health respondents provided a urine sample taken from their first stream of urine on the day of the wave 3 interview, which was tested for chlamydia via Ligase Chain Reaction (LCR™) amplification technology in the Abbott LCx® Probe System.32
Independent Variables
Neighborhood Social Context during Adolescence
The neighborhood was defined as the census tract of residence; consistent with previous research.12 Wave 1 neighborhood measures were derived from the 1990 US Census and provided to researchers in Add Health’s contextual data set. Three indicators of the neighborhood social context were examined—racial and ethnic concentration, concentrated poverty, and residential instability. Racial and ethnic concentration consisted of two measures—(1) Black residential concentration measured as the proportion of Black residents in the census tract and (2) ethnic residential concentration measured via the mean of three standardized items: proportion of Latino/Hispanic residents, proportion of linguistically isolated residents, and proportion of foreign-born residents (α = 0.95). Concentrated poverty was measured as the mean of four standardized items: proportion of households below poverty, proportion of households on public assistance, total unemployment rate, and proportion of female-headed households with children (α = .82). Residential instability was measured as the mean of two standardized items: proportion of households living in the census tract for 5 years or more and proportion of owner occupied homes (α = .82).
STI Risk Factors
Sexual risk behaviors and depression measures were included in the analysis as potential mediators of the relationship between the neighborhood context during adolescence and young adults’ acquisition of chlamydia. Sexual risk behaviors included: age at first vaginal intercourse (18–27 years, 16–17 years, 10–15 years, or never—reference ); STI in the year prior to wave 1 (yes = 1); multiple vaginal sex partners in the year prior to wave 3 (three or more partners in the past year; yes = 1); condom used at last sex (yes = 1); level of binge drinking in year prior to wave 3 (number of days during the past year drank five or more drinks ranging from never to every/almost every day); and drug use during the month prior to wave 3 (if used marijuana, methamphetamine, cocaine, or other illegal drug; yes = 1). Depression was based on nine items from the CES-D available at both waves; measurement was consistent with prior research investigating depression and STI.27 Items were summed and respondents who scored greater than 10 on the scale were considered depressed. A categorical measure was then created for those who reported depression only at wave 1 (past), depression only at wave 3 (current), depression at both waves (chronic), or no depression at either wave (reference).
Control Variables
A host of neighborhood and individual control variables from both waves were included in the analyses. Wave 3 neighborhood control measures were included to examine associations between the contemporaneous neighborhood and young adults’ acquisition of chlamydia: racial and ethnic concentration, concentrated poverty, residential instability, and urbanicity (proportion of residents in the census tract living in an urban area). The variables were measured identically to the wave 1 neighborhood context measures except they were derived from the 2000 US Census. Wave 1 neighborhood urbanicity was also included as a control variable. The following individual-level sociodemographic factors derived from waves 1 and 3 in-home interviews were included: gender (male = 1), race and ethnicity (non-Hispanic Black, Hispanic, non-Hispanic “other”, or non-Hispanic white—reference); foreign birth (yes = 1), heterosexual orientation at wave 3 (yes = 1); parental economic hardship at wave 1 (received food stamps, housing assistance, or AFDC in the past year; yes = 1), lived in a two parent household at wave 1 (yes = 1), respondent economic hardship at wave 3 (received food stamps, housing assistance, or AFDC in the past year; yes = 1); employed at least 10 h weekly at wave 3 (yes = 1); attending high school or college at wave 3 (yes = 1); married at wave 3 (yes = 1); living with parents at wave 3 (yes = 1); and the number of times participants moved between waves 1 and 3 (continuous measure). In addition, a measure for antibiotic use in the month prior to wave 3 was included to adjust for potential treatment of an undiagnosed STI (yes = 1).
Analysis
Descriptive statistics were conducted using SAS survey procedures, version 9.2 (SAS Institute, Cary, NC) to examine the prevalence of chlamydia and the sample characteristics. Multilevel modeling for non-linear outcomes was employed for multivariate analyses using HLM 6.08 software (Scientific Software International, Lincolnwood, IL). Because Add Health does not provide survey weights for multilevel models analyzing neighborhoods, all multilevel analyses were conducted unweighted. However, school stratification variables were included in the multilevel analyses to adjust for the sampling design as directed by Add Health (personal communication, Kim Chantala, Add Health User’s Conference, 2008). These variables included geographic area (northeast, west, midwest, and south—reference), school size, school urbanicity, school type (public or private), and ethnic mix (proportion of students who were non-Hispanic White).29,30 Sensitivity analyses were conducted using standard logistic regression (all neighborhood measures disaggregated to the individual level), weighted (to account for attrition, oversampling) and adjusted for the complex survey design. Findings of both analytic strategies were consistent with one another, thus we present the findings from the multilevel analyses due to our primary interest in the wave 1 neighborhood effects.
We examined five random intercepts logistic regression models estimated using LaPlace estimation procedures. We focused the first two models on the contemporaneous relationships between neighborhood social context during young adulthood and young adults’ odds of being infected with chlamydia. The wave 3 neighborhood measures were disaggregated to the individual-level for analysis because our key interest was in the longitudinal effects of neighborhood social context at wave 1 on subsequent chlamydia infection. The intercept random effect adjusts standard errors of wave 1 neighborhood coefficients for clustering at that wave (clustering within neighborhood at wave 3 was substantially reduced due to geographic dispersion of the sample). Models 3 and 4 focused on the associations between wave 1 neighborhood measures and chlamydia infection, such that model 3 included wave 1 neighborhood racial and ethnic concentration and in model 4, neighborhood concentrated poverty and residential instability were added to the analysis. We selected this model building strategy as prior research found neighborhood poverty mediated the relationship between neighborhood racial concentration and trichomoniasis, thus we wanted to examine these potential relationships. Model 5 examined the extent to which STI risk behaviors and depression mediated the relationships between the wave 1 neighborhood context and young adults’ acquisition of chlamydia. In model 5, chlamydia infection at wave 3 is modeled as a function of wave 1 sociodemographic characteristics, STI risk factors, and depression at the individual level, with the effects of relevant wave 1 neighborhood social context measures estimated as a separate level in the model. Specifically, Yij takes on a value of unity if person i in wave 1 neighborhood j has chlamydia at wave 3 (otherwise Yij = 0), and let μij denote the probability Yij = 1.
At level 1, the log-odds of chlamydia at wave 3 are as follows:
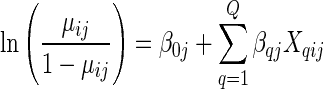 |
where β0j is the intercept, βqj are coefficients describing the effects of Q individual level covariates X (sociodemographic, STI-risk factors, and depression) on the log odds of chlamydia at wave 3.
At level 2,
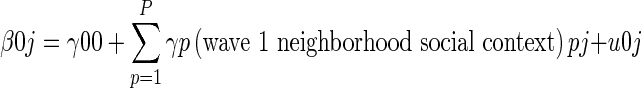 |
Where γ00 is the overall grand mean, γp are the wave 1 neighborhood social context measures of interest (racial and ethnic concentration, concentrated poverty, and residential instability) that may independently contribute to chlamydia risk (along with additional neighborhood controls), and u0j is the neighborhood-level error term.
Results
Descriptive statistics are presented in Table 1, including unweighted and weighted proportions and means. Findings were consistent with the exception of race and ethnicity due to oversampling and weighted adjustments. The unweighted findings indicated 4.6 % of young adults in the study sample had chlamydia at wave 3. The mean age was 22 years, 47 % were male, 89 % reported they were heterosexual, and nearly 18 % were married. The majority self-identified as non-Hispanic white (55 %) and 8 % were foreign born. The young adults reported greater household economic hardship during adolescence (17 %) compared to young adulthood (7 %). Descriptive statistics of additional control measures are presented in Table 1.
Table 1.
Characteristics of the unweighted and weighted sample of young adults aged 18–27 years, waves 1–3 of the National Longitudinal Study of Adolescent Health (Add Health), N = 11,460
| Unweighted sample | Weighted sample | |
|---|---|---|
| % | % (SE) | |
| Positive urine screen for Chlamydia | 4.6 | 4.1 (0.36) |
| Male | 47.2 | 50.5 (0.68) |
| Race and ethnicity | ||
| Hispanic | 16.3 | 11.6 (1.73) |
| Black | 20.5 | 15.3 (1.97) |
| Other | 8.0 | 4.5 (0.79) |
| White (reference) | 55.2 | 68.6 (2.91) |
| Foreign born | 7.9 | 5.7 (0.85) |
| Heterosexual orientation (w3) | 89.3 | 89.3 (0.45) |
| Parental economic hardship (w1) | 17.0 | 17.3 (1.27) |
| Two parent household (w1) | 54.5 | 56.8 (1.28) |
| Economic hardship (w3) | 7.0 | 6.9 (0.51) |
| Employed (w3) | 70.1 | 70.2 (1.01) |
| Enrolled in school (w3) | 38.0 | 37.0 (1.53) |
| Married (w3) | 17.6 | 17.2 (0.99) |
| Living with parents (w3) | 39.9 | 39.1 (1.33) |
| Antibiotics prior month (w3) | 13.5 | 13.7 (0.49) |
| Age at first vaginal intercourse | ||
| 18–25 years | 27.6 | 26.2 (0.98) |
| 16–17 years | 30.7 | 31.0 (0.62) |
| 10–15 years | 29.1 | 30.3 (1.01) |
| Has not has vaginal intercourse | 12.6 | 12.5 (0.56) |
| STI in prior year (w1) | 2.2 | 2.2 (0.27) |
| 3+ sex partners prior year (w3) | 14.7 | 15.0 (0.53) |
| Condom use last sex (w3) | 56.7 | 56.3 (0.73) |
| Drug use prior year (w3) | 22.7 | 24.5 (0.79) |
| Depression | ||
| Depressed wave 1 only | 12.3 | 11.0 (0.49) |
| Depressed wave 3 only | 15.3 | 15.1 (0.55) |
| Depressed waves 1 and 3 | 6.4 | 6.1 (0.37) |
| Never depressed (reference) | 66.0 | 67.7 (0.70) |
| Mean (SD) | Mean (SE) | |
| Age in years (w1) range 11–21 years | 15.6 (1.73) | 15.4 (0.12) |
| Number times moved since wave 1 range 0–10 | 2.1 (2.14) | 2.1 (0.07) |
| Binge drinking prior year (w3) range 0–6 | 1.20 (1.56) | 1.34 (0.05) |
Multivariate results are presented in two tables with Table 2 including models 1 and 2 and Table 3 including models 3–5. Because the prevalence of chlamydia in our sample was low (4.6 %), the presented odds ratios approximate relative risk ratios. Models 1 and 2 examined the associations between the contemporary neighborhood control variables and young adults’ acquisition of chlamydia. In model 1, no significant relationships between wave 3 neighborhood racial or ethnic concentration and young adults’ odds of having a chlamydia infection were found. Neighborhood concentrated poverty and residential instability measures were included simultaneously in model 2; no significant associations between either of the indicators and young adults’ acquisition of chlamydia were found. Models 3–5 focused on the longitudinal associations between the neighborhood context of exposure during adolescence and young adults’ acquisition of chlamydia, including potential mediating relationships. Specifically, in model 3, neither the racial nor the ethnic concentration of the neighborhood of exposure during adolescence increased the odds of young adults’ acquisition of chlamydia. Model 4 included the indicators for neighborhood residential instability and concentrated poverty. No significant associations between living in a residentially unstable neighborhood during adolescence and young adults’ acquisition of chlamydia were found. However, young adults who lived in a neighborhood with higher concentrations of poverty during their adolescence (wave 1) had a higher odds of chlamydia at wave 3 compared to their more advantaged peers (AOR = 1.25, 95 % CI = 1.08, 1.45). Furthermore in model 5, the lagged effect of exposure to neighborhood poverty during adolescence on young adults’ odds of chlamydia at wave 3 was statistically significant and the size of the effect relatively unchanged after accounting for potential mediating relationships with young adults’ STI risk factors and depression (AOR = 1.23, 95 % CI = 1.06, 1.42).
Table 2.
Multilevel logistic regression: relationships between the neighborhood social context and chlamydia in young adulthood—National Survey of Adolescent Health (Add Health), waves 1–3, N = 11,460
| Model 1 | Model 2 | |
|---|---|---|
| AOR (95 % CI) | AOR (95 % CI) | |
| Level 1 fixed effects | ||
| STI risk factors | ||
| Age at first vaginal intercourse | ||
| 18–25 years | ||
| 16–17 years | ||
| 10–15 years | ||
| Has not has vaginal intercourse | ||
| STI in prior year (w1) | ||
| 3+ sex partners prior year (w3) | ||
| Condom use last sex (w3) | ||
| Binge drinking prior year (w3) | ||
| Drug use prior year (w3) | ||
| Depression | ||
| Depressed wave 1 only | ||
| Depressed wave 3 only | ||
| Depressed waves 1 and 3 | ||
| Never depressed (reference) | ||
| Wave 3 neighborhood context | ||
| Proportion Black concentration | 1.08 (0.96, 1.21) | 1.04 (0.90, 1.21) |
| Immigrant concentration | 1.01 (0.89, 1.15) | 0.99 (0.86, 1.14) |
| Concentrated poverty | 1.06 (0.89, 1.25) | |
| Residential instability | 0.99 (0.86, 1.15) | |
| Proportion urban | 0.94 (0.69, 1.28) | 0.96 (0.69, 1.31) |
| Level 2 fixed effects | ||
| Wave 1 neighborhood context | ||
| Proportion Black concentration | ||
| Immigrant concentration | ||
| Concentrated poverty | ||
| Residential instability | ||
| Proportion urban | ||
| Intercept | 0.03 (0.02, 0.04)*** | 0.03 (0.02, 0.04)*** |
| Random effect | ||
| Tau | 0.02435 | 0.02563 |
aUnweighted analysis adjusted for wave 1 stratification variables of geographic region, school urbanicity, school size, and ethnic mix
bAll models also included the following control variables: gender, age, race and ethnicity, foreign birth, sexual orientation, wave 1 family structure, number of residential move between waves, wave 1 and 3 economic hardship, and wave 3 marital status, employment, school attendance, living with parents, and antibiotic use
*p < 0.05; **p < 0.01; ***p < 0.001
Table 3.
Multilevel logistic regression: relationships between the neighborhood social context and chlamydia in young adulthood—National Survey of Adolescent Health (Add Health), waves 1–3, N = 11,460a
| Model 3 | Model 4 | Model 5 | |
|---|---|---|---|
| AOR (95 % CI) | AOR (95 % CI) | AOR (95 % CI) | |
| Level 1 fixed effectsb | |||
| STI risk factors | |||
| Age at first vaginal intercourse | |||
| 18–25 years | 1.60 (0.99, 2.56) | ||
| 16–17 years | 1.94 (1.25, 3.02)** | ||
| 10–15 years | 1.80 (1.15, 2.82)* | ||
| Has not has vaginal intercourse | 1.00 | ||
| STI in prior year (w1) | 0.92 (0.51, 1.66) | ||
| 3+ sex partners prior year (w3) | 1.52 (1.19, 1.94)** | ||
| Condom use last sex (w3) | 1.03 (0.84, 1.26) | ||
| Binge drinking prior year (w3) | 1.02 (0.95, 1.11) | ||
| Drug use prior year (w3) | 1.28 (0.98, 1.66) | ||
| Depression | |||
| Depressed wave 1 only | 0.94 (0.70, 1.25) | ||
| Depressed wave 3 only | 0.87 (0.66, 1.16) | ||
| Depressed waves 1 and 3 | 1.30 (0.90, 1.86) | ||
| Never depressed (reference) | 1.00 | ||
| Wave 3 neighborhood context | |||
| Proportion Black concentration | |||
| Immigrant concentration | |||
| Concentrated poverty | |||
| Residential instability | |||
| Proportion urban | |||
| Level 2 fixed effects | |||
| Wave 1 neighborhood context | |||
| Proportion Black concentration | 1.07 (0.97, 1.18) | 0.95 (0.84, 1.08) | 0.96 (0.85, 1.09) |
| Immigrant concentration | 0.94 (0.83, 1.07) | 0.91 (0.80, 1.05) | 0.91 (0.80, 1.05) |
| Concentrated poverty | 1.25 (1.08, 1.45)** | 1.23 (1.06, 1.42)** | |
| Residential instability | 0.92 (0.79, 1.06) | 0.92 (0.80, 1.06) | |
| Proportion urban | 0.76 (0.58, 1.01) | 0.79 (0.59, 1.06) | 0.80 (0.60, 1.06) |
| Intercept | 0.03 (0.02, 0.04)*** | 0.03 (0.02, 0.04)*** | 0.02 (0.01, 0.04)*** |
| Random effect | |||
| Tau | 0.02060 | 0.03706 | 0.00018 |
aUnweighted analysis adjusted for wave 1 stratification variables of geographic region, school urbanicity, school size, and ethnic mix
bAll models also included the following control variables: gender, age, race and ethnicity, foreign birth, sexual orientation, wave 1 family structure, number of residential move between waves, waves 1 and 3 economic hardship, and wave 3 marital status, employment, school attendance, living with parents, and antibiotic use
*p < 0.05; **p < 0.01; ***p < 0.001
Discussion
Our study was one of the first to find evidence that exposure to neighborhood poverty during adolescence increases the likelihood of chlamydia infection during young adulthood, above and beyond numerous STI risk factors occurring at both time-points. These findings are in accordance with life course theory,19 and prior research on the longitudinal effects of neighborhood poverty and other health outcomes such as body mass index, weight gain,33 asthma, diabetes, hypertension, heart disease, and stroke.34 However, in contrast to the lagged effects of neighborhood poverty on chlamydia infection, our study did not find significant longitudinal relationships between the neighborhood racial and ethnic concentration or residential instability and young adults’ STI acquisition. While it is plausible these neighborhood measures have no longitudinal effects on acquisition of chlamydia, alternative explanations should be explored. For example, the lagged effects of living in neighborhoods with high concentrations of Black or Hispanic residents during adolescence on young adults’ acquisition of chlamydia may vary by the social processes occurring within the neighborhoods (e.g., social cohesion, trust, norms) or by individual characteristics (race/ethnicity, SES; coping skills). Thus, researchers should employ more comprehensive data on the social processes characterizing neighborhoods and their potential interaction with compositional factors. The potential for cross-level interactions with key individual level factors should also be explored. In addition, Add Health data do not include measures for contiguous census tracts, thus our neighborhood measures do not capture residence in larger segregated regions that may amplify STI risk. Future research using indices of residential segregation is needed to strengthen our understanding of the depth of community exclusion from others on STI outcomes.35 Last, the multiple neighborhoods to which we belong over our life course could cumulatively influence our health or the effects of neighborhood exposures may be more salient at critical phases in the life course. For example, Wodtke, Harding, and Elwert36 recently found prolonged exposure to neighborhood socioeconomic disadvantage from aged 2 to 17 years significantly decreased the likelihood of graduating from high school. Furthermore, the authors note the impact on graduation was much larger in their study using the duration of exposure to neighborhood disadvantage measure compared to prior research measuring neighborhood disadvantage at only one time-point.36 Although we sequentially examined the contemporary and lagged effects of neighborhood exposures on acquisition of chlamydia, information on the neighborhood of residence between waves of data collection is not available in Add Health. This lack of information could have led to an underestimation of the effects of the contemporary neighborhood conditions on young adults’ acquisition of chlamydia leading to the non-significant associations found in our study. Thus, when data permit, researchers should consider exploring neighborhood effects cumulatively or at critical time points in the life course using more advanced multilevel statistical modeling.36–38
Our study also examined the extent to which sexual risk-taking behaviors and depression occurring during the transition from adolescence to young adulthood mediated the relationships between exposure to neighborhood poverty during adolescence and young adults’ acquisition of chlamydia, however, no significant effects were found. Methodological and developmental factors may account for the unanticipated findings. First, as in most studies, the measurement of sexual risk behaviors and depression were based on adolescent and young adult self-report, thus under-reporting of behaviors due to social desirability concerns or poor recall may have occurred. Second, although exposure to neighborhood poverty during adolescence has been found to increase the likelihood of depression24,25 and/or sexual risk taking20–23 among adolescents, the effect of neighborhood poverty on these outcomes may not persist into young adulthood. Specifically, the numerous cognitive and developmental changes that occur during the transition from adolescence into young adulthood may enhance young adults’ decision-making capacities regarding engagement in high-risk behavior. Third, the effect of neighborhood poverty on sexual risk taking and depression also may vary by the duration of exposure to neighborhood poverty36 as well as other risk and protective factors occurring at the individual and/or neighborhood level (e.g., coping skills, personality, individual income, neighborhood cohesion, etc.).
The findings of this research illustrate the deleterious effect of exposure to neighborhood poverty during adolescence on the acquisition of chlamydia during young adulthood. Further research on the mechanisms through which exposure to neighborhood poverty during one phase of the life course contributes to future STI risk is greatly needed. For example, neighborhood of residence during adolescence could influence opportunities for future partner selection as prior research indicates socioeconomic homophily in partner selection is common.39 Thus, young adults who lived in an impoverished neighborhood during their adolescence may have a pool of higher risk sexual partners to choose from compared to their peers from more advantaged neighborhoods. Furthermore, sustained exposure to neighborhood poverty during adolescence into young adulthood may increase the risk further as geographic poverty is linked to higher STI rates in the community,7–11 thus the probability of subsequent contact with an infected sexual partner is significantly higher compared to those in communities with lower STI rates.
In addition to potential network explanations, chronic stress associated with living in adverse neighborhood contexts may impair immune system function and increase biological vulnerability to STI.40 Chronic stress is hypothesized to increase infectious disease risk, in part through dysregulation of the hypothalamic–pituitary–adrenal axis and the subsequent irregularities to immune function, particularly inflammation and cortisol secretion.41 Research on the linkages between neighborhood conditions and biological measures of health is burgeoning, and findings are suggestive that neighborhood disadvantage may have deleterious effects on immune function. Specifically, studies found neighborhood socioeconomic disadvantage,42 and neighborhood deprivation and low safety43 were associated with higher interleukin-6 levels whereas short-term increases in neighborhood burglary rates were linked to higher CRP levels among men, but not among women.44 With respect to cortisol, neighborhood violence has been linked to irregularities in the diurnal curve among a sample of adults, including lower cortisol levels upon waking and a slower decline in levels over the earlier part of the day.45 Neighborhood cohesion, poverty, and disorder also were examined, but the findings were less consistent and significant effects were modest. Others found high levels of perceived (e.g., disorder, violence, safety, etc.) or observed (e.g., crime, vacant housing, etc.) neighborhood stressors or low levels of perceived neighborhood social support were associated with a blunted cortisol diurnal curve due to a flatter rate of cortisol decline throughout the day.46 However, most studies to date have focused on adult samples, thus research investigating these relationships with adolescent samples and over critical periods in the life course is warranted.
Several limitations to our study warrant discussion. First, Add Health does not contain data on neighborhood social processes or individual stress measures, thereby limiting exploration of potential mechanisms that may explain how neighborhood poverty during adolescence contributed to chlamydia infection in young adulthood. Second, Add Health employed a school-based design and only those young adults who attended school at wave 1 were in the sample. Third, control measures for anal intercourse were not included in this study as 2,579 young adults did not complete the section of the interview that included these behaviors; therefore sexual orientation was used as a crude proxy.
Despite these limitations, our study highlights the deleterious effects of exposure to neighborhood poverty during adolescence on chlamydia infection in young adulthood, above and beyond numerous individual and neighborhood risk factors. Further research is needed on the potential mechanisms linking exposure to neighborhood poverty during adolescence to chlamydia in young adulthood. Adolescents and young adults experience a disproportionate burden of chlamydia infection in the USA and our findings strengthen the evidence that comprehensive STI prevention strategies addressing neighborhood poverty are needed to address the sexual health needs of this vulnerable population.
Acknowledgments
This research uses data from Add Health, a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill, and funded by grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Information on how to obtain the Add Health data files is available on the Add Health website (http://www.cpc.unc.edu/addhealth). No direct support was received from grant P01-HD31921 for this analysis.
Funding
This study was funded by the Robert Wood Johnson Foundation Nurse Faculty Scholar program awarded to the first author.
Contributor Information
Jodi L. Ford, Phone: +1-614-2926862, FAX: +1-614-2924948, Email: ford.553@osu.edu
Christopher R. Browning, Phone: +1-614-2922983, Email: browning.90@osu.edu
References
- 1.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Atlanta: U.S. Department of Health and Human Services; 2011.
- 2.Healthy People 2010. Sexually transmitted infections. Available at: http://www.healthypeople.gov/2010/Document/HTML/Volume2/25STDs.htm Accessed May 5, 2012.
- 3.Healthy People 2020. Sexually transmitted diseases. Available at: http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=37 Accessed May 5, 2012.
- 4.Shaw CR, McKay HD. Juvenile delinquency and urban areas. Chicago: The University of Chicago Press; 1969. [Google Scholar]
- 5.Sampson RJ, Raudenbush S, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277(5328):918–24. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 6.Wilson WJ. The truly disadvantaged: the inner city, the underclass, and public policy. Chicago: University of Chicago Press; 1987. [Google Scholar]
- 7.Krieger N, Waterman PD, Chen JT, et al. Monitoring socioeconomic inequalities in sexually transmitted infections, tuberculosis, and violence: geocoding and choice of area-based socioeconomic measures—the public health disparities geocoding project (US) Public Health Rep. 2003;118:240–60. doi: 10.1093/phr/118.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen D, Spear S, Scribner R, et al. “Broken Windows” and the risk of gonorrhea. Am J Public Health. 2000;90:230–6. doi: 10.2105/AJPH.90.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen DA, Mason K, Bedimo A, et al. Neighborhood physical conditions. Am J Public Health. 2003;93:467–71. doi: 10.2105/AJPH.93.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du P, McNutt LA, O’Campo P, et al. Changes in community socioeconomic status and racial distribution associated with gonorrhea rates: an analysis at the community level. Sex Transm Dis. 2009;36:430–8. doi: 10.1097/OLQ.0b013e31819b8c2f. [DOI] [PubMed] [Google Scholar]
- 11.Zierler S, Krieger N, Tang Y, et al. Economic deprivation and AIDS incidence in Massachusetts. Am J Public Health. 2000;90:1064–73. doi: 10.2105/AJPH.90.7.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford JL, Browning CR. Neighborhood social disorganization and the acquisition of trichomoniasis among young adults in the United States. Am J Public Health. 2011;101:1696–703. doi: 10.2105/AJPH.2011.300213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biello KB, Pettigrew MM, Niccolai LM. Multiple chlamydia infection among young women: comparing the role of individual- and neighbourhood-level measures of socioeconomic status. Sex Transm Infect. 2011;87:560–2. doi: 10.1136/sextrans-2011-050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infect Dis. 2005;191(Suppl 1):S115–22. doi: 10.1086/425280. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan MS, Crespo CJ, Huguet N, Marks G. Ethnic/racial homogeneity and sexually transmitted disease: a study of 77 Chicago community areas. Sex Transm Dis. 2009;36:108–11. doi: 10.1097/OLQ.0b013e31818b20fa. [DOI] [PubMed] [Google Scholar]
- 16.Upchurch DM, Mason WM, Kusonoki Y, Kriechbaum MJ. Social and behavioral determinants of self-reported STD among adolescents. Perspect Sex Reprod Health. 2004;36:276–87. doi: 10.1363/3627604. [DOI] [PubMed] [Google Scholar]
- 17.Laumann EO, Youm Y. Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sex Transm Dis. 1999;26:250–61. doi: 10.1097/00007435-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–45. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- 19.Halfon N, Hochstein M. Life course health development: an integrated framework for developing health, policy, and research. Milbank Q. 2002;80:433–79. doi: 10.1111/1468-0009.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Browning CR, Burrington LA, Leventhal T, Brooks-Gunn J. Neighborhood structural inequality, collective efficacy, and sexual risk behavior among urban youth. J Health Soc Behav. 2008;49(3):269–85. doi: 10.1177/002214650804900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browning CR, Leventhal T, Brooks-Gunn J. Neighborhood context and racial differences in early adolescent sexual activity. Demography. 2004;41(4):697–720. doi: 10.1353/dem.2004.0029. [DOI] [PubMed] [Google Scholar]
- 22.Browning CR, Olinger-Wilbon M. Neighborhood structural disadvantage, social organization, and number of short-term sexual partnerships. J Marriage Fam. 2003;65:730–45. doi: 10.1111/j.1741-3737.2003.00730.x. [DOI] [Google Scholar]
- 23.Cubbin C, Brindis CD, Jain S, et al. Neighborhood poverty, aspirations and expectations, and initiation of sex. J Adolesc Health. 2010;47:399–406. doi: 10.1016/j.jadohealth.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Aneshensel CS, Sucoff CA. The neighborhood context of adolescent mental health. J Health Soc Behav. 1996;37:293–310. doi: 10.2307/2137258. [DOI] [PubMed] [Google Scholar]
- 25.Dupéré V, Leventhal T, Lacourse É. Neighborhood poverty and suicidal thoughts and attempts in late adolescence. Psychol Med. 2009;39:1295–306. doi: 10.1017/S003329170800456X. [DOI] [PubMed] [Google Scholar]
- 26.Ford JL, Rechel M. Parental perceptions of the neighborhood context and adolescent depression. Public Health Nurs. 2012;29:390–402. doi: 10.1111/j.1525-1446.2012.01015.x. [DOI] [PubMed] [Google Scholar]
- 27.Khan MR, Kaufman JS, Pence BW, et al. Depression, sexually transmitted infection, and sexual risk behavior among young adults in the United States. Arch Pediatr Adolesc Med. 2009;163:644–52. doi: 10.1001/archpediatrics.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brawner BM, Gomes MM, Jemmott LS, Deatrick JA, Coleman CL. Clinical depression and HIV risk-related sexual behaviors among African-American adolescent females: unmasking the numbers. AIDS Care. 2012;24:618–25. doi: 10.1080/09540121.2011.630344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris KM. The National Longitudinal Study of Adolescent Health (Add Health), waves I and II, 1994–1996; wave III, 2001–2002; wave IV, 2007–2009 [machine-readable data file and documentation]. Chapel Hill, NC: Carolina Population Center, University of North Carolina at Chapel Hill; 2009.
- 30.Harris KM, Halpern CT, Whitsel E, et al. The National Longitudinal Study of Adolescent Health: Research Design. Available at: http://www.cpc.unc.edu/projects/addhealth/design. Accessed March 15, 2012.
- 31.Chantala K, KalsbeekWD, Andraca E. Non-response in wave III of the Add Health Study. Available at: http://www.cpc.unc.edu/projects/addhealth/data/guides/W3nonres.pdf. Accessed March 15, 2012.
- 32.Schmitz, JL. Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) testing. In Biomarkers wave III of the Add Health study. Available at: http://www.cpc.unc.edu/projects/addhealth/data/using/guides/biomark.pdf. Accessed March 13, 2012.
- 33.Burdette AM, Needham BL. Neighborhood environment and body mass index trajectories from adolescence to adulthood. J Adolesc Health. 2012;50:30–7. doi: 10.1016/j.jadohealth.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Johnson RC, Schoeni RF. Early-life origins of adult disease: national longitudinal population-based study of the United States. Am J Public Health. 2011;101:2317–24. doi: 10.2105/AJPH.2011.300252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acevedo-Garcia D, Lochner KA. Residential segregation and health. In: Kawachi I, Berkman LF, editors. Neighborhoods and Health. New York: Oxford; 2003. pp. 265–87. [Google Scholar]
- 36.Wodtke GT, Harding DJ, Elwert F. Neighborhood effects in temporal perspective. Am Sociol Rev. 2011;76:713–36. doi: 10.1177/0003122411420816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beretvas SN. Cross-classified and multiple-membership models. In: Hox J, Roberts JK, editors. Handbook of advanced multilevel analysis. London: Routledge Academic; 2010. pp. 313–34. [Google Scholar]
- 38.Naess O, Leyland AH. Analysing the effect of area of residence over the life course in multilevel epidemiology. Scand J Public Health. 2010;38(5 Suppl):119–26. doi: 10.1177/1403494810384646. [DOI] [PubMed] [Google Scholar]
- 39.Bearman PS, Moody J, Stovel K. Chains of affection: the structure of adolescent romantic and sexual networks. AJS. 2004;110:44–91. [Google Scholar]
- 40.Aiello AE, Simanek AM, Galea S. Population levels of psychological stress, herpesvirus reactivation and HIV. AIDS Behav. 2010;14:308–17. doi: 10.1007/s10461-008-9358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pedersen AF, Bovbjerg DH, Zachariae R. Stress and susceptibility to infectious disease. In: The handbook of stress science: biology, psychology, and health (Eds. Contrada RJ, Baum A). 2011. New York: Springer. 425–446.
- 42.Petersen KL, Marsland AL, Flory J, Votruba-Drzal E, Muldoon MF, Manuck SB. Community socioeconomic status is associated with circulating interleukin-6 and c-reactive protein. Psychosom Med. 2008;70:646–52. doi: 10.1097/PSY.0b013e31817b8ee4. [DOI] [PubMed] [Google Scholar]
- 43.Nazmi A, Diez Roux A, Ranjit N, Seeman TE, Jenny NS. Cross-sectional and longitudinal associations of neighborhood characteristics with inflammatory markers: findings from the multi-ethnic study of atherosclerosis. Health Place. 2010;16:1104–12. doi: 10.1016/j.healthplace.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Browning CR, Cagney KA, Iveniuk J. Neighborhood stressors and cardiovascular health: crime and C-reactive protein in Dallas, USA. Soc Sci Med. 2012;75:1271–9. doi: 10.1016/j.socscimed.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 45.Do DP, Diez Roux AV, Hajat A, et al. Circadian rhythm of cortisol and neighborhood characteristics in a population-based sample: the multi-ethnic study of atherosclerosis. Health Place. 2011;17:625–32. doi: 10.1016/j.healthplace.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karb RA, Elliott MR, Dowd JB, Morenoff JD. Neighborhood-level stressors, social support, and diurnal patterns of cortisol: the Chicago community adult health study. Soc Sci Med. 2012;75:1038–47. doi: 10.1016/j.socscimed.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]


