Abstract
The present study evaluated the antidiabetic potential of Butea monosperma Lam. Kuntze (Fabaceae) leaves and the stem bark using various in vitro techniques. The samples were studied for their effects on glucose adsorption, diffusion, amylolysis kinetics, enteric enzymes and glucose transport across yeast cells. Both the samples adsorbed glucose and the adsorption of glucose increased with increase in glucose concentration. The samples also inhibited movement of glucose across the dialysis membrane to varying degree in both glucose diffusion and amylolysis kinetic experiment models. B. monosperma leaves inhibited α-amylase, α-glucosidase and sucrase enzymes in succession to varying degrees, whereas the bark inhibited only α-amylase to a significant extent and slightly activated α-glucosidase and sucrase enzymes. The extracts of both leaves and bark promoted glucose uptake by yeast cells compared to control. Enhancement of glucose uptake was dependent on both the sample and glucose concentration. It was directly proportional to the sample concentration and inversely proportional to the molar concentration of the glucose. From the results of the study, it is inferred that, B. monosperma leaves and bark possesses antidiabetic activity. However, these effects need to be confirmed using in vivo models and clinical trials for its effective utilization as therapeutic agents.
Keywords: Butea monosperma, GDRI, α-amylase, α-glucosidase, Sucrase, Glucose uptake
Introduction
Diabetes mellitus is a serious chronic metabolic disorder that has a significant impact on the health, quality of life and life expectancy, as well as on the health care system (Dey et al. 2002). Type 2 diabetes represents a syndrome with a disorder of carbohydrate metabolism; the most prominent clinical feature is hyperglycemia (Dey et al. 2002). With increasing incidence of diabetes, search for new alternative therapies for its prevention and treatment is the need of the hour (Al Faris et al. 2011). Medicinal plants continue to play an important role in the treatment of diabetes, particularly in developing countries where most people have limited resources and do not have an access to modern treatment. The increased demand is also due to the side effects associated with the use of insulin and oral hypoglycemic agents (Ali et al. 2006). To date, over 400 traditional medicinal plant treatments for diabetes have been reported but only a small number of these have received scientific and medical evaluation to assess their efficacy.
One such well-known traditionally used medicinal plant is Butea monosperma Lam. Kuntze (Fabaceae), commonly known as palash, (Bavarva and Narasimhacharya 2008). It is a medium sized tree, native of the mountainous regions of India and Burma (Anonymous 1988). Various medicinal properties are attributed for different parts of this plant in Ayurveda. The root bark is used as aphrodisiac, analgesic and anthelmintic (Anonymous 1988). The flowers are used for the treatment of liver disorders (Wagner et al. 1986) and the seeds as anthelmintic (Lal et al. 1978). Flowers are reported to have astringent, diuretic, and anti-inflammatory activity. Alcoholic concentrate of petals exhibit antiestrogenic activity and decoction of flowers is useful in diarrhoea and show anti-implantation activity (Khanna et al. 1966; Kirtikar and Basu 1989).The stem bark is used for the treatment of dyspepsia, diarrhoea, dysentery, diabetes, ulcers, sore throat and snake bites (Jayaweera 1981; Varier 1993). Roots are reported to be useful in the treatment of filariasis, night blindness, helminthiasis, piles, ulcers and tumors (Raj and Kurup 1968).
Since, several mechanisms have been proposed for the antidiabetic effects of medicinal plants, such as inhibition of carbohydrate metabolizing enzymes, manipulation of glucose transporters, β-cell regeneration and enhancing insulin releasing activity (Tiwari and Rao 2002), the present study was planned to evaluate the antidiabetic potential of Butea monosperma leaves and the stem bark using suitable in vitro model systems in order to elucidate the possible mechanism of antidiabetic action, which has not been documented yet.
Materials and methods
P-nitrophenyl-α-D-glucopyranoside was purchased from Sisco Research Laboratory, India. Acarbose (ACB) and wheat bran (WB) were purchased from a local store. α-amylase (Sigma Aldrich, India) and glucose oxidase peroxidase kit (Agappe Diagnostics, India), dialysis bags (12,000 MW cutoff; Himedia laboratories, India) were used. All the chemicals used in the study were of extra pure analytical grade. The leaves and the bark of B. monosperma were collected from Kollegal, Chamarajanagar district, Karnataka, India, subsequently identified by Dr. Shivprasad Hudeda, JSS Ayurvedic College, Mysore and a voucher specimen is retained in the laboratory for future reference (RL2 003/2008-09). The bark and leaves were cleaned, dried in a hot air oven (50 °C), powdered, passed through 60 mesh sieve (BS) and stored in an airtight container at 4 °C till further use.
Preparation of aqueous extracts
Aqueous extracts were prepared by extracting the powders of B. monosperma leaves (BML) and B. monosperma bark (BMB) with hot water (70 °C) in a mechanical shaker (24 h), filtered and freeze dried.
Determination of glucose adsorption capacity
Glucose adsorption capacity of the samples was determined as reported earlier (Ahmed et al. 2011). Briefly, samples (1%) were added to 25 mL of glucose solution of increasing concentration (5, 10, 20, 50 & 100 mM), the mixture was stirred well, incubated in a shaker water bath at 37 °C for 6 h, centrifuged at 4,000 × g for 20 min and the glucose content in the supernatant was determined. Glucose bound was calculated using the following formula.
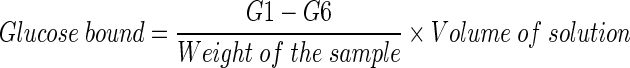 |
*G1: glucose concentration of original solution, G6: glucose concentration after 6 h
In case of fiber, acarbose (0.2%) was added to 25 mL of glucose solution (20 mM). The solution mixtures were dialyzed against 200 ml of distilled water at 37 °C. The glucose content in the dialysate was determined after 6 h. Glucose bound was calculated as follows:
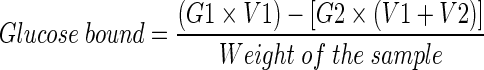 |
*G1: glucose concentration in retentate before start of diffusion, G2: glucose concentration in dialysate after 6 h, V1: volume of retentate, V2: volume of dialysate.
Effect of the selected samples on in vitro glucose diffusion
The glucose-dietary fiber system comprising of 25 mL of glucose solution (20 mM) and the samples (1%) and acarbose (0.2%) were dialyzed in dialysis bags against 200 mL of distilled water at 37 °C in a shaker water bath. The glucose content in the dialysate was determined at 60, 120, 180 and 240 min using glucose oxidase peroxidase diagnostic kit (Ahmed et al. 2011). A control test was carried out without sample. The GDRI was calculated with the following formula,
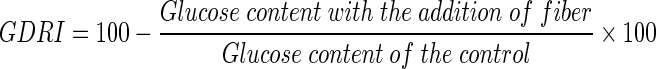 |
Effect of the samples on in vitro amylolysis kinetics
Forty grams of potato starch was added to ≈900 ml of 0.05 M phosphate buffer (pH 6.5). The solution after stirring at 65 °C for 30 min was made up to a final volume of 1,000 mL to give a 4% (w/v) starch solution. The starch-α-amylase-dietary fiber system comprising the above starch solution (25 mL), α-amylase (0.4%), and test sample (1%) were dialyzed in a dialysis bags against 200 mL of distilled water at 37 °C (pH 7.0) in a shaker water bath. The glucose content in the dialysate was determined at 60, 120, 180 and 240 min. A control test was carried out without sample (Ahmed et al. 2011).
α-amylase inhibitory activity
α-amylase inhibitory activity was studied using enzyme-starch system. Sample (1%) was mixed by stirring with 25 mL of potato starch (4%) in a beaker; 100 mg of α-amylase was added to the starch solution, stirred vigorously and incubated at 37 °C for 60 min. After the incubation period 0.1 M NaOH (2 mL) was added to terminate enzyme activity. The mixture was centrifuged for 15 min (3,000 × g) and glucose content was determined in the supernatant. A control test was also run without the addition of test sample (Ou et al. 2001).
α-glucosidase inhibitory activity
α-glucosidase inhibitory activity was studied according to the method of Honda and Hara (1993), wherein crude enzyme solution containing α-glucosidase and sucrase prepared from the rat small intestine was used (Ahmed et al. 2009). The enzyme solution (10 μL) and 50 μL of sample emulsion (10 mg mL-1) were incubated together with 200 μL maleate buffer (pH 6.0) for 10 min at 37 °C. The enzyme reaction was started by adding 200 μL of p-nitrophenyl-α-D-glucopyranoside solution (2 mM) and incubated at 37 °C. After 30 min, the reaction was terminated by treating the mixture in a boiling water bath for 5 min and after the addition of 1.0 mL of disodium hydrogen phosphate solution (0.1 M), the absorption of the liberated p-nitrophenol was read at 400 nm. An untreated enzyme solution was used as the control.
Sucrase inhibitory activity
The enzyme solution (10 μL) and 10 μL of sample emulsion (10 mg mL−1) were incubated together with 180 μL maleate buffer (pH 6.0) for 10 min at 37 °C. The enzyme reaction was started by adding 100 μL sucrose solution (60 mM). After 30 min, the reaction was terminated by adding 200 μL of 3,5-dinitrosalysilic acid reagent and treating the mixture in a boiling water bath for 5 min. The absorbance of the solution was read at 540 nm. An untreated enzyme solution was used as the control (Honda and Hara 1993).
The percent inhibitory activities of α-amylase, α-glucosidase and sucrase were calculated using the following formula.
 |
Where, Abs control is the absorbance of the control reaction (containing all reagents except the test sample), and Abs sample is the absorbance of the test sample. All the experiments were carried out in triplicates.
Glucose uptake in yeast cells
Yeast cells were prepared according to the method of Cirillo (1962). Briefly, commercial baker’s yeast was washed by repeated centrifugation (3,000 × g; 5 min) in distilled water until the supernatant fluids were clear and a 10% (v/v) suspension was prepared in distilled water.
Various concentrations of extracts (1–5 mg) were added to 1 mL of glucose solution (5–25 mM) and incubated together for 10 min at 37 °C. Reaction was started by adding 100 μL of yeast suspension, vortex and further incubated at 37 °C for 60 min. After 60 min, the tubes were centrifuged (2,500 × g, 5 min) and glucose was estimated in the supernatant.
The percent increase in glucose uptake by yeast cells was calculated using the following formula.
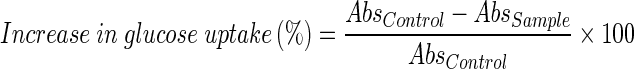 |
Where, Abs control is the absorbance of the control reaction (containing all reagents except the test sample), and Abs sample is the absorbance of the test sample. All the experiments were carried out in triplicates.
Statistical analysis
All determinations were carried out in triplicates and data were analyzed by ANOVA followed by Tukey’s multiple comparisons test for significant differences using SPSS 14.0 software. Values were considered significant at p ≤ 0.05.Graphs were plotted using Origin 8.1 software.
Results and discussion
Glucose adsorption capacity of Butea monosperma leaves and the bark
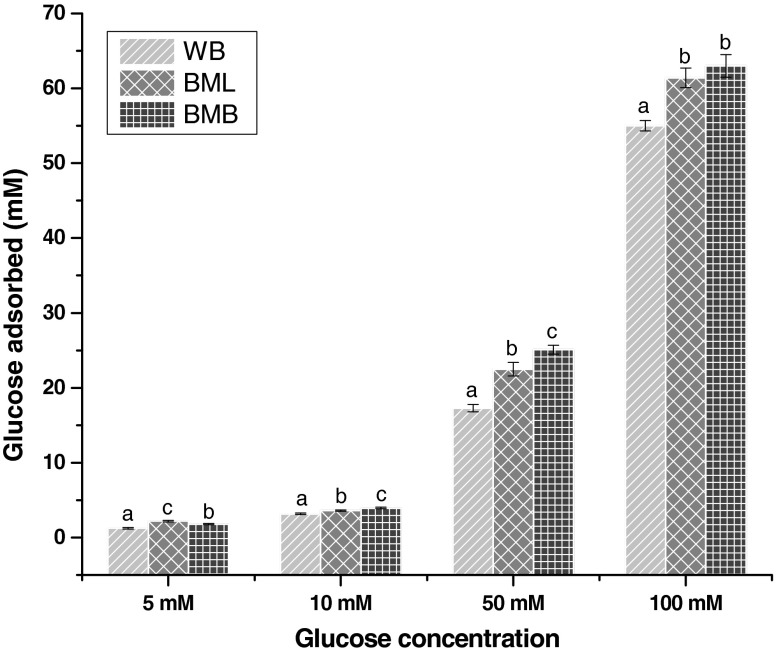
The effect of BML, BMB and wheat bran (WB) on glucose adsorption capacity is shown in Fig. 1. At 5 mM concentration of glucose BML adsorbed significantly higher (p ≤ 0.05) amount of glucose than BMB and WB. As the concentration increased, glucose adsorption capacity of BMB increased significantly (p ≤ 0.05) and adsorbed more glucose than BML and WB. However, at all concentrations the glucose adsorption capacity of BMB and BML were significantly higher (p ≤ 0.05) than that of WB. It is noteworthy that, glucose adsorption capacity of all the samples was directly proportional to the molar concentration of glucose. Changes in the adsorption rate of glucose by the BML, BMB and WB may be attributed to their difference in the physical entrapment of the solvent, their affinity to bind substrate and nutrient composition particularly, total dietary fiber content. Dietary fibers can bind glucose, but they might show no adsorption capacity for glucose when the concentration of glucose is decreased (Ou et al. 2001). In vivo and in vitro studies of glucose adsorption have shown that the delay in glucose absorption in the gastrointestinal tract is determined mainly by the viscosity of soluble polysaccharides (Chau et al. 2003). The abilities of both insoluble and soluble fibers from different sources, to adsorb glucose have been reported, this may be because of the difference in the fiber content and composition of BML and BMB in comparison with commercial fiber WB (Adiotomre et al. 1990; Brigenti et al. 1995; Ou et al. 2001).
Fig. 1.
Glucose binding capacity of the samples at different concentrations of glucose. WB wheat bran; BML Butea monosperma leaf; BML Butea monosperma bark. Values are mean ± SD of triplicate determinations. Bars carrying different letters differ significantly from each other (p ≤ 0.05)
Effect on in vitro glucose diffusion
The effect of the samples on retarding glucose diffusion across the dialysis membrane is shown in Table 1. The rate of glucose diffusion was found to increase with time from 30 to 180 min. Both BML and BMB caused significant retardation (p ≤ 0.05) of glucose diffusion compared to control at various time intervals. The retardation of glucose diffusion by BML was significantly higher (p ≤ 0.05) than BMB and WB. These effects were reflected with higher GDRI values for BML followed by BMB and WB (Table 1). It is well known that dietary fibers interfere with diffusion of glucose, which indicates that, plant fibers can modulate postprandial glucose and insulin concentrations in non-insulin dependent diabetic subjects if administered with meals. In particular, water-soluble fibers are reported to be more effective than insoluble fibers such as wheat bran (Pastors et al. 1991). From the results, it was observed that the GDRI was more in WB followed by BML and BMB. This phenomenon can be explained in terms of adsorption of glucose by the water soluble dietary fibers as, water soluble fibers affect the diffusion more significantly than the water insoluble dietary fiber (Ou et al. 2001). Reports show that the diffusion of glucose was affected by adsorption and viscosity of dietary fibers (Ou et al. 2001) thus, diffusion rate of glucose was slow, although the concentration in the dialysis bag was high. As the adsorption saturated, the diffusion of glucose was affected only by the viscosity of dietary fibres.
Table 1.
Effect of selected samples on glucose diffusion and glucose dialysis retardation index (GDRI)
| Sample | Glucose content in the dialysate (mM) | |||
|---|---|---|---|---|
| 30 | 60 | 120 | 180 | |
| Control | 0.94c ± 0.01 | 1.23d ± 0.01 | 1.76c ± 0.01 | 1.93c ± 0.01 |
| WB | 0.75b ± 0.01 (29.2) | 1.18c ± 0.01 (4.0) | 1.60b ± 0.01 (9.0) | 1.78b ± 0.01 (7.7) |
| BML | 0.61a ± 0.01 (34.7) | 0.75a ± 0.01 (38.1) | 1.56a ± 0.01 (10.9) | 1.71a ± 0.01 (10.8) |
| BMB | 0.64a ± 0.01 (31.8) | 1.05b ± 0.01 14.0 | 1.55a ± 0.01 (11.9) | 1.72a ± 0.01 (11.0) |
WB wheat bran; BML Butea monosperma leaf powder; BML Butea monosperma bark powder
Values in parenthesis indicate glucose dialysis retardation index (GDRI)
Mean values (n = 3) with different superscript letters in columns differ significantly from each other (p ≤ 0.05)
Effect on amylosis kinetics
The effects of BML and BMB on the amylolysis kinetics are shown in the Table 2. Inhibition of α-amylase and glucose diffusion retardation index (GDRI) was found to be 53%, 40% and 32.4% in WB, BML and BMB, respectively at 60 min. A higher GDRI indicates a higher retardation index of glucose by the sample. The effect of insoluble dietary fiber in the inhibition of glucose diffusion in the small intestine is suggested to be due to the adsorption or inclusion of the smaller sugar molecules within the structure of the fiber particles (Nishimune et al. 1991; Jenkins et al. 1978). The retardation of glucose diffusion is also due to the inhibition of α-amylase, thereby limiting the release of glucose from the starch. The inhibition of α-amylase activity by medicinal plants might be attributed to several possible factors such as fiber concentration, the presence of inhibitors on fibers, encapsulation of starch and enzyme by the fibers present in the sample, thereby reducing accessibility of starch to the enzyme, and direct adsorption of the enzyme on fibers, leading to decreased amylase activity (Ou et al. 2001).
Table 2.
Effect of selected samples on starch digestibility and glucose dialysis retardation index (GDRI)
| Sample | Glucose content in the dialysate (mM) | |||
|---|---|---|---|---|
| 30 | 60 | 120 | 180 | |
| Control | 0.0 | 0.19c ± 0.01 | 0.26c ± 0.01 | 0.35c ± 0.01 |
| WB | 0.0 (100) | 0.09a ± 0.01 (52.7) | 0.15a ± 0.01 (42.4) | 0.20a ± 0.01 (42.9) |
| BML | 0.0 (100) | 0.10ab ± 0.01 (40.0) | 0.21b ± 0.01 (19.5) | 0.28b ± 0.01 (20.0) |
| BMB | 0.0 (100) | 0.12b ± 0.01 (32.4) | 0.21b ± 0.01 (19.1) | 0.33c ± 0.01 (5.7) |
WB wheat bran, BML Butea monosperma leaf powder, BML Butea monosperma bark powder
Values in parenthesis indicate glucose dialysis retardation index (GDRI)
Mean values (n = 3) with different superscript letters in columns differ significantly from each other (p ≤ 0.05)
Effect on carbohydrate hydrolyzing enzymes
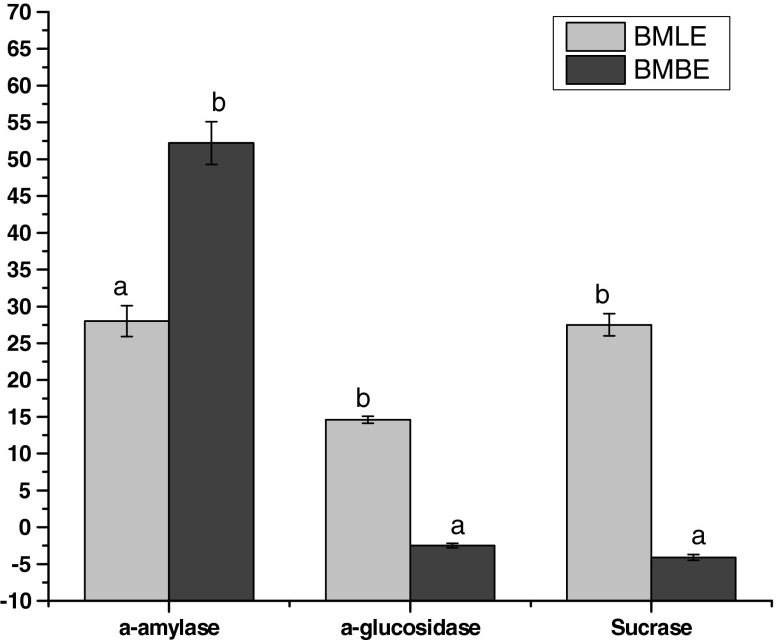
One therapeutic approach for treating diabetes is to decrease the postprandial hyperglycemia. This is done by retarding the absorption of glucose through the inhibition of carbohydrate hydrolyzing enzymes (α-amylase, α-glucosidase and sucrase) in the digestive tract. Inhibitors of these enzymes delay and prolong carbohydrate digestion time, causing a reduction in the rate of glucose absorption and consequently blunting the postprandial plasma glucose rise (Ali et al. 2006). The effect of aqueous extracts of B. monosperma leaves (BMLE) and bark (BMBE) on the activities of brush border enzymes such as α-amylase, α-glucosidase and sucrase is presented in Fig. 2. Both the sample extracts inhibited α-amylase to varying degrees and among two, BMBE (52%) exhibited significantly higher (p ≤ 0.05) inhibition of α-amylase than BMLE (28%). BMLE inhibited α-glucosidase and sucrase to an extent of 14.6% and 27.5% respectively, while BMBE activated the enzymes to an extent of 2.5% and 4.5%, respectively.
Fig. 2.
Effect of Butea monosperma on α-amylase, α-glucosidase and sucrase. WB wheat bran; BMLE Butea monosperma leaf extract; BMBE Butea monosperma bark extract. Values are mean ± SD of triplicate determinations. Bars carrying different letters differ significantly from each other (p ≤ 0.05)
The inhibition of a-amylase might be attributed to several possible factors such as fiber concentration, the presence of inhibitors on fibers, encapsulation of starch and enzyme by the fibers present in the sample thereby reducing accessibility of starch to the enzyme and direct adsorption of the enzyme on fibers leading to decreased amylase activity (Sheela and Augusti 1992). The differences of inhibition between sucrase and α-glucosidase may derive from the affinities of the esterified polyphenols for the enzyme proteins (Honda and Hara 1993). A slight increase in the activities α-glucosidase and sucrase by the bark extract may be due to a conformational change of the phytochemicals derived from binding of compounds to the enzyme (Nickavar et al. 2008).
Effect on glucose transport across yeast cells
The rate of glucose transport across cell membrane in yeast cells system is presented in Fig. 3. The amount of glucose remaining in the medium after a specific time serves as an indicator of the glucose uptake by the yeast cells. The rate of uptake of glucose into yeast cells was linear in all the 5 glucose concentrations. The BMBE exhibited significantly higher (p ≤ 0.05) activity than BMLE at all concentrations. However, the percent increase in the glucose uptake by the yeast cells was inversely proportional to the glucose concentration and was found to decrease with increase in the molar concentration of the glucose solution.
Fig. 3.
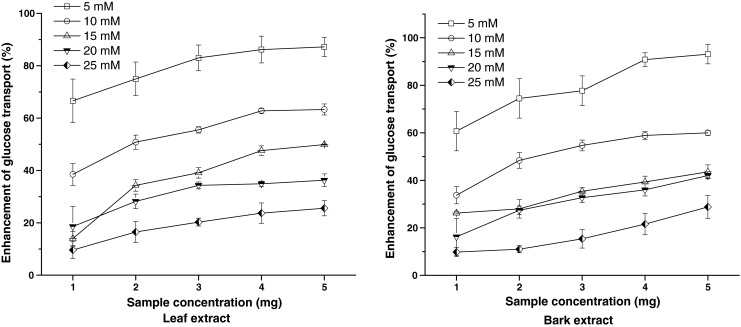
Effect of Butea monosperma leaf and bark extracts on the uptake of glucose by yeast cells. Values are mean ± SD of triplicate determinations
The mechanism of glucose transport across the yeast cell membrane has been receiving attention as in vitro screening method for hypoglycemic effect of various compounds/medicinal plants. Recent studies on the transport of nonmetabolizable sugars and certain metabolizable glycosides suggest that sugar transport across the yeast cell membrane is mediated by stereospecific membrane carriers. It is reported that in yeast cells (Saccharomyces cerevisiae) glucose transport is extremely complex and it is generally agreed that glucose is transported in yeast is by a facilitated diffusion process. Facilitated carriers are specific carriers that transport solutes down the concentration gradient. This means that effective transport is only attained if there is removal of intracellular glucose (Illiano and Cuatrecasas 1971; Teysink et al. 1998).
Conclusion
From the results of the present study, it is inferred that Butea monosperma leaves and bark possesses antidiabetic properties as implied by the in vitro and ex vivo experiments. Further, its hypoglycemic effect is mediated by increasing glucose adsorption, decreasing glucose diffusion rate, by inhibiting the activities of the enteric enzymes such as α-amylase, α-glucosidase and sucrose and at cellular level by promoting glucose transport across the cell membrane as reflected by simple in vitro model of yeast cells.
References
- Adiotomre EMA, Edwards CA, Brydon W. Dietary fiber: in vitro methods that anticipate nutrition and metabolic in humans. Am J Clin Nutr. 1990;52:128–134. doi: 10.1093/ajcn/52.1.128. [DOI] [PubMed] [Google Scholar]
- Ahmed F, Sairam S, Urooj A. Effect of various Ayurvedic formulations and medicinal plants on carbohydrate hydrolyzing enzymes and glucose uptake by yeast cells-An in vitro study. J Pharm Res. 2009;2:563–568. [Google Scholar]
- Ahmed F, Sairam S, Urooj A. In vitro hypoglycemic effects of selected dietary fiber sources. J Food Sci Technol. 2011;48(3):285–289. doi: 10.1007/s13197-010-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Faris NA, Al Othman ZA, Ahmad D. Effects of Mesembrrybryanthemum forsskalei Hochst seeds in lowering glucose/lipid profile in streptozotocin-induced diabetic rats. J Food Sci Technol. 2011;48(5):616–621. doi: 10.1007/s13197-010-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali H, Houghton PJ, Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol. 2006;107:449–455. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- The wealth of India. New Delhi: Council of Scientific and Industrial Research; 1988. p. 341. [Google Scholar]
- Bavarva JH, Narasimhacharya AVRL. Preliminary study on antihyperglycemic and antihyperlipaemic effects of Butea monosperma in NIDDM rats. Fitoterapia. 2008;79:328–331. doi: 10.1016/j.fitote.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Brigenti F, Pellegrini N, Casiraghi MC, Testolin G. In vitro studies to predict physiological effects of dietary fiber. Eur J Clin Nutr. 1995;49:381–388. [PubMed] [Google Scholar]
- Chau CF, Huang YL, Lee MH. In vitro hypoglycemic effect of different insoluble fiber-rich fractions prepared from the peel of citrus Sinensis L.cv. Liucheng. J Agric Food Chem. 2003;51:6623–6626. doi: 10.1021/jf034449y. [DOI] [PubMed] [Google Scholar]
- Cirillo VP. Mechanism of Glucose transport across the yeast cell membrane. J Bacteriol. 1962;84:485–491. doi: 10.1128/jb.84.3.485-491.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey L, Anoja S, Attele YCS. Type two diabetes; Alternative medicine review. Thorne Res. 2002;7:45–58. [PubMed] [Google Scholar]
- Honda M, Hara Y. Inhibition of rat small intestinal sucrase and á-glucosidase activities by tea polyphenols. Biosci Biotech Biochem. 1993;57:123–124. doi: 10.1271/bbb.57.123. [DOI] [PubMed] [Google Scholar]
- Illiano G, Cuatrecasas P. Glucose transport in fat cell membranes. J Biochem. 1971;246:2472–2479. [PubMed] [Google Scholar]
- Jayaweera DMA. Medicinal plant used in Ceylon. Part 3. Colombo: National Science Council of Sri Lanka; 1981. p. 161. [Google Scholar]
- Jenkins DJA, Wolever TMS, Leeds AR, Gassul MA, Haisman P, Dilawari J, Goff DV, Metz GL, Alberti KGMM. Dietary fibers, fiber analogues and glucose tolerance: importance of viscosity. Brit Med J. 1978;1:1392–1394. doi: 10.1136/bmj.1.6124.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna U, Handa S, Choudhruy RR. The effect of Butea monosperma on the fertility of female rats. Indian J Pharmacol. 1966;28:343–347. [Google Scholar]
- Kirtikar KR, Basu BD. Indian medicinal plants. Dehradun: Shiva Offset Press; 1989. p. 785. [Google Scholar]
- Lal J, Chandra S, Sabir M. Modified method for isolation of palasonin—the anthelmintic principle of Butea frondosa seeds. Indian J Pharmacol Sci. 1978;40:97–98. [Google Scholar]
- Nickavar B, Abolhasani L, Izadpanah H. α-amylase inhibitory activities of six Salvia species. Iranian J Pharm Res. 2008;7:297–303. [Google Scholar]
- Nishimune T, Yakushiji T, Sumimoto T, Taguchi S, Konishi Y, Nakahara S, Kunita N. Glycemic response and fiber content of some foods. Am J Clin Nutr. 1991;54:414–419. doi: 10.1093/ajcn/54.2.414. [DOI] [PubMed] [Google Scholar]
- Ou S, Kwok KC, Li Y, Fu L. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J Agric Food Chem. 2001;49:1026–1029. doi: 10.1021/jf000574n. [DOI] [PubMed] [Google Scholar]
- Pastors JG, Blaisdell PW, Balm TK, Asplin CM, Pohl SL. Psyllium fiber reduces rise in postprandial glucose and insulin concentrations in patients with non-insulin-dependent diabetes. Am J Clin Nutr. 1991;53:1431–1435. doi: 10.1093/ajcn/53.6.1431. [DOI] [PubMed] [Google Scholar]
- Raj RK, Kurup PA. Anthelmintic activity, toxicity nd other pharmacological activities of palasonin, the active principle of seeds and its piperazine salt. Indian J Med Res. 1968;56:13–18. [PubMed] [Google Scholar]
- Sheela CG, Augusti KT. Antidiabetic effects of S-allyl cysteine sulphoxide isolated from garlic Allium sativum Linn. Indian J Exp Biol. 1992;30:1491. [PubMed] [Google Scholar]
- Teysink B, Jasper A, Diderich HV, Westerhoff DKV, Walsh MC. Intracellular glucose concentration in derepressed yeast cells consuming glucose is high enough to reduce the glucose transport rate by 50% J Bacteriol. 1998;180:556–562. doi: 10.1128/jb.180.3.556-562.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari AK, Rao JM. Diabetes mellitus and multiple therapeutic approaches of phytochemicals: present status and future prospects. Curr Sci. 2002;23:30–33. [Google Scholar]
- Varier SPV. Indian medicinal plants, vol. 1. 1. Madras: Orient Longman Limited; 1993. p. 284. [Google Scholar]
- Wagner H, Geyer B, Fiebig M, Kiso Y, Hikino H. Isobutrin and butrin, the antihepatotoxic principles of Butea monosperma flowers. Planta Med. 1986;2:77–79. doi: 10.1055/s-2007-969083. [DOI] [PubMed] [Google Scholar]