Abstract
Quality changes of anchovy (Stolephorus heterolobus) muscle during 7 days of refrigerated storage with ice and without ice were studied using several indicators: changes of ATP degradation products, K-value, TVB-N, TMA-N, Lactic acid, biogenic amines, sensory and microbiological analysis. During 7-day of refrigerated storage with ice and without ice, K-value, TVB-N, TMA-N and D, L-lactic acids contents increased with longer storage time (p ≤ 0.05). Major biogenic amines found in anchovy muscle during refrigerated storage were cadaverine, agmatine and tyramine, followed by putrescine and histamine. Skin and external odour by sensory evaluation, progressive decreases were observed as refrigeration time progressed. Storage of anchovy with ice resulted in a better maintenance of sensory quality, better control microbial activity, and the slowing down of biochemical degradation mechanisms. This result introduces the use of refrigerated storage with ice as a practical preliminary chilling for anchovy during industrial processing.
Keywords: Anchovy, quality changes, ATP-degradation, Biogenic amines
Introduction
Anchovy (Stolephorus heterolobus) is the most important fish species in Thailand. Yearly harvesting of anchovy in Thailand has increased from 57,800 t in 1987 to 134,700 t in 2009. In Thailand, most anchovy captures are raw materials for producing boiled and dried products. Most of the quality problems found in boiled and dried anchovy products are directly related to the initial quality of the fresh raw materials, which declines continuously due to post-mortem changes during preliminary refrigerated storage, occurring before drying process. Since fresh anchovy are not processed within a short time, it is always kept at low temperatures during storage; delaying the drying process results in the deterioration of final anchovy products. Unfortunately, the data available on anchovy (Stolephorus heterolobus) quality changes in low temperature storage before processing are scant and inconclusive.
Fish have attracted considerable attention as nutrient sources in the human diet. Quality changes in fish muscle depend upon endogenous enzymes, microbial contamination, and post-catch handling (Iwamoto et al. 1987). The rate of quality loss depends on the fish species and the storage condition (Olafsdöttir et al. 1997). To decrease the processes involved in quality loss, fish should be refrigerated immediately. Freshness greatly contributes to the quality of fish. Freshness and technological functionality of fish muscle are rapidly lost if stored at high temperatures. Traditionally, flaked ice has been used to cool fresh fish to a final temperature slightly above 0 °C in order to maintain freshness during storage or transport (Heen 1982). Several authors have studied ice storage effects on the quality of different fish varieties (Mazorra-Manzano et al. 2000; Márquez-Ríos et al. 2007; Kilinc et al. 2007; Scherer et al. 2005). Fish stored in ice show significant deterioration losses of nutritional value due to the effects of a variety of degradation mechanisms (Ashie et al. 1996).
The objective of this study was to investigate quality changes of anchovy in Thailand by investigating the practical application of 2 different chilling methods available to the boiled and dried anchovy industry in Thailand in terms of their performance and effects on fish deterioration. Basic knowledge of quality changes of anchovy would greatly benefit its utilization and processing for human food as well as improve commercial practices.
Materials and methods
Fish samples
Fresh anchovy (Stolephorus heterolobus), approximately 5–10 cm in length from the Andaman coast, were captured at night in January 2009. In the boat, fish were stored in a self-draining box with ice. When the boat reached port, fish were transported by truck within 2 h to the Agricultural Products Processing Technology Research Unit, Faculty of Science and Industrial Technology, Prince of Songkla University, Surat Thani Campus. Fish were immediately washed with tap water and randomly divided into 70.0 kg groups. The first lot (I) was surrounded by flake ice at a 2:1 fish to ice ratio and then placed into polystyrene boxes with holes for draining. They were then stored in a refrigerated room at 4 °C. To maintain the flaked ice, melted ice was removed and replaced with new, flaked ice in equal amounts daily. The second lot (WI) was placed in the polystyrene boxes without flaked ice and stored in a refrigerated room at 4 °C. A sample of 2.5 kg anchovies, randomly chosen, was taken from the boxes immediately after being acquired and everyday for analysis during 7-days of refrigerated storage.
Chemicals
Standards for ATP-related compounds, and biogenic amines were purchased from Sigma-Aldrich Chemical Co. Ltd (St. Louis, MO, USA). These compounds were: (adenosine 5′-triphosphate disodium salt, adenosine 5′-diphosphate sodium salt, adenosine 5′-monophosphate sodium salt, inosine 5′-monophosphate disodium salt, inosine and hypoxanthine), biogenic amines standards (tyramine hydrochloride, putrescine dihydrochloride, cadaverine dihydrochloride, agmatine sulphate and histamine dihydrochloride). High performance liquid chromatography (HPLC)-grade organic solvents were purchased from the British Drug House (BDH) (Poole, UK) or Merck (Darmstadt, Germany). Other chemicals and organic solvents used in this study were analytical grade without further purification.
Determination of ATP-related compounds
Nucleotide extracts were prepared and concentrations determined according to the method of Veciana-Nogues et al. (1997) using HPLC with some modification. In brief, 10.0 g of fish muscle was homogenized with 15 ml of 0.6 N HClO4 at 0 °C for 1 min with a homogenizer. The homogenate was centrifuged at 1500 × g for 10 min and 10.0 ml of supernatant was neutralized to pH 6.5 with 0.1 N KOH and left to stand for 30 min at 4 °C. KClO4 was removed by filtration through a 0.2 μm cellulose acetate membrane syringe filter and stored at −80 °C until analysis. Reversed phase (RP)-HPLC was performed using an Agilent 1100 series (Palo Alto, CA, USA) equipped with a Hypersil ODS column (4.0 × 250 mm, 5 μm, Agilent Technologies, Palo Alto, CA, USA) and UV-visible detector (model G1379A) at 254 nm. The mobile phase used was 0.1 M phosphate buffer pH 7.0 (0.04 M KH2PO4 and 0.06 M K2HPO4) with a flow rate 0.75 ml/ min. The content of ATP-related compounds were identified by their relative retention times and quantified based upon their peak areas using standard curves for ATP, adenosine diphosphate (ADP), adenosine monophosphate (AMP), inosine monophosphate (IMP), inosine (HxR), and hypoxanthine (Hx).
Determination of K-value
K-value was calculated in accordance with the method of Saito et al. (1959) using this formula; 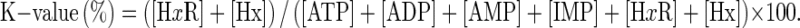
Microbiological analysis
The viable mesophilic and psychrophilic bacterial counts were performed by the pour plate method using plate count agar (Difco™, 0479–17) according to the method of Harrigan and McCance (1976). Approximately 10 g of fish muscle was dissected aseptically from the refrigerated whole fish specimens, mixed with 90.0 ml of 0.1% peptone water (Difco™, 0118-17-0), and homogenized in a stomacher for 1.0 min. Serial dilutions from the microbial extracts were prepared in 0.1% peptone water. The plates were incubated at 30 °C for 48 h and 5 °C for 72 h for mesophilic counts and psychrophilic counts, respectively.
Determination of lactic acids
Lactic acids content were extracted according to the method of Wongso and Yamanaka (1996) and determined by HPLC in accordance with the method of Aguiar et al. (2005). In brief, 5 g of fish muscle was homogenized with 15.0 ml of 6% HClO4 at 0 °C for 1 min. This suspension was centrifuged at 1500 × g for 10 min, and then the supernatant was neutralized with KOH, and made up to 25.0 ml with distilled water. It was then filtered through a 0.2 μm cellulose acetate membrane syringe filter and stored at −80 °C until analyzed. HPLC was performed using a Shimadzu 6A (Kyoto, Japan) equipped with an ionic exchange Aminex HPX-87H column (7.8 × 300 mm, 5 μm, Bio-Rad Laboratory (Hemel Hemstead, UK), and a UV-visible detector (model SPD-10AV, Shimadzu, Kyoto, Japan) at 210 nm. The mobile phase used was 0.005 M of H2SO4 (pH 2.1) at a flow rate 0.75 ml/min. The amount of D, L-lactic acid was quantified, based upon peak area using the appropriate standard curves.
Determination of biogenic amines
Biogenic amines were determined in accordance with the method of Özogul et al. (2002) with some modification. In brief, 10.0 g of fish muscle was homogenized with 50 ml of 1% TCA at 0 °C for 1 min and filtered through Whatman No. 1 filter paper. For derivatization, 2.0 ml of supernatant was added to 1.0 ml of 2.0 M NaOH, followed by 1.0 ml of 2% benzoyl chloride and shaken with a vortex mixer for 1 min. This mixture was left at 25 °C for 30 min then 2.0 ml of saturated NaCl was added. Amines were extracted twice with 2.0 ml of diethyl ether and the pooled ether extract evaporated under a stream of N2 gas. The residue was dissolved with acetonitrile before analysis. HPLC was performed using a Shimadzu 6A (Kyoto, Japan) equipped with a Spherisorb 5 Si C18 column (4.6 × 250 mm) (Phenomenex, Macclesfield, UK) and a diode array detector (model SPD-M20A, Shimadzu, Kyoto, Japan) at 254 nm. The mobile phase used was a gradient solution of acetonitrile and water at flow rate 1 ml/min. Biogenic amines content was quantified based upon peak area using appropriate standard curves.
Determination of total volatile base nitrogen (TVB-N)
TVB-N was measured in accordance with the method of Woyewoda et al. (1986) using distillation and titration. In brief, 10.0 g of fish muscle was homogenized with 300 ml of distilled water. This homogenate was transferred to a 1000 ml round bottom distillation flask containing 2.0 g of MgO. The distillation flask was connected to a vertical distillation apparatus and heated for 25 min. The condensate was received in a flask containing 2.0% of boric acid solution and titrated back to the original color using a 0.05 N H2SO4 standard solution. TVB-N was expressed as mg N/100 g sample.
Determination of trimethylamine nitrogen (TMA-N)
TMA-N was measured in accordance with the method of Woyewoda et al. (1986) using a spectrophotometer. In brief, 50.0 g of fish muscle was homogenized in 100 ml of 7.5% TCA solution and centrifuged at 4 °C for 10 min at 2000 × g. The supernatant was filtered through Whatman No. 4 filter paper. Then 1.0 ml of 10% formaldehyde, 10.0 ml of toluene, and 3.0 ml of 25% KOH were added to 2 ml of extract. The mixture was vigorously agitated for 10 min, and then 7.0 ml of the supernatant was transferred to a tube containing 0.5 g anhydrous Na2SO4. An aliquot (5 ml) of the clarified solution was added to 5.0 ml of picric acid to develop the color. The absorbance was measured at 410 nm by using UV-vis spectrophotometer (model Lambda EZ201 UV/vis spectrophotometer, Perkin Elmer, Waltham, MA, USA). TMA-N contents were calculated from a standard curve and expressed as mg N/100 g sample.
Sensory evaluation
Sensory evaluation was performed acceding to Rodríguez et al. (2005) with some modification by using 7 trained panelists who had extensive experience in the evaluation of fish muscle according to four category scale was used for sensory assessment of skin guidelines as: highest quality (E; very intense pigmentation and transparent mucus), good quality (A; milky mucus, insignificant and pigmentation losses), fair quality (B; slightly greyish mucus and pigmentation without shine) and unacceptable (C; widely opaque mucus and important pigmentation loss) and external odour guidelines as: highest quality (E; sharply seaweedy and shellfish smell), good quality (A; weakly seaweedy and shellfish smell), fair quality (B; incipiently putrid and rancid) and unacceptable (C; putrid and rancid). Sensory assessment of the anchovy included the following parameters of skin and external odour.
Statistical analysis
Student t-test and one-way analysis of variances (ANOVA) for a completely random design using the least significant difference at the level of 0.05 were used. The data values were expressed as mean ± SD (n = 3) for each specific storage time.
Results and discussion
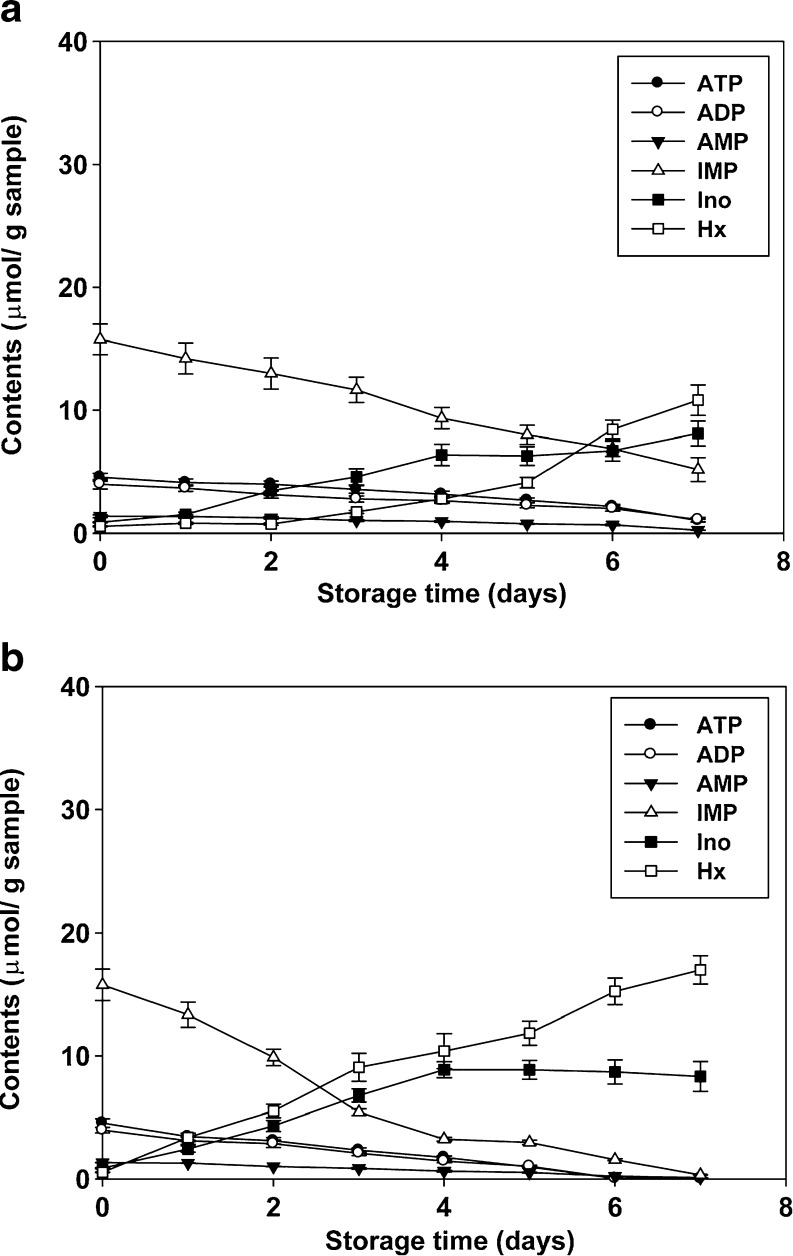
ATP degradation in post-mortem fish muscle has been considered one of the earliest indices in assessing fish freshness (Kassemsarn et al. 1963). The pathway of ATP degradation in fish muscle has been extensively documented as a degradative sequence to ADP, AMP, IMP, Ino and Hx (Ehira and Uchiyama 1987). In this study, the mean total molar concentration for ATP and derivatives was 27.1 ± 2.5 μmol/g. This value is higher than the total molar concentration for ATP and derivatives that have been reported in several fish species (Mazorra-Manzano et al. 2000; Murata and Sakaguchi 1986; Ocaño-Higuera et al. 2009). The presence of variations in total molar concentration result from season, species, and physiological conditions. The ATP degradation was found to be more rapid in anchovy during refrigerated storage without ice. The degradation of ATP-related compounds during storage is often accelerated at higher storage temperatures (Ponce de Leon et al. 1994; Özogul et al. 2008). The ATP breakdown patterns of anchovy during refrigerated storage with ice and without ice for 7 days are shown in Fig. 1a and b. This study revealed a rapid degradation to IMP after death, followed by a progressive degradation to Hx and Ino throughout 7-days of ice and without ice storage.
Fig. 1.
Changes in nucleotide degradation product (μmol/g sample) of anchovy muscle during refrigerated storage with ice (a) and without ice (b). Values are mean±SD (n = 3)
The initial ATP content of anchovy during refrigerated with ice and without ice was 4.6 ± 0.32 μmol/g, while the cumulated nucleotide was the IMP (15.8 ± 1.2 μmol/g) during the same period. At the beginning of refrigerated storage, higher initial IMP contents were reported in several fish species (Aubourg et al. 2007; Özogul et al. 2008; Ocaño-Higuera et al. 2009). The IMP is predominately accumulated in fish muscle at the early stage of low temperature storage, probably due to the degradation of ATP within the first 24 h of post-mortem (Haard 1992). Therefore, the high concentration of IMP in this study reflected a rapid degradation of ATP to IMP. According to IMP, changes in nucleotide degradation have been found to contribute directly to the sensory quality of fish, showing a taste enhancing effects through combination with glutamic acid. Meanwhile, Hx contributes to bitterness in low temperature stored fish (Lindsay 1994; Chruch 1998; Kawai et al. 2002). A marked decrease in the IMP content of anchovy was observed from an initial concentration of 15.8 ± 1.2 μmol/g to 5.2 ± 0.97 μmol/g during refrigerated storage with ice and 0.32 ± 0.01 μmol/g for refrigerated without ice at the end of storage. Similar results regarding rapid IMP degradation in several fish during ice or chilled storage has been reported by different authors (Özogul et al. 2008; Aubourg et al. 2007). Hx contents tend to increase with longer storage times. At day 7, anchovy during refrigerated storage without ice had a higher Hx content (17.0 ± 1.2 μmol/g) than anchovy stored in ice (10.9 ± 1.2 μmol/g). The presence of larger amounts of Hx in anchovy muscle correlated well with autolytic deterioration from bacteria spoilage (Woyewoda et al. 1986).
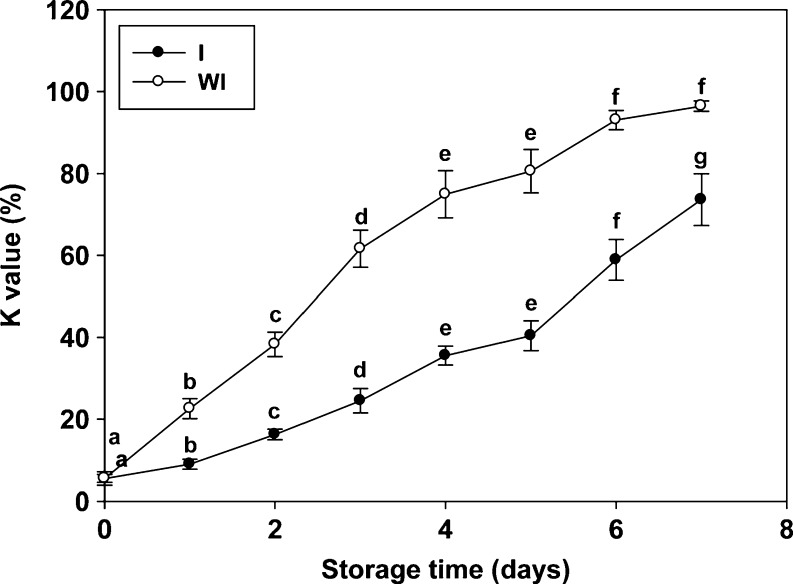
K-value is one of the indices that has an early and linear response with time when fish are stored at low temperatures. The K-value has been proposed as an index for assessing ATP degradation as a measure of the loss of freshness during the storage of fish (Woyewoda et al. 1986). K-value changes in anchovy muscle during refrigerated storage with ice and without ice for this study are shown in Fig. 2. The K-value of the anchovy muscle increased along with the storage time (p ≤ 0.05). At the beginning of storage, the initial K-value was 5.5 ± 1.7%. This value is similar to that given in a previous report where the initial value of K in fish muscles immediately after capture was lower than 10% (Sikorkski et al. 1990). The K-value increased sharply during the 7 days of refrigerated storage with ice and without ice (p ≤ 0.05), reaching K-values of 73.6 ± 6.3 and 96.5 ± 1.2%, respectively. The increase in K-value was noticeable as the impact of storage temperature. In this study, K-value clearly can be seen slowly increasing during refrigerated storage with ice as compared to its rapid increase without ice storage. This finding was probably due to the decomposition of IMP, which is carried out by the activity of 5-nucleotidase. This enzyme was found to have short stability at iced storage; therefore, this finding could be explained by the effectiveness of low temperature to minimize the activity of 5-nucleotidase (Ehira and Uchiyama 1987). The increase in the K-value indicated the progressive deterioration and increased unacceptability of the anchovy muscle during refrigerated storage.
Fig. 2.
Changes in K-value (%) in anchovy muscle during refrigerated storage with ice (I) and without ice (WI). Values are mean ± SD (n = 3). Different letters within the same storage conditions indicate significant differences (p ≤ 0.05)
The initial quality of the anchovy used in this study was high as indicated by a low initial amount of bacteria (<3.0 logCFU/g) before storage at a low temperature. Initial psychrophilic and mesophilic viable counts of anchovy muscle were 2.0 ± 0.13 and 1.3 ± 0.11 logCFU/g. Total viable psychrophilic and mesophilic bacterial counts increased throughout the storage period (Table 1). The refrigerated storage of anchovy muscle delayed microbial growth and the lag phase for 24 h (P > 0.05). After that time, a significant increase in psychrophilic and mesophilic viable counts were observed from day 3 to 7 (p ≤ 0.05) during both refrigerated storage conditions. At the end of refrigerated storage with ice, psychrophilic and mesophilic viable counts were 6.1 ± 0.43 and 3.1 ± 0.21 logCFU/g, while refrigerated storage without ice psychrophilic and mesophilic viable counts were 8.0 ± 0.76 and 4.1 ± 0.25 logCFU/g. With regard to the increasing bacteria content during refrigerated storage in several fish, the results had been reported by different authors (Reza et al. 2009; Rodríguez et al. 2004; Özogul et al. 2008).
Table 1.
Bacteria in anchovy muscle during refrigerated storage with ice (I) and without ice (WI). Values are mean ± SD (n = 3)
| Storage time | Psychrophilic bacteria (log CFU/g) | Mesophilic bacteria (log CFU/g) | ||
|---|---|---|---|---|
| (days) | I | WI | I | WI |
| 0 | 2.0 ± 0.13Aa | 2.0 ± 0.13Aa | 1.3 ± 0.11Aa | 1.3 ± 0.11Aa |
| 1 | 2.0 ± 0.19ABa | 2.1 ± 0.17Aa | 1.6 ± 0.13ABa | 1.6 ± 0.16Aa |
| 2 | 2.5 ± 0.24Ba | 3.4 ± 0.23Bb | 1.9 ± 0.12Ba | 2.0 ± 0.11Ba |
| 3 | 3.0 ± 0.23Ca | 4.1 ± 0.31Ca | 2.0 ± 0.23BCa | 2.2 ± 0.16BCa |
| 4 | 4.7 ± 0.35Da | 5.3 ± 0.45Db | 2.3 ± 0.24Ca | 2.5 ± 0.14Ca |
| 5 | 5.0 ± 0.39DEa | 6.4 ± 0.56Eb | 2.6 ± 0.19CDa | 2.7 ± 0.23Ca |
| 6 | 5.5 ± 0.48Ea | 7.3 ± 0.58EFb | 2.9 ± 0.16Da | 3.3 ± 0.21Db |
| 7 | 6.1 ± 0.43Ea | 8.0 ± 0.76Fb | 3.1 ± 0.21Da | 4.1 ± 0.25Eb |
In each column, different capital letters indicate significant differences (p ≤ 0.05) and in each row, different small letters within the same microbiological indicate significant differences (p ≤ 0.05)
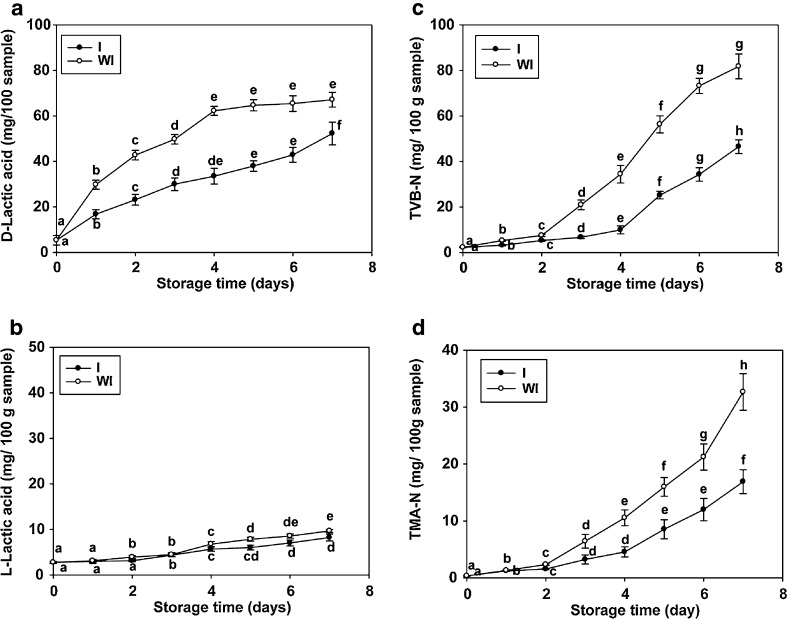
D and L-lactic acids are the final products of glycolysis in fish during low temperature storage. Changes in lactic acids content in anchovy muscle during refrigerated storage with ice and without ice are shown in Fig. 3a and b. At the beginning of storage, the mean D-lactic acid content was 5.4 ± 2.1 mg/100 g, while the L-lactic acid content was 2.1 ± 1.5 mg/100 g. The D-lactic acid content of anchovy muscle increased markedly (p ≤ 0.05) in the first days of both storage conditions, while L-lactic acid slightly increased after 1 day of storage (p ≤ 0.05). At the end of storage, D-lactic acid contents of anchovy muscle during refrigerated storage with ice and without ice were 52.3 ± 5.0 and 67.1 ± 3.2 mg/100 g, respectively, while L-lactic acid contents of anchovy muscle during refrigerated storage with ice and without ice were 8.2 ± 0.76 and 9.7 ± 0.32 mg/100 g, respectively. Lactic acids contribute to the pH level, which is one of the indices for freshness of fish and fishery products. The accumulation of organic acids in anchovy muscle could be attributable to glycolysis, which was enhanced by extended storage.
Fig. 3.
Chemical changes in anchovy muscle during refrigerated storage with ice (I) and without ice (WI). Values are mean ± SD (n = 3). Different letters within the same storage conditions indicate significant differences (p ≤ 0.05)
Biogenic amines are generated by microbial decarboxylation of amino acids in food products. The adverse effects on human health and food quality of biogenic amines, such as histamine (His), putrescine (Put), cadaverine (Cad), tyramine (Try), and agmatine (Agm) makes it important to monitor their levels during fish storage (Chu and Bjeldanes 1981; Stratton et al. 1991). The concentrations of biogenic amines present in the muscle of anchovy during refrigerated storage with ice and without ice are shown in Table 2. Although, His, Put, Cad, Agm and Try were detected in all anchovy muscle during the storage time, Cad, Agm and Try were the major amines formed, followed by Put and His. According to the results, these biogenic amines increased with longer storage times (p ≤ 0.05). In this study, His and Put levels increased more slowly than those of Cad, Agm, and Try. At the end of storage, histamine, a toxic amine and causative agent of histamine fish poisoning (Lehane 2000) had a low concentration. This concentration probably resulted from the low level of histamine precursors and would account for lower production of histamine in fish (Chytiri et al. 2004). Our results are in agreement with those of Marks Rupp and Anderson (2005) who reported that histamine is not always found in spoiled fish, thus Cad may be a better indicator for their decomposition. From the results, Cad, Agm, and Try were the most useful indicators for freshness among biogenic amines since these amines increased in proportion to storage time. Therefore, anchovy muscle during refrigerated storage with ice managed to extend the biogenic amines’ formation by inhibiting microbial growth compared to anchovy muscle during refrigerated storage without ice.
Table 2.
Biogenic amines (mg/100 g sample) in anchovy muscle during refrigerated storage with ice (I) and without ice (WI). Values are mean ± SD (n = 3)
| Storage time | HIS | PUT | CAD | AGM | TRY | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (days) | I | WI | I | WI | I | WI | I | WI | I | WI |
| 0 | 0.33 ± 0.03Aa | 0.33 ± 0.03Aa | 0.98 ± 0.09Aa | 0.98 ± 0.19Aa | 1.5 ± 0.12Aa | 1.5 ± 0.12Aa | 4.2 ± 0.43Aa | 4.2 ± 0.43Aa | 1.2 ± 0.21Aa | 1.2 ± 0.21Aa |
| 1 | 0.39 ± 0.06Aa | 0.38 ± 0.06Aa | 1.1 ± 0.15ABa | 1.2 ± 0.21ABa | 3.6 ±0.23Ba | 4.0 ± 0.31Ba | 6.3 ± 0.59Ba | 7.0 ± 0.53Ba | 1.3 ± 0.31Aa | 1.4 ± 0.22Aa |
| 2 | 0.76 ± 0.05Ba | 0.77 ± 0.08Ba | 1.5 ± 0.28Ba | 1.5 ± 0.19Ba | 4.7 ± 0.37Ca | 5.0 ± 0.46Ca | 8.3 ± 0.76Ca | 9.1 ± 0.79Ca | 4.0 ± 0.34Ba | 4.0 ± 0.23Ba |
| 3 | 0.85 ± 0.06BCa | 0.87 ± 0.11Ba | 2.0 ± 0.27BCa | 2.0 ± 0.20Ca | 7.9 ± 0.65Da | 11.2 ± 1.18Db | 9.1 ± 1.01Ca | 15.4 ± 1.23Db | 4.6 ± 0.39Ba | 4.5 ± 0.34Ba |
| 4 | 0.99 ± 0.08Ca | 2.3 ± 0.15Cb | 2.4 ± 0.21Ca | 4.0 ± 0.31Db | 10.2 ± 1.19Ea | 16.8 ± 1.34Eb | 13.0 ± 1.29Da | 19.7 ± 1.34Eb | 5.8 ± 0.65Ca | 9.9 ± 0.56Cb |
| 5 | 1.4 ± 0.17Da | 3.0 ± 0.19Db | 2.9 ± 0.35CDa | 4.3 ± 0.18Db | 14.6 ± 1.21Fa | 22.3 ± 2.38Fb | 16.7 ± 1.42Ea | 23.4 ± 1.99Fb | 6.3 ± 0.77Ca | 11.4 ± 0.98CDb |
| 6 | 1.7 ± 0.18DEa | 3.8 ± 0.21Eb | 3.2 ± 0.24Da | 4.8 ± 0.16Eb | 19.9 ± 2.01Ga | 27.9 ± 3.87Fb | 23.7 ± 2.09Fa | 32.1 ± 2.57Gb | 8.0 ± 0.59Da | 13.4 ± 1.12Db |
| 7 | 2.2 ± 0.19Ea | 4.9 ± 0.23Fb | 4.0 ± 0.24Ea | 5.3 ± 0.21Fb | 24.0 ± 3.02Ga | 40.0 ± 3.02Gb | 29.9 ± 3.21Ga | 40.1 ± 3.16Hb | 9.6 ± 0.87Ea | 16.9 ± 1.44Eb |
In each column, different capital letters indicate significant differences (p ≤ 0.05), and in each row, different small letters within the same biogenic amine indicate significant differences (p ≤ 0.05)
Determination of TVB-N is widely used to assess the freshness of fish, and this value correlates well with sensory changes during spoilage. TVB-N is a term that includes measurement of trimethylamine, dimethylamine, ammonia and other compounds related to fish spoilage. At the beginning of storage, the TVB-N content of the sample was 2.3 ± 0.22 mg/100 g. This content level was probably due to the endogenous production of ammonia from the enzymatic degradation of protein, amino acids, and nucleotides immediately post-mortem. However, a higher level of TVB-N was reported by Mol et al. (2006) for their anchovy muscle 12.3 ± 1.1 mg/ 100 g sample. The pattern of change in TVB-N content in anchovy muscle during the 7-day storage period is shown in Fig. 3c. TVB-N content increased with increasing storage time in both refrigerated storage conditions (p ≤ 0.05). TVB-N content increased rapidly from days 2 to 7 of refrigerated storage. At the end of refrigerated storage, the mean TVB-N content in anchovy muscle under refrigerated storage with ice and without ice were 46.6 ± 3.0 and 81.8 ± 5.5 mg/100 g sample, respectively. A similar behavior was observed by Özogul et al. (2008) for wild white grouper. The limit for TVB-N content in edible fish has been reported to be 30.0 mg/100 g (Woyewoda et al. 1986). In this study, the TVB-N contents of anchovy muscle under refrigerated storage with ice and without ice were exceeded after 6 and 4 days of storage. Thus, the refrigerated storage with ice is important in controlling TVB-N content in anchovy muscle.
TMA-N is considered a valuable tool in the evaluation of the quality of fish stored in ice because of its rapid accumulation in muscle under refrigerated conditions (Kryzmien and Elias 1990). In this study, the production of TNA-N followed a similar pattern to TVB-N during refrigerated storage, where a significant increase was observed with storage time (p ≤ 0.05). The initial mean TMA-N content of anchovy muscle was 0.35 ± 0.06 mg/100 g. The TMA-N content of the anchovy increased slightly during the first 2 days of refrigerated storage with ice (1.6 ± 0.13 mg/100 g) and refrigerated storage without ice (2.3 ± 0.14 mg/100 g) (p ≤ 0.05) (Fig. 3d). After this time, a marked increase in TMA-N content was observed until day 7 of refrigerated storage with ice (16.9 ± 2.1 mg/100 g) and refrigerated storage without ice (32.7 ± 3.2 mg/100 g) (p ≤ 0.05). Results agreed with previous reports; it has been mentioned that TMA-N rapidly increases during cold storage of anchovy (Mol et al. 2006). In general, the upper limit for TMA-N before consumer rejection of fish is usually 5.0 to 10.0 mg/100 g (Sikorkski et al. 1990). The high correlation between TMA-N and TVB-N levels during fish storage indicated by other authors (Burt et al. 1976; Ruiz-Capillas and Moral 2001) were also observed in anchovy muscle during 7- days of refrigerated storage with ice (0.991) and without ice (0.949) in this study.
Fresh anchovy was generally considered to have very high acceptability for consumers. However, fresh anchovy is susceptible to spoilage caused by both microbiological and chemical reactions. Different attributes were analyzed and compared between during refrigerated storage with ice and without ice. Changes in the skin and external odour of anchovy muscle during 7 days of refrigerated storage with ice and without ice were observed. According to the results of anchovy skin and external odour by sensory evaluation, progressive decreases in average score were observed in anchovy from both conditions as refrigeration time progressed. Skin and external odour were detected as unacceptable (C) in anchovy muscle during refrigerated storage in ice and without ice at days 6 and 4, respectively. The results of the sensory analysis indicated that the shelf-lives of anchovy are affected by the storage in ice. Thus, a profitable effect of the presence of ice was concluded. Similar results with regard to the acceptability of fish muscle, during refrigerated storage in anchovy had been reported by different authors (Yerlikaya et al. 2005; Kilinc 2007).
In general, the upper limit for mesophilic viable counts before the rejection of freshwater and marine fish usually exceeded 7.0 logCFU/g. Thus, the increase in mesophilic and psychrophilic viable counts with increasing storage time could be used to indicate the deterioration and unacceptability of anchovy muscle at the end of storage. The presence of larger psychrophilic and mesophilic viable counts of anchovy muscle during refrigerated storage with ice and without ice highly correlated with the greater TVB-N and TMA-N contents. The number of mesophilic aerobic bacteria remained lower than 7.0 logCFU/g during refrigerated storage of anchovy with ice and without ice even if they were unacceptable according to the results of sensory analysis. In this study, psychrophilic aerobic bacteria counts were around 5.0 logCFU/g per sample, and fish were accepted as spoilage based on sensory evaluations on the 6th and 4th days of iced and without iced storage when TVB-N and TMA-N values reached the limit of fish spoilage (Scott et al. 1992; Köse and Erdem 2004). According to the results, have concentrated on sensory and chemical changes rather microbiological associated with anchovy during refrigerated storage with ice and without ice.
Conclusion
In this study, the effects of ice on practical industrial refrigerated storage conditions in Thailand on the quality of anchovy were evaluated. The results of sensory, chemical, biological and microbiological analyses described here show that the refrigerated storage of anchovy with ice allows better maintenance of quality and enhances the shelf-life of fish to 6 days. On the basis of this information, it is highly recommended that the commercial and practical industrial chilling method of anchovy be modified in order to extend the shelf life of this product.
Acknowledgements
This work was supported in part by a grant in aid from the the Thailand Research Fund to Chatchawan Chotimarkorn under the project title, “Improvement of Quality and Processing of the Dried Anchovy (S. heterolobus) for Commercial Practices.”
References
- Aguiar A, Nascimento RAA, Ferretti LP, Gonçalves AR. Determination of organic acids and ethanol in commercial vinegars. Braz J Food Technol. 2005;5:51–56. [Google Scholar]
- Ashie I, Smith J, Simpson B. Spoilage and shelf-life extension of fresh fish and shellfish. Crit Rev Food Sci. 1996;36:87–121. doi: 10.1080/10408399609527720. [DOI] [PubMed] [Google Scholar]
- Aubourg SP, Quitral V, Larraín MA, Rodríguez A, Gómez J, Maier L, Vinagre J. Autolytic degradation and microbial activity in farmed Coho salmon (Oncorhynchus kisutch) during chilled storage. Food Chem. 2007;104:369–375. doi: 10.1016/j.foodchem.2006.11.066. [DOI] [Google Scholar]
- Burt JR, Gibson DM, Jason AC, Sanders HR. Comparison of methods of freshness assessment of wet fish II. Instrumental and chemical assessment of boxed experimental fish. J Food Technol. 1976;11:73–89. doi: 10.1111/j.1365-2621.1976.tb00704.x. [DOI] [Google Scholar]
- Chruch N. MAP fish and crustaceans- sensory enhancement. Food Sci Tech Today. 1998;12:73–83. [Google Scholar]
- Chu CH, Bjeldanes LF. Effects of diamines, polyamines and tuna fish extract on the binding of histamine to mucin in vitro. J Food Sci. 1981;47:7980–7988. [Google Scholar]
- Chytiri S, Paleologos E, Savvaidis I, Kontominas MG. Relation of biogenic amines with microbial and sensory changes of whole and filleted freshwater rainbow trout (Oncorhynchus mykiss) stored on ice. J Food Portect. 2004;67:960–965. doi: 10.4315/0362-028x-67.5.960. [DOI] [PubMed] [Google Scholar]
- Ehira S, Uchiyama H. Determination of fish freshness using the K-value and comments on some other biochemical changes in relation to freshness. In: Kramer DE, Liston J, editors. Seafood Quality Determination. Amsterdam: Elsevier Science Publisher B.V; 1987. pp. 185–207. [Google Scholar]
- Haard N. Biochemistry and chemistry of color and color change in seafood. In: Flinck J, editor. Advance in seafood biochemistry, composition and quality. Louisiana: Technomic Publishing Co; 1992. pp. 305–360. [Google Scholar]
- Harrigan WF, McCance ME. Biochemical test for bacteria. In: Harrigan WF, McCance ME, editors. Laboratory methods in food and dairy microbiology. London: Academic Press Inc; 1976. pp. 15–22. [Google Scholar]
- Heen E. Development in chilling & freezing of fish. Int J Refrig. 1982;5:45–49. doi: 10.1016/0140-7007(82)90011-1. [DOI] [Google Scholar]
- Iwamoto M, Yamanaka H, Watabe S, Hashimoto K. Effects of storage temperature on rigor mortis and ATP degradation in place (Paralichthys olivaceus) muscle. J Food Sci. 1987;52:1541–1547. doi: 10.1111/j.1365-2621.1987.tb05867.x. [DOI] [Google Scholar]
- Kassemsarn BO, Sanz PB, Murray J, Jones NR. Nucleotide degradation in the muscle of iced hassock (Gadus agelefinus) lemon sole (Pleuronectes microcephalus) and plaice (Pleuronectes platessa) J Food Sci. 1963;28:28–37. doi: 10.1111/j.1365-2621.1963.tb00155.x. [DOI] [Google Scholar]
- Kawai M, Okiyama A, Ueda Y. Taste enhancements between various amino acids and IMP. Chem Senses. 2002;27:739–745. doi: 10.1093/chemse/27.8.739. [DOI] [PubMed] [Google Scholar]
- Kilinc B. Microbiological, sensory, and color changes of anchovy (Engraulis encrasicholus) patties during refrigerated storage. J Muscle Foods. 2007;20:129–137. doi: 10.1111/j.1745-4573.2009.00139.x. [DOI] [Google Scholar]
- Kilinc B, Cakli S, Cadum A, Dincer T, Tolasa S. Comparison of effects of slurry ice and flake ice parameters on the quality of aquacultured sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) stored at 4°C. Food Chem. 2007;104:1611–1617. doi: 10.1016/j.foodchem.2007.03.002. [DOI] [Google Scholar]
- Köse S, Erdem ME. An investigtion of quality changes in anchovy (Engraulis encraicolus L, 1758) stored at different temperature. Turk J Vet Anim Sci. 2004;28:575–582. [Google Scholar]
- Kryzmien ME, Elias L. Feasibility study on the determination of fish freshness by trimethylamine headspace analysis. J Food Sci. 1990;55:1228–1233. doi: 10.1111/j.1365-2621.1990.tb03903.x. [DOI] [Google Scholar]
- Lehane L. Update on histamine fish poisoning. Med J Australia. 2000;173:149–152. doi: 10.5694/j.1326-5377.2000.tb125575.x. [DOI] [PubMed] [Google Scholar]
- Lindsay RC. Flavor of fish. In: Shahidi F, Botta JR, editors. Seafood chemistry, processing technology and quality. London: Blackie Academic & Professional; 1994. pp. 75–84. [Google Scholar]
- Marks Rupp HS, Anderson CR. Determination of putrescine and cadaverine in seafood finfish and shell fish by liquid chromatography using pyrene excimer fluorescence. J Chromatogra A. 2005;1094:60–69. doi: 10.1016/j.chroma.2005.07.088. [DOI] [PubMed] [Google Scholar]
- Márquez-Ríos E, Morán-Oalacio EF, Lugo-Sánchez ME, Ocano-Higuera VM, Pacheco-Aguilar R. Postmortem biochemical behavior of giant squid (Dosidicus gigas) mantle muscle stored in ice and its relation with quality parameters. J Food Sci. 2007;72:C356–C362. doi: 10.1111/j.1750-3841.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- Mazorra-Manzano MA, Pacheco-Aguilar R, Diaz-Rojas EI, Lugo-Sanchez ME. Postmotem changes in black skipjack muscle during storage in ice. J Food Sci. 2000b;65:774–779. doi: 10.1111/j.1365-2621.2000.tb13585.x. [DOI] [Google Scholar]
- Mol S, Erkan N, Ücok D, Tosun SY. Effect of psychrophilic bacteria to estimate fihs quality. J Muscle Foods. 2006;18:120–128. doi: 10.1111/j.1745-4573.2007.00071.x. [DOI] [Google Scholar]
- Murata M, Sakaguchi M. Storage of yellowtail (Seriola quinquerediata) white and dark muscle in ice: Changes in content of adenine nucleotide and related compound. J Food Sci. 1986;55:321–326. doi: 10.1111/j.1365-2621.1986.tb11120.x. [DOI] [Google Scholar]
- Ocaño-Higuera VM, Marquez-Ríos E, Canizales-Dávila M, Castillo-Yáñez FJ, Pacheco-Aguilar R, Lugo-Sánchez ME, García-Orozco KD, Graciano-Verdugo AZ. Posmortem changes in cazon fish muscle stored on ice. Food Chem. 2009;116:933–938. doi: 10.1016/j.foodchem.2009.03.049. [DOI] [Google Scholar]
- Olafsdöttir G, Martinsdöttir E, Oehlenschläger J, Dalgard P, Jensen B, Underland I, Mackie I, Henehan G, Nielsen J, Nilsen H. Methods to evaluate fish freshness in research and industry. Trends Food Sci Tech. 1997;8:285–265. doi: 10.1016/S0924-2244(97)01049-2. [DOI] [Google Scholar]
- Özogul F, Taylor KDA, Özogul Y. Biogenic amines formation in Atlantic herring (Clupea harengus) stored under modified atmosphere packaging using a rapid HPLC method. Int J Food Sci Tech. 2002;37:515–522. doi: 10.1046/j.1365-2621.2002.00608.x. [DOI] [Google Scholar]
- Özogul F, Özogul Y, Kuley E. Nucleotide degradation and biogenic amine formation of wild white grouper (Epinephelus aeneus) stored in ice and at chill temperature (4°C) Food Chem. 2008;108:933–941. doi: 10.1016/j.foodchem.2007.11.070. [DOI] [PubMed] [Google Scholar]
- Ponce de Leon S, Inoue N, Shinano H. Self life of sardine and bacterial flora in brine containing acetic acid during immersed storage. Fisheries Sci. 1994;60:429–433. [Google Scholar]
- Reza MS, Bapary MAJ, Ahasan CT, Islam MN, Kamal M. Shelf life of several marine fish species of Bangladesh during ice storage. Int J Food Sci Tech. 2009;44:1485–1494. doi: 10.1111/j.1365-2621.2007.01613.x. [DOI] [Google Scholar]
- Rodríguez Ó, Losada V, Aubourg SP, Barros-Velázquez JB. Enhanced shelf-life of chilled European hake (Merluccius merluccius) stored in slurry ice as determined by sensory analysis and assessment of microbiological activity. Food Res Int. 2004;37:749–757. doi: 10.1016/j.foodres.2004.03.008. [DOI] [Google Scholar]
- Rodríguez Ó, Losada V, Aubourg SP, Barros-Velázquez JB. Sensory, microbial and chemical effects of a slurry ice system on horse mackerel (Trachurus trachurus) J Sci Food Agr. 2005;85:235–242. doi: 10.1002/jsfa.1960. [DOI] [Google Scholar]
- Ruiz-Capillas C, Moral A. Correlation between biochemical and sensory quality indices in hake stored in ice. Food Res Int. 2001;34:441–447. doi: 10.1016/S0963-9969(00)00189-7. [DOI] [Google Scholar]
- Saito T, Arai K, Matuyoshi M. A new method for estimating the freshness of fish. B Jpn Soc Sci Fish. 1959;24:749–750. doi: 10.2331/suisan.24.749. [DOI] [Google Scholar]
- Scherer R, Augusti PR, Steffens C, Bochi VC, Hecktheure LH, Lazzari R, Radünz-Neto J, Pomblum SCG, Emanuelli T. Effect of slaughter method on postmortem changes of grass carp (Ctenopharyngodon idella) stored in ice. J Food Sci. 2005;70:C348–C353. doi: 10.1111/j.1365-2621.2005.tb09965.x. [DOI] [Google Scholar]
- Scott DN, Fletcher GC, Charles JC, Wong RJ. Spoilage changes in the deep water fish, smooth Oreo Dory during storage in ice. Int J Food Sci Tech. 1992;27:577–587. doi: 10.1111/j.1365-2621.1992.tb01225.x. [DOI] [Google Scholar]
- Sikorkski ZE, Kolakowska A, Burt JR. Postharvest biochemical and microbial changes. In: Sikorski ZE, editor. Seafood: Resources, nutritional composition and preservation. Boca Raton: CRC Press; 1990. pp. 55–75. [Google Scholar]
- Stratton JE, Hutkins RW, Taylor SL. Biogenic-amines in cheese and other fermented foods. A review. J Food Protect. 1991;54:460–470. doi: 10.4315/0362-028X-54.6.460. [DOI] [PubMed] [Google Scholar]
- Veciana-Nogues MT, Izquierdo-Pulido M, Vidal-Carou MC. Determination of ATP related compounds in fresh and canned tuna fish by HPLC. Food Chem. 1997;59:467–472. doi: 10.1016/S0308-8146(96)00243-9. [DOI] [Google Scholar]
- Wongso S, Yamanaka H. Changes in content of extractive components in the adductor muscle of noble scallop during storage. Fisheries Sci. 1996;62:815–820. [Google Scholar]
- Woyewoda AD, Shaw SJ, Ke PJ, Burns BG (1986) Recommended laboratory methods for assessment of fish quality. In: Halifax NS (ed) Canadian Technical Report of Fisheries and Aquatic Science No. 1448, Minster of Supply and Services, Canada, pp 125–134
- Yerlikaya P, Gokuglu N, Uran H. Quality changes of fish patties produced from anchovy during refrigerated storage. Eur Food Res Tech. 2005;220:287–291. doi: 10.1007/s00217-004-1035-x. [DOI] [Google Scholar]