Abstract
Pomegranate juice’s valuable nutritional components may be reduced during its processing or storage. This work examined the effect of frozen storage at −25 °C on some chemical characteristics of pomegranate juice. Total anthocyanin content of pomegranate juice, which was measured using the pH differential method, decreased by 11% after 20 days of frozen storage. Phenolic components, measured using a Folin and Ciocalteu assay by means of a UV–vis spectrophotometer, decreased by 29% after 20 days of frozen storage. Antioxidant activity, measured based on the radical scavenging properties of the juice using the 2,2-diphenyl-1-picrylhydrazyl method, decreased by 50% after 20 days of frozen storage. Pomegranate juice has 5 major anthocyanins, including Cyanidin 3-glucoside, Cyanidin 3,5-diglucoside, Delphinidin 3-glucoside, Pelargonidin 3-glucoside and Pelargonidin 3,5-diglucoside are 5 major anthocyanins of pomegranate juice. They were measured using the LC-MS method and results showed that Pelargonidin 3,5- diglucoside had the greatest decrease. Also, the LC-MS method showed that ellagic acid decreased by 15%.
Keywords: Anthocyanin, Frozen, Juice, Phenolic, Pomegranate, LC-MS
Introduction
The pomegranate (Punica granatum L., Punicaceae), mainly produced in the Middle East, has been reported to have a number of nutritional and health benefits (Aviram et al. 2004; Fuhrman et al. 2005; Lansky and Newman 2007; Türk et al. 2008). Pomegranate juice is a potential source of anthocyanins, ellagic acid, phytoestrogenic flavonoids, tannins and organic acids, some of which are antioxidants (Poyrazoğlu et al. 2002; Mousavinejad et al. 2009; Tezcan et al. 2009). Since the pomegranate is a seasonal fruit, selecting the best conditions to preserve its juice is important. Pomegranate juice can be concentrated for consumption when the fresh fruit is not available, and can be stored in chilled and frozen conditions to preserve it from chemical and microbial spoilages.
Freezers are one of the common instruments to preserve food materials such as fruit juices. In the food industry, a storage temperature of −18 °C effectively reduces chemical and microbial spoilage of foods.
This raises an important question for juice consumers: is the juice’s nutritional value preserved during prolonged frozen storage? To response to this question Cortés et al. (2005) evaluated the effect of frozen condition on contents of carotenoids and the ascorbic acid content in orange-carrot juice. After storing the juice at −40 °C for 132 days, they concluded that ascorbic acid decreased by 4.1% during storage; however, the activity of vitamin A increased. Antheraxanthin and the mixture of 9-cis- violaxantin and neoxanthin decreased after storage but other carotenes were constant. In a similar study, Lee and Coates (1999) evaluated the changes in vitamin C in frozen orange juice. They found that vitamin C content decreased from 40.6 mg/100 ml to 32.8 mg/100 ml after 24 months’ storage at −23 °C.
Since the nutritional value of pomegranate juice is an important factor that makes it preferable to some other juices, its preservation is valuable from the point of view of consumer health. However, there is only one study about changes in pomegranate juice characteristics during concentration and clarification of the juice using membrane technology (Mirsaeedghazi et al. 2009), and no study examining its characteristics after frozen storage. In this work the nutritional components of pomegranate juice (total anthocyanins content, phenolic components and antioxidant activity) were evaluated before freezing and during frozen storage. Also, to determine the most sensitive anthocyanins the juice was analyzed using the LC-MS method during 20 days’ storage at −25 °C.
Material and methods
Juice preparation and storage conditions
Pomegranate fruits vt. Saveh Black Leather were obtained from a horticultural research center in Saveh, Iran. The fruits were washed and manually cut into small pieces. The outer leathery skin enclosing the hundreds of fleshy sacs was also removed. The juice was then extracted by manual pressing of the sacs. The extracted juice was pureed (with passing from mesh filter No. 10 to remove the large extra particles) and placed in polyethylene terephthalate (PET) bottles, which were stored in the freezer at a temperature of −25 °C. The samples’ temperature was controlled during storage using an infrared digital thermometer scanner. The samples were defrosted at different times during the storage period (5, 10, 15, and 20 days) in same conditions which the blank sample was stored and the chemical characteristics were analyzed after complete defrosting.
Measurement of phenolic compounds and total anthocyanin content
Total phenolic contents of the juice were measured using a Folin and Ciocalteu assay by means of a UV–vis spectrophotometer (CECIL, model 2502, Cambridge, England) at 760 nm. Gallic acid was used as the external standard. The total phenolic content was recorded as mg of gallic acid in 100 mL juice (Çam et al. 2009). Total anthocyanin content of pomegranate juice was measured according to Çam et al. (2009) by using the pH differential method and reported as cyanidin-3-glucoside.
Evaluation of antioxidant activity
Antioxidant activity was measured based on the radical scavenging properties of the juice using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method, and the results were expressed as an efficient concentration (EC 50), i.e., the concentration of antioxidant required for the 50% scavenging of DPPH radicals when a steady state is reached (Çam et al. 2009).
LC/MS analysis
The samples were centrifuged and filtered through a 0.45 μm PTFE filter into testing vials. The LC system was a Waters 2690 and consisted of a binary pump, an in-line degasser, an autosampler, a column thermostat, and an Quattro-Ultima triple quadrupole mass spectrometer (Micromass). Chromatograms were processed using Micromass-MassLynx (ver. 3.5) software. The detection of the analyte was in the multiple reaction monitoring mode (MRM) using an electrospray positive ionization (ESI positive). The monitored ions are listed in Table 1. Chromatographic separation was performed at 30 ◦C on a reversed phase XTerra MS C18 column (150 × 4.6 mm; particle size 5 μm), protected by an in-line filter. The liquid chromatographic conditions were described previously [Mirsaeedghazi et al. 2010].The specific LC-MS conditions for each analyte are mentioned in Table 2.
Table 1.
The main parameters of LC/MS analysis of each target compound
| Compound | Cone voltage (V) | Collision energy (eV) | Mother ion (m/z) | Daughter ion (m/z) |
|---|---|---|---|---|
| Cyanidin 3,5-diglucoside | 31 | 34 | 611 | 287 |
| Delphinidin 3- glucoside | 31 | 34 | 465 | 303 |
| Pelargonidin 3,5- diglucoside | 31 | 34 | 595 | 271 |
| Cyanidin 3-glucoside | 31 | 34 | 449 | 287 |
| Pelargonidin 3- glucoside | 31 | 34 | 433 | 271 |
| Ellagic acid | 135 | 34 | 301 | 284 |
Table 2.
The specific LC/MS conditions for each analyte
| Compound | ESI capillary voltage (kV) | Source temperature (°C) | Desolvation temperature (°C) | Cone gas flow (L/h) | Desolvation gas flow (L/h) | Multiplier (V) |
|---|---|---|---|---|---|---|
| Anthocyanins | 3.5 | 120 | 200 | 40 | 425 | 525 |
| Ellagic acid | 3.0 | 120 | 220 | 40 | 425 | 525 |
Statistical analysis of the data
All physicochemical tests were performed three times and the means were recorded. Statistical analysis of the data was performed using one-way ANOVA and mean values were compared applying a Duncan multiple range test using Minitab 15 software.
Results and discussion
Changes in total anthocyanins, phenolic compounds and antioxidant activity
Anthocyanins, tannins and ellagic acid are the major phenolic components in pomegranate juice (Mousavinejad et al. 2009). Evaluation of the frozen pomegranate juice showed that total anthocyanins decreased by about 11% after 20 days’ frozen storage (−25 °C) (Table 3). Evaluation of total phenolic contents showed a 29% decrease after 15 days’ storage at −25 °C (Table 3). One of the most important nutritional parameters of pomegranate juice is its antioxidant activity, which is attributed to the high content and unique composition of its soluble phenolic compounds, especially punicalagin (Tezcan et al. 2009; Borochov-Neori et al. 2009). The antioxidant activity of pomegranate juice during frozen storage was halved after 20 days (Table 3). These results showed that frozen storage, even at lower temperatures than industrial freezing conditions (−18 °C), cannot preserve the antioxidant activity, anthocyanin content, and phenolic components of pomegranate juice. Results were in contrast with the results of Lohachoompol et al. (2004) about frozen storage of blueberry juice. They concluded that antioxidant activity and total anthocyanin content of blueberry juice did not change during storage at frozen condition. Such different results can probably attribute to dissimilar main anthocyanin in blueberry juice compared to pomegranate juice. Also, our results were in contrast with the results of Polinati et al. (2010) that evaluated the effect of storage at −18 and −70 °C on phenolic components and antioxidant activity of orange and apple juices. They concluded that polyphenolic content and antioxidant activity of both juices did not decrease even at −18 °C. However, our results were same as the results of Poiana et al. (2010). They evaluated the effect of storage at −18 °C on chemical composition of strawberry, sweet cherry and sour cherry juices and concluded that freezing can decrease bioactive components in all juices. But, their other results were not same as our results. Bioactive components in these juices did not change during storage at −18 °C up to 4 months.
Table 3.
Changes in chemical properties of pomegranate juice during frozen storage at −25 °C
| Time (Day) | |||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | |
| Total anthocyanins (mg/100 mL) | 18.5a,* | nd | 17.7b | nd | 16.4c |
| Efficient concentration 50 (mL juice/g DPPH) | 0.003a | nd | 0.005b | nd | 0.006c |
| Total phenolic content (mg/100 mL) | 406a | 392b | 323c | 288d | nd |
nd no data
*In each row, means (from three replications) with the same letter are not significantly different (p > 0.05)
Analytical method validation for LC-MS method
Since fragment ions from the direct cleavage of the anthocyanidins and glycosides are the major peaks in the MS/MS spectrum and are easily distinguished, mass spectrum provides the most valuable information for anthocyanin identification. This is especially useful in simultaneously quantifying the different anthocyanins in the samples. Using HPLC in tandem with ESI-MS/MS allowed the separation of the anthocyanins and the acquisition of the molecular weight and useful fragmentation ions in a single run. The aims of the analytical method developed in this study were to obtain adequate separation and peak resolution of the target compounds in a shorter run-time.
Each compound was quantified by comparing its peak area against the standard curve obtained specifically for the reference solutions containing that compound. To obtain the standard curves, five different concentrations of ellagic acid (1–10 μg/100 μL) and five different concentrations (1–40 μg/100 μL) of each anthocyanin type (Cyanidin 3-glucoside, Cyanidin 3,5-diglucoside, Delphinidin 3-glucoside, Pelargonidin 3-glucoside, Pelargonidin 3,5-diglucoside) were analyzed.
The concentrations of all analytes were determined automatically by the instrument data system using the external standard method. The calibration curve models were determined by the least squares analysis. Distribution of the residuals (% difference of the back-calculated concentrations from the nominal concentrations) was investigated. The calibration model was accepted, if the residuals were within ±20% at the lower limit of quantification (LLOQ) and within ±15% at all other calibration levels and at least 2/3 of the standards met this criterion, including highest and lowest calibration levels. The lower limit of quantification was established as the lowest calibration standard with an accuracy and precision less than 20%. The within- and between-run precision (expressed as coefficient of variation, CV%) and accuracy (expressed as relative difference between obtained and theoretical concentration, Bias%) of the assay procedure were determined by analysis of five different samples at each of the lower, medium, and higher levels of the considered concentration range and one different sample of each on the same day on five different occasions, respectively.
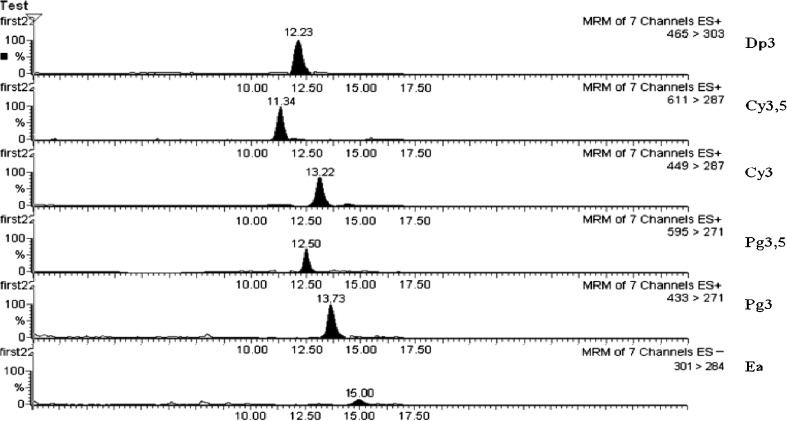
The LOQ for all the analytes was determined as 0.6 μg/mL except for pelargonidin 3- glucoside and ellagic acid, for which it was 1 μg/mL. There is a considerable concern about the matrix effect in LC/MS analysis. To evaluate the matrix effect and the selectivity of the analytical method, this work used an approach developed in our previous studies (Ahmadkhaniha et al. 2009a, b, 2010; Mirsaeedghazi et al. 2010). In brief¸ the analytes were quantified in a mixture of test samples (real pomegranate juices) from 10 different sources by two methods; the standard addition and the spiked calibration curve. Then, the results were compared by statistical analysis. The results showed that there are no significant differences between the two quantification methods. So, effect of matrix in the developed LC/MS method is absent. Figure 1 is a sample ion chromatogram showing adequate separation of each compound for quantification. The integrity and purity of analyte signals were confirmed by comparing their mass spectrum ion ratios before and after fortification with the analyte standards at different concentrations.
Fig. 1.
Sample ion chromatogram of different anthocyanins and ellagic acid obtained from LC/MS method
Changes in individual anthocyanins and ellagic acid
These results showed that total anthocyanins decreased during frozen storage of pomegranate juice. This raises the question: do all anthocyanins contribute to this reduction, or do some of them remain at high levels? To respond to this question, individual anthocyanins were evaluated with the LC–MS method. Results showed that cyanidin 3-glucoside, cyanidin 3,5-diglucoside, pelargonidin 3- glucoside, pelargonidin 3,5- diglucoside and delphinidin 3- glucoside decreased by 4.8%, 3.5%, 4.6%, 6% and 3.4% respectively after 20 days’ storage at −25 °C (Table 4). As illustrated, pelargonidin 3,5- diglucoside decreased the most compared to the other anthocyanins; however, all anthocyanins decreased after storage to some degree.
Table 4.
Changes in main anthocyanins and ellagic acid of pomegranate juice during frozen storage at −25 °C
| Time (Day) | |||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | |
| Cyanidin 3-glucoside (μg/mL) | 101.2a | 100.6b | 100.1c | 99.7d | 96.3e |
| Cyanidin 3,5-diglucoside (μg/mL) | 165.3a | 163.0b | 162.1c | 161.8d | 159.5e |
| Pelargonidin 3- glucoside (μg/mL) | 17.5a | 17.3b | nd | 17.3d | 16.7e |
| Pelargonidin 3,5- diglucoside (μg/mL) | 68.3a | 66.9b | 66.5c | 66.2d | 64.2e |
| Delphinidin 3- glucoside (μg/mL) | 100.9a | 100.1b | 99.8c | 99.7d | 97.4e |
| Ellagic acid (μg/mL) | 10.2a | 10.1b | 9.9c | 9.7d | 8.6e |
nd no data
*In each row, means (from three replications) with the same letter are not significantly different (p > 0.05)
Also, evaluation of ellagic acid as one of pomegranate juice’s major antioxidant components using the LC–MS method showed that it decreased by 15.5% after 20 days’ storage.
Conclusion
Evaluation of total anthocyanins and phenolic contents in pomegranate juice stored at −25 °C showed that they decreased after 20 days’ frozen storage due to oxidation. The same results were seen for antioxidant activity due to reduction of the major antioxidant components in juice namely anthocyanins and phenolic components. To evaluate the contribution of each individual anthocyanin, they were analyzed using the LC–MS method. The results showed that pelargonidin 3,5- diglucoside had the most reduction between all anthocyanins analyzed; however, all other anthocyanins decreased, as well. Also, ellagic acid as a major phenolic component decreased during frozen storage. This study concludes that frozen storage, even at −25 °C, cannot preserve the nutritional value of pomegranate juice.
References
- Ahmadkhaniha R, Shafiee A, Rastkari N, Kobarfard F. Accurate quantification of endogenous androgenic steroids in cattle’s meat by gas chromatography mass spectrometry using a surrogate analyte approach. Anal Chim Acta. 2009;631:80–86. doi: 10.1016/j.aca.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Ahmadkhaniha R, Kobarfard F, Rastkari N, Khoshayand MR, Amini M, Shafiee A. Assessment of endogenous androgen levels in meat, liver and testis of Iranian native cross-breed male sheep and bull by gas chromatography mass Spectrometry. Food Addit Contam A. 2009;26:453–465. doi: 10.1080/02652030802627475. [DOI] [PubMed] [Google Scholar]
- Ahmadkhaniha R, Shafiee A, Rastkari N, Khoshayand MR, Kobarfard F. Quantification of endogenous steroids in human urine by gas chromatography mass spectrometry using a surrogate analyte approach. J Chromatogr B. 2010;878(11–12):845–852. doi: 10.1016/j.jchromb.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Aviram M, Rosenblat M, Gaitini D, Nitecki S, Hoffman A, Dornfeld L, Volkova N, Presser D, Attias J, Liker H, Hayek T. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr. 2004;23:423–433. doi: 10.1016/j.clnu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Borochov-Neori H, Judeinstein S, Tripler E, Harari M, Greenberg A, Shomer I, Holland D. Seasonal and cultivar variations in antioxidant and sensory quality of pomegranate (Punica granatum L.) fruits. J Food Compos Anal. 2009;22:189–195. doi: 10.1016/j.jfca.2008.10.011. [DOI] [Google Scholar]
- Çam M, Hışıl Y, Durmaz G. Classification of eight pomegranate juices based on antioxidant capacity measured by four methods. Food Chem. 2009;112:721–726. doi: 10.1016/j.foodchem.2008.06.009. [DOI] [Google Scholar]
- Cortés C, Esteve M, Frígola A, Torregrosa F. Changes in carotenoids including geometrical isomers and ascorbic acid content in orange-carrot juice during frozen storage. Eur Food Res Tech. 2005;221:125–131. doi: 10.1007/s00217-004-1117-9. [DOI] [Google Scholar]
- Fuhrman B, Volkova N, Aviram M. Pomegranate juice inhibits oxidized LDL uptake and cholesterol biosynthesis in macrophages. J Nutr Biochem. 2005;16:570–576. doi: 10.1016/j.jnutbio.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Lee HS, Coates GA. Vitamin C in frozen, fresh squeezed, unpasteurized, polyethylene bottled orange juice: a storage study. Food Chem. 1999;65:165–168. doi: 10.1016/S0308-8146(98)00180-0. [DOI] [Google Scholar]
- Lohachoompol V, Srzednicki G, Craske J. The change of total anthocyanins in blueberries and their antioxidant effect after drying and freezing. J Biomed Biotechnol. 2004;5:248–252. doi: 10.1155/S1110724304406123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsaeedghazi H, Emam-Djomeh Z, Mousavi SMA. Concentration of pomegranate juice by membrane processing: membrane fouling and changes in juice properties. J Food Sci Tech. 2009;46(6):538–542. [Google Scholar]
- Mirsaeedghazi H, Emam-Djomeh Z, Mousavi SMA, Ahmadkhaniha R, Shafiee A. Effect of membrane clarification on the physiochemical properties of pomegranate juice. Int J Food Sci Tech. 2010;45:1457–1463. doi: 10.1111/j.1365-2621.2010.02284.x. [DOI] [Google Scholar]
- Mousavinejad G, Emam-Djomeh Z, Rezaei K, Haddad Khodaparast MH. Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars. Food Chem. 2009;115:1274–1278. doi: 10.1016/j.foodchem.2009.01.044. [DOI] [Google Scholar]
- Poiana MA, Moigradean D, Alexa E. Influence of home-scale freezing and storage on antioxidant properties and color quality of different garden fruits. Bulg J Agric Sci. 2010;16(2):163–171. [Google Scholar]
- Polinati RM, Faller ALK, Fialho E. The effect of freezing at −18 °C and −70 °C with and without ascorbic acid on the stability of antioxidant in extracts of apple and orange fruits. Int J Food Sci Tech. 2010;45:1814–1820. doi: 10.1111/j.1365-2621.2010.02333.x. [DOI] [Google Scholar]
- Poyrazoğlu E, Gökmen V, Artιk N. Organic acids and phenolic compounds in pomegranate (Punica granatum L.) grown in Turkey. J Food Compos Anal. 2002;15:567–575. [Google Scholar]
- Tezcan F, Gültekin-Özgüven M, Diken T, Özçelik B, Erim FB. Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem. 2009;115:873–877. doi: 10.1016/j.foodchem.2008.12.103. [DOI] [Google Scholar]
- Türk G, Sönmez M, Aydin M, Yüce A, Gür S, Yüksel M, Aksu EH, Aksoy H. Effects of pomegranate juice consumption on sperm quality, spermatogenic cell density, antioxidant activity and testosterone level in male rats. Clin Nutr. 2008;27:289–296. doi: 10.1016/j.clnu.2007.12.006. [DOI] [PubMed] [Google Scholar]