Abstract
Anthocyanin pigments in black soybean (Glycine max (L.) Merr.) varieties as Tawonkong (TW) and Geomjeongkong-2 (G2) were identified to evaluate their potentials as nutritional function, natural colorant or functional foods. Anthocyanin extraction was conducted with acidified methanol with 0.1 M HCl (85:15, v/v). Identification of anthocyanin was conducted by comparison with purified standards by HPLC and mass spectrometry (MS). G2 showed six different types of pigments by HPLC, whereas TW showed seven pigments. Three major anthocyanins (peaks 1, 3, 4) were detected in both varieties and peak 1 was characterized as delphinidin-3-O-β-D-glucoside, peak 3 as cyanidin-3-O-β-D-glucoside (C3G), and peak 4 as petunidin-3-O-β-D-glucoside by comparison of chromatographic properties with authentic standards and MS. Minor peaks 5, 6 and 7 in TW were tentatively identified as pelargonidin-3-O-glucoside, pelargonidin-3-O-(6″-malonylglucoside) and cyanidin on the basis of MS. MS with major ions at 287 and 449 of peak 2 were exactly same as those of peak 3 meaning that peak 2 has cyanidin and other hexose different from glucose. After acid hydrolysis of fractioned peak 2, HPLC showed the hexose as galactose, and peak 2 was identified as C3Glactose. The most abundant anthocyanin in black soybean (Glycine max (L.) Merr.) was C3G and G2 showed the higher amount of total anthocyanins than TW (p < 0.001).
Keywords: Anthocyanin, Black soybean, Natural colorant, Mass spectrometry (MS)
Introduction
Anthocyanins are groups of reddish or purple flavonoids that have been used widely as natural coloring agents in the food industry (Bridle and Timberlake 1997). They have been recognized as health-promoting functional food ingredients due to their antioxidant activity (Nam et al. 2006; Philpott et al. 2006; Satue-Gracia et al. 1997), which has been reported to reduce the risk of coronary heart disease (Stocker and O’Halloran 2004; Waterhouse 1995). Anthocyanins are also known to have anti-cancer (Hyun and Chung 2004; Kamei et al. 1995; Zhao et al. 2004), hypoglycemic (Tsuda et al. 2003), and anti-inflammatory effects (Tsuda et al. 2002) and have been used in the treatment of various circulatory disorders (Bettini et al. 1985). Furthermore, these functions provide synergic effects with various nutrients in vivo, so their nutritional values are of great interest recently.
Consumptions of black soybeans are rapidly growing due to their nutritional values and potentials to develop as healthy functional food ingredients in Korea. Anthocyanins, a group of reddish or purple flavonoids, are reported to be the primary pigments in the black soybean varieties (Choung et al. 2001; Kuroda and Wada 1933; Lee et al. 2009; Yoshikura and Hamaguchi 1969). Black soybeans contain anthocyanins mainly in the seed coat. The black pigmentation is due to accumulation of anthocyanins in the epidermis palisade layer of the seed coat (Todd and Vodkin 1993), which could be separated into anthocyanin-rich fractions for use as functional colorants or functional food ingredients. Although an extensive scientific literature on the composition of anthocyanins in fruits and vegetables exists (Francis 2000; Mazza and Gao 2005), reports on anthocyanins in different varieties of black soybean are limited with controversial results. Several groups (Choung et al. 2001; Kuroda and Wada 1933; Yoshida et al. 1996; Yoshikura and Hamaguchi 1969) have identified only one to three different anthocyanins [cyanidin-3-O-β-D-glucoside (C3G), delphinidin-3-O-β-D-glucoside (D3G) and petunidin-3-O-β-D-glucoside (Pt3G)], while Lee et al. (2009) recently reported nine different anthocyanins, which included three major anthocyanins and several other anthocyanins. These studies show substantial differences in anthocyanin contents and compositions among varieties. Therefore, further investigation including minor pigments is required to produce an anthocyanin profile of the black soybean from different varieties.
The present study was aimed at characterizing anthocyanin composition in different varieties of black soybean to identify an anthocyanin-rich variety for the future industry utilization or development of functional foods. The identity of anthocyanin compounds was based on the data obtained from HPLC and liquid chromatography-mass spectrometry (LC-MS).
Materials and methods
Sample preparation
Two different varieties of black soybean, Tawonkong (TW) and Geomjeongkong-2 (G2), were selected for use in this study. The black soybeans were cultivated and grown for experimental purposes at the experimental field of Rural Development Administration, Suwon, South Korea, during the summer of 2004. After harvest, the seeds were cleaned in distilled water to remove extraneous matters and subsequently dried at 105 °C for 2 h. TW and G2 varieties showed moisture content of 7.1 ± 0.5% and 6.6 ± 0.5%, respectively. The dried seeds were stored at 4 °C until they were used.
Chemicals
The pure standard materials of C3G, D3G and Pt3G were the generous gift from the Department of Herbal Medicine Resources, Kangwon National University, Republic of Korea. Analytical grade hexane, hydrochloric acid (HCl), amyl alcohol, HPLC grade methanol, formic acid, acetonitrile (ACN) and acetic acid were purchased from Sigma Chemical Co., (St. Louis, MO, USA). Glucose, fructose, galactose and sucrose were used for the standards of sugar analyses and purchased from Fruka analytical (Buchs, Switzerland).
Anthocyanin extraction
For anthocyanin extraction, seed coats of the two black soybean varieties were manually removed with tweezers and freeze-dried. Thirty grams of each sample were grounded with pestles and mortars after frozen with liquid nitrogen. Anthocyanins were extracted according to the method described by Abdel-Aal et al. (2006) with slight modifications after grounded samples were soaked in 30 mL hexane for 24 h to remove fats and oils. Three grams of the ground materials were extracted twice by mixing with 30 mL of methanol acidified with 0.1 M HCl (85:15, v/v) and shaking on a shaker at 4 °C for 24 h. The crude extracts were filtered with Whatman No. 1 paper.
Analysis of anthocyanins and LC-MS confirmation of anthocyanin identity
Anthocyanins in the partially purified extracts were separated and quantified with reverse phase HPLC equipped with an Xterra MS C18 column (3.5 μm, 4.6 × 250 mm), Sykamm UV–vis detector (Sykamm, Erensing, Germany). The column was eluted with a mobile phase consisting of H2O:methanol:formic acid (75:20:5, v/v/v) with flow rate of 0.5 mL/min. The separated anthocyanins were detected and measured at 530 nm, and the identity of anthocyanins was based on the congruence of retention times and UV–vis spectra with those of pure authentic standards. The anthocyanins were further identified with LC-MS after separation of each peak carried out by semi-preparative HPLC with GROM-SIL 120 ODS-5 ST 0.5 μm (150 × 20 mm) column and Younglin M720 UV–vis detector (Younglin, Seoul, Korea) at 530 nm. The eluent of preparative column was same as the analytical HPLC system with flow rate of 10 mL/min, and injection volume of the sample was 500 μL. Each peak sample was collected separately and concentrated approximately 5-fold by freeze-drying.
Confirmation of identity of each peak was carried out by LC-mass spectrometry (Shimadzu, Tokyo, Japan) employing electrospray ionization (ESI) and operating in a single quadrupole mode. The instrument was scanned over the m/z range of 50 to 1000 in the ESI positive ion mode. The LC-MS was eluted with ACN and 2.5% acetic acid (90:10, v/v) with flow rate of 0.4 mL/min, and the injection mode was FIA (Flow injection analysis).
Identification of sugar moiety of the unknown peak
After the aglycone of the unknown anthocyanin was confirmed with LC-MS, the sugar moiety was confirmed by HPLC chromatography after acid hydrolysis. Acid hydrolysis was carried out according to the standard procedure (Schou 1927). Briefly, 10 mL of 2 M HCl were added to 3 mL of anthocyanin solution. The hydrolysis was carried out for 1 h at 100 °C in a water bath. The hydrolysate was extracted with amyl alcohol and used for aglycone analysis. The aqueous phase after extraction was concentrated and used for sugar analysis.
The HPLC condition to identify sugar moiety was equipped with a Prevail Carbohydrate ES (GRACE, 5 μm, 4.6 × 250 mm) and ELSD 3300 detector (Alltech, Deerfield, IL, USA). The column was eluted with a mobile phase consisting of 75% ACN with flow rate of 0.7 mL/min. The identity of sugar moiety was based on the congruence of retention time (RT) with a pure authentic standard.
Statistical analysis
The data were reported as means of three replicates with standard deviation. The differences of quantities of anthocyanins between two varieties were analyzed with Student’s t-test using SPSS (14.0).
Results and discussion
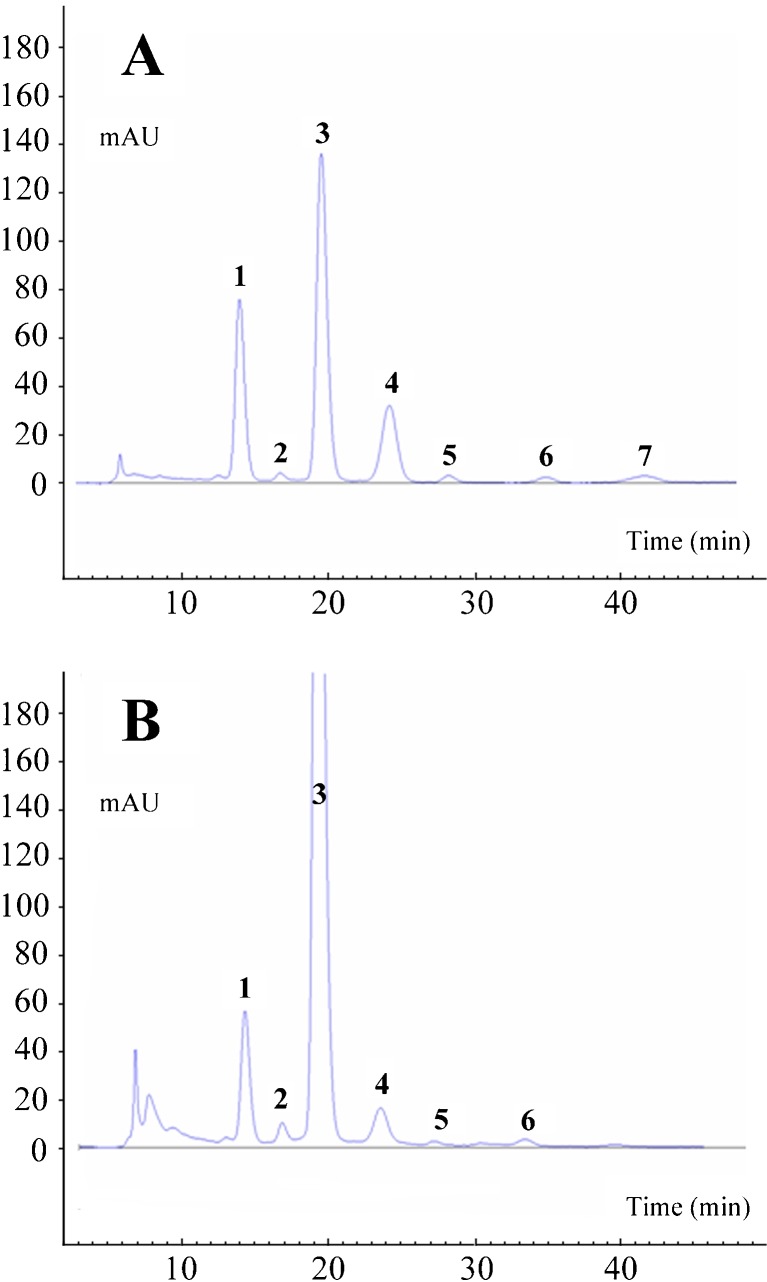
The HPLC chromatogram of acidic methanol extract obtained from TW showed seven distinct peaks while G2 showed six peaks (Fig. 1 and Table 1). Identification was conducted on the basis of the RTs of components separated by HPLC. Table 2 shows the identification methods and characterized anthocyanins
Fig. 1.
HPLC chromatograms of anthocyanin pigments separated from Tawonkong (a) and Geomjeongkong-2 (b) varieties of black soybean (Glycine max (L.) Merr.) by Xterra MS C18 column
Table 1.
Results of HPLC analysis from standards and black bean varieties
| Peak numbera | Compound Name (Standards) | mg/g Anthocyanin (peak area mAU) | |
|---|---|---|---|
| TW | G2 | ||
| 1 | D3G | 3.8 ± 0.03 (504.1 ± 3.4***) | 2.6 ± 0.02 (343.7 ± 2.5) |
| 2 | unknown | − (22.0 ± 0.8) | − (44.4 ± 1.2***) |
| 3 | C3G | 4.6 ± 0.05 (1073.6 ± 12.1) | 15.1 ± 0.06 (3503.7 ± 16.8***) |
| 4 | Pt3G | 4.7 ± 0.02 (361.7 ± 1.5***) | 2.0 ± 0.01 (155.0 ± 1.0) |
| 5 | unknown | − (26.5 ± 1.2***) | − (12.7 ± 0.7) |
| 6 | unknown | − (30.2 ± 1.7) | − (27.6 ± 1.3) |
| 7 | unknown | − (49.0 ± 1.0) | |
| Total | 13.1 ± 0.08 (2067.1 ± 19.0) | 19.7 ± 0.09 (4087.1 ± 20.5***) | |
TW Tawonkong, G2 Geomjeongkong-2, D3G delphinidin-3-O-β-D-glucoside, C3G cyanidin-3-O-β-D-glucoside, Pt3G petunidin-3-O-β-D-glucoside
aPeak number according to the HPLC chromatogram shown in Fig. 1
***: p < 0.001 by Student’s t-test
Table 2.
Identification of unknown peaks of HPLC by LC/MS in black soybean varieties
| Peak numbera | Identified anthocyanin | Identification methods | Major ions (m/z) |
|---|---|---|---|
| 1 | D3G | LC/MS and HPLC standard | 465, 303 |
| 2 | C3Gal | LC/MS | 449, 287 |
| 3 | C3G | LC/MS and HPLC standard | 449, 287 |
| 4 | Pt3G | LC/MS and HPLC standard | 479, 317 |
| 5 | Pg3G | LC/MS | 433, 271 |
| 6 | Pg-MalG | LC/MS | 519, 271 |
| 7 | Cyanidin | LC/MS | 287 |
D3G delphinidin-3-O-β-D-glucoside, C3Gal cyanidin-3-O-galactoside, C3G cyanidin-3-O-β-D-glucoside, Pt3G petunidin-3-O-β-D-glucoside, Pg3G pelargonidin-3-O-glucoside, Pg-MalG pelargonidin-3-O-(6″- malonyl glucoside)
aPeak number according to the HPLC chromatogram shown in Fig. 1
The major peaks (1, 3 and 4) have been identified as D3G, C3G and Pt3G, respectively, by the congruence of the RTs with authentic standards and also confirmed by fragmentation patterns arising from MS created by ESI in the positive mode. A typical ESI positive MS shows two ions. One is the protonated molecular ion designated as [MH]+ and the other is a fragmented ion designated as [MH without X]+ arising from loss of the saccharide moiety (X). However, one observes a true molecular ion [M]+ and a fragmented ion [M without X]+ because the anthocyanins have a natural positive charge. The MS of the compound showing ions at m/z 303 and 465 in peak 1 suggests that the aglycone is delphinidin (m/z 303), and the difference of m/z 162 suggests a hexose. Consequently, the ions at m/z 287 in peak 3 and m/z 317 in peak 4 suggest the aglycones are cyanidin and petunidin. Peaks 3 and 4 also showed the differences of m/z 162 between two major MS ions in each peak, suggesting a hexose. Unfortunately, ESI-MS do not distinguish the various hexoses. We could confirm the hexose as glucose in these cases by comparisons of RTs with authentic standards from HPLC. For G2 variety, these three major anthocyanins represented about 98% (Table 1) of the total peak area with three unknown peaks (2, 5 and 6). However, for TW variety, other than three major anthocyanins (peaks 1, 3 and 4) which represented about 93% of the total peak area (Table 1), four unidentified minor peaks (2, 5, 6 and 7) were detected. The three major anthocyanins in black soybean seed coats, D3G, C3G and Pt3G, were previously identified by Kuroda and Wada (1933), Yoshikura and Hamaguchi (1969), Choung et al. (2001) and Lee et al. (2009).
The MS analysis of peak 2 showed m/z 287 and m/z 449, which are exactly same MS results as peak 3, suggesting that they are isomers containing cyanidin (m/z 287) as an aglycone. The two isomers could be either structural or positional isomers; otherwise, they just have different hexoses. ESI-MS cannot distinguish the point of attachment to the aglycone as well as various hexoses. Therefore, we conducted an identification of the hexose from peak 2 after acid hydrolysis. The typical structures of anthocyanins are composed of a central flavonoid ring structure (aglycone) and a saccharide moiety connected to aglycone. The typical anthocyanins can be cleaved to aglycone and saccharide moieties by acid hydrolysis (Strack and Wray 1989). The saccharide moiety liberated by acid hydrolysis of the purified anthocyanin of peak 2 was identified as galactose by the results of HPLC analysis. Therefore, anthocyanin of peak 2 is cyanidin-3-galactoside (C3Gal). This result is in agreement with other study (Lee et al. 2009) conducted with another variety (Cheongja 3) of black soybean cultivar (Glycine max (L.) Merr.) grown in Korea.
Peaks 5, 6 and 7 of TW variety showed major MS ions of m/z 271-m/z 433, m/z 271-m/z 519 and m/z 287, respectively. The ions at m/z 271 in peaks 5 and 6, and m/z 287 in peak 7 suggest the aglycones are pelargonidin and cyanidin, respectively. The difference between two ions of peak 5 is m/z 162 suggesting a hexose. Meanwhile, the difference between two ions of peak 6 is m/z 248 suggesting malonylglucoside. MS peak m/z 248 is tentatively accepted as 6″-malonylglucoside (Abdel-Aal et al. 2006; Moreno et al. 2005), which means malonyl is linked to sugar unit. MS results of m/z 162 is hexose and m/z 86 (m/z 248 – m/z 162) indicates malonyl. Therefore, we presumptively conclude peaks 5 and 6 as pelargonidin-3-glucoside (Pg3G) and pelargonidin malonylglucoside (Pg-MalG), respectively. Peak 7 showed a molecular ion at m/z 287 suggesting a free anthocyanin aglycone of cyanidin. The first appearance of free anthocyanin aglycone of cyanidin was reported by Lee et al. (2009) as the case of C3Gal. Naturally occurring free anthocyanin aglycones are reported to be unstable due to limited solubility in water and they are rapidly destroyed by alkalis and lights. Therefore, they rarely appear in nature (Dao et al. 1998). Macz-Pop et al. (2006) first reported natural occurrence of free anthocyanin aglycones of cyanidin, peonidin and pelargonidin in Phaseolus vulgaris. Lee et al. (2009) reported the appearance of free cyanidin in Korean black soybean cultiva Glycine max, but they used other variety (Cheongja 3) different from the varieties used in this study. In the present study, only TW showed peak 7 of free cyanidin.
C3G was the most abundant anthocyanin of the black soybean varieties investigated. D3G came second, while Pt3G was the third major anthocyanin of the Korean black soybean varieties used. Choung et al. (2001) found one or two major anthocyanin(s) in two varieties and three anthocyanins in six varieties of black soybean in which C3G was reported to be the most abundant (or only) anthocyanin (0.94 – 15.98 mg/g), whereas D3G (0.64 – 3.71 mg/g) is the second. In the present study, the amounts of anthocyanin compounds quantified from the pigment with authentic standards available were 13.1 and 19.7 mg/g for TW and G2, respectively. G2 showed significantly higher amount of total anthocyanins (p < 0.001), but D3G, Pt3G and Pg3G were higher in TW than in G2 (p < 0.001). The amount of anthocyanin in the black soybean variety of G2 in the present study is higher than those in nine other black soybean varieties cultivated in Korea, but lower than one variety of YJ 100–1 (Choung et al. 2001). Black soybeans analyzed in the present study contain more diverse kinds of anthocyanins than rice varieties (Abdel-Aal et al. 2006; Kim et al. 2008), corn, wheat or barley varieties (Abdel-Aal et al. 2006), kidney bean varieties (Choung et al. 2003) and other Korean black soybean varieties (Choung et al. 2001). In addition, slightly different chromatograms of 9 anthocyanins were reported recently in Cheongja 3 variety of black soybean (Lee et al. 2009) grown in Korea. These results suggest that black soybean can be an abundant source of anthocyanin.
Conclusions
This study showed that Korean black soybeans are abundant with various kinds of anthocyanins. Out of the seven anthocyanin compounds observed, D3G, C3G and Pt3G were confirmed by LC/MS and the congruence of RTs of pure authentic standards. Pg3G, Pg-MalG and cyanidin were presumptively characterized by LC/MS. Aglycone of C3Gal was identified by MS and saccharide part was confirmed by HPLC after acid analysis. C3G was the most abundant anthycyanin. And D3G came second, while Pt3G was the third major anthocyanin of the Korean black soybean varieties investigated.
Acknowledgment
This study was supported by the research grant from Soonchunhyang University.
References
- Abdel-Aal E-SM, Young JC, Rabalski I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J Agric Food Chem. 2006;54:4696–4704. doi: 10.1021/jf0606609. [DOI] [PubMed] [Google Scholar]
- Bettini V, Fiori A, Martino R, Mayellaro R, Ton P. Study of the mechanism whereby anthocyanosides potentiate the effect of catecholamines on coronary vessels. Fitoterapia. 1985;2:67–72. [Google Scholar]
- Bridle P, Timberlake CF. Anthocyanins as natural foods colours-selected aspects. Food Chem. 1997;58:103–109. doi: 10.1016/S0308-8146(96)00222-1. [DOI] [Google Scholar]
- Choung MG, Baek IY, Kang ST, Han WY, Shin DC, Moon HP, Kang KW. Isolation and determination of anthocyanins in seed coats of black soybean (Glycin max (L.) Merr.) J Agric Food Chem. 2001;49:5848–5851. doi: 10.1021/jf010550w. [DOI] [PubMed] [Google Scholar]
- Choung MG, Choi BR, An YN, Chu YH, Cho YS. Anthocyanin profile of Korean cultivated kidney bean. J Agric Food Chem. 2003;51:7040–7043. doi: 10.1021/jf0304021. [DOI] [PubMed] [Google Scholar]
- Dao LT, Takeota GR, Edwards RH, JdeJ B. Improved method for the stabilization of anthocyanins. J Agric Food Chem. 1998;46:3564–3569. doi: 10.1021/jf980359v. [DOI] [Google Scholar]
- Francis FJ. Anthocyanins and betalains composition: composition and applications. Cereal Foods World. 2000;45:208–213. [Google Scholar]
- Hyun JW, Chung HS. Cyanidin and malvidin from Oryza sativa cv. Heugjinjubyeo mediate cytotoxicity against human monocytic leukemia cells by arrest of G2/M phase and induction of apoptosis. J Agric Food Chem. 2004;52:2213–2217. doi: 10.1021/jf030370h. [DOI] [PubMed] [Google Scholar]
- Kamei H, Kojima T, Hasegawa M, Koide T, Umeda T, Yukawa T, Terabe K. Suppression of tumor cell growth by anthocyanins in vitro. Cancer Invest. 1995;13:590–594. doi: 10.3109/07357909509024927. [DOI] [PubMed] [Google Scholar]
- Kim MK, Kim H, Koh K, Kim HS, Lee YS, Kim YH. Identification and quantification of anthocyanin pigments in colored rice. Nutr Res Prac. 2008;2:46–49. doi: 10.4162/nrp.2008.2.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda C, Wada M. The colouring matter of kuno-mame. Proc Imper Acad. 1933;9:17. [Google Scholar]
- Lee JH, Kang NS, Shin SO, Shin SH, Lim SG, Suh DY, Baek IY, Park KY, Ha YJ. Characterization of anthocyanins in the black soybean (Glycine max L.) by HPLC-DAD-ESI/MS analysis. Food Chem. 2009;112:226–231. doi: 10.1016/j.foodchem.2008.05.056. [DOI] [Google Scholar]
- Macz-Pop GA, Rivas-Gozalo JC, Perez-Alonso JJ, Gonzalez-Paramas AM. Natural occurrence of free anthocyanin aglycones in beans (Phaseolus vulgaris L.) Food Chem. 2006;94:448–456. doi: 10.1016/j.foodchem.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Mazza G, Gao L. Blue and purple grains. In: Abdel-Aal E-SM, Peter PJ, editors. Specialty Grains for Food and Feed. St. Paul: American Association of Cereal Chemists; 2005. pp. 45–67. [Google Scholar]
- Moreno YS, Sanchez GS, Hernandez DR, Lobato NR. Characterization of anthocyanin extracts from maize kernels. J Chromatogr Sci. 2005;43:483–487. doi: 10.1093/chromsci/43.9.483. [DOI] [PubMed] [Google Scholar]
- Nam SH, Choi SP, Kang MY, Koh HJ, Kozukue N, Friedman M. Antioxidative activities of bran from twenty one pigmented rice cultivars. Food Chem. 2006;94:613–620. doi: 10.1016/j.foodchem.2004.12.010. [DOI] [Google Scholar]
- Philpott M, Gould KS, Lim C, Ferguson LR. In situ and in vitro antioxidant activity of sweetpotato anthocyanins. J Agric Food Chem. 2006;54:1710–1715. doi: 10.1021/jf060666y. [DOI] [PubMed] [Google Scholar]
- Satue-Gracia M, Heinonen IM, Frankel EN. Anthocyanins as antioxidants on human low-density lipoprotein and lecithinliposome system. J Agric Food Chem. 1997;45:3362–3367. doi: 10.1021/jf970234a. [DOI] [Google Scholar]
- Schou SA. Light absorption of several anthocyanins. Helv Chim Acta. 1927;10:907–915. doi: 10.1002/hlca.192701001114. [DOI] [Google Scholar]
- Stocker R, O’Halloran RO. Dealcoholized red wine decreases atherosclerosis in apolipoprotein E gene-deficient mice independently of inhibition of lipid peroxidation in the artery wall. Am J Clin Nutr. 2004;79:123–130. doi: 10.1093/ajcn/79.1.123. [DOI] [PubMed] [Google Scholar]
- Strack D, Wray V. Methods in Plant Biochemistry. London: Academic; 1989. [Google Scholar]
- Todd JJ, Vodkin LO. Pigmented soybean (Glycine max) seed coats accumulate proanthocyanidins during development. Plant Physiol. 1993;102:663–670. doi: 10.1104/pp.102.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda T, Horio F, Osawa T. Cyanidin 3-O-β-glucoside suppresses nitric oxide production during a zymosan treatment in rats. J Nutr Sci Vitaminol. 2002;48:305–310. doi: 10.3177/jnsv.48.305. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. Dietary cyanidin 3-O-β-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia. J Nutr. 2003;133:2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- Waterhouse AL (1995) Wine and heart disease. Chem Ind 338–341
- Yoshida K, Sato Y, Okuno R, Kameda K, Isobe M, Kondo T. Structural analysis and measurement of anthocyanin from colored seed coats of Vigna, Phaseolus, and Glycine legumes. Biosci Biotechnol Biochem. 1996;60:589–593. doi: 10.1271/bbb.60.589. [DOI] [Google Scholar]
- Yoshikura K, Hamaguchi Y. Anthocyanins of the black soybean. Eiyo To Shokuryo. 1969;22:367. doi: 10.4327/jsnfs1949.22.367. [DOI] [Google Scholar]
- Zhao C, Giusti MM, Malik M, Moyer MP, Magnuson BA. Effects of commercial anthocyanin-rich extracts on colonic cancer and nontumorigenic colonic cell growth. J Agric Food Chem. 2004;52:6122–6128. doi: 10.1021/jf049517a. [DOI] [PubMed] [Google Scholar]