Abstract
The effects of zero-trans chemically interesterified (in-es) and non-interesterified (non-in-es) cottonseed (CO), hazelnut (HO) and olive oil (OO) and their blends (25, 50 and 75%) with palm oil (PO) were studied in the production of cookies. All the experimental shortenings had zero-trans fatty acids (TFA) while the shortening contained 14.20% TFA. Incorporation of CO in PO considerably increased the linoleic acid content whereas the raising of HO and OO ratio in the blend increased the oleic acid content. Zero-TFA and lower saturated /unsaturated fatty acid ratio (SFA/UFA) of some of the experimental shortenings indicated an important in nutritional properties of cookies produced from these experimental shortenings. Cookies with in-es shortenings showed significantly higher (p < 0.05) spread ratios and L Hunter color than their non-in-es shortenings added counterparts. It can be concluded that chemical interesterification is a promising method to produce cookie shortenings with zero-TFA.
Keywords: Interesterified fat, Hydrogenated fat, Trans fatty acid, Shortening, Cookie
Introduction
Fats are probably the most important ingredients used in cookie formulation and textural characteristics of cookies are mostly provided by their high fat content (Zoulias et al. 2002; Manley 2000). Several studies have reported a relationship between trans fatty acids (TFA) and risk of cardiovascular disease (Block et al. 2008; Jakobsen et al. 2006; Willett 2006), breast cancer, shortening of pregnancy period, risks of preeclampsia, disorders of nervous system and vision in infants, colon cancer, diabetes, obesity and allergy (Dhaka et al.2011). Also Micha and Mozaffarian stated that a comprehensive strategy to eliminate the use of industrial TFA in both developed and developing countries, including education, food labeling, and policy and legislative initiatives, would likely prevent tens of thousands of CHD events worldwide each year (Micha and Mozaffarian 2008 ). TFA are considered to be harmful more than saturated fatty acid (SFA) (Stender et al.2006; Ascherio and Willett 1997). In part due to these concerns WHO and FAO recommend that intake of TFA per day should not exceed 4% in food and consequently, some countries legally limited TFA in foods (Aini and Miskandar 2007; Stender et al.2006). This limitation has leaded the biscuit processors to investigate alternative methods to produce products with lower TFA to respond to the growing demands by the health conscious consumer for healthier products.
The adverse effects of hydrogenation; the formation of saturated and TFA (Tavella et al. 2000; Ascherio and Willett 1997) make the use of interesterified (in-es) oils an attractive alternative. Interesterification which mainly based on rearranging the distribution of fatty acids on the glycerol backbone without changing their chemical composition have been used to improve the cold spreadability of butterfat-canola oil blends (Rousseau and Marangoni 1999) and the production of margarine (Petrauskaite et al.1998), cheese (Javidipour and Tuncturk 2007), shortening (Öztürk et al.2008; Rodriguez et al.2001), frankfurter (Vural et al.2004; Vural and Javidipour 2002) and Turkish-type salami (Javidipour et al.2005; Javidipour and Vural 2002). Dogan et al. (2007) successfully made cakes with zero-TFA by using in-es cottonseed (CO) and palm oil (PO) blends without any considerably adverse change in sensorial properties. However, Park et al. (1983) found that the loss of tocopherols accelerated the autoxidation of randomized soybean oil, Basturk et al. (2007) indicated that based on peroxide and anisidine values and reaction rate constants the oxidative stability of in-es CO, PO and soybean oils were higher than their non-interesterified (non-in-es) counterparts. The aim of this study was to produce cookies high in unsaturated fatty acids with zero-TFA content.
Materials and methods
Materials
Refined, bleached and deodorized palm oil (PO) were purchased from Etsun Inc (Adana, Turkey), CO, hazelnut (HO), olive oil (OO) and hydrogenated shortening (HS) were purchased from local supermarkets in Ankara, Turkey. The commercial soft wheat flour (Ankara Flour Inc., Ankara, Turkey) used in this study consisted of 10.6% protein (Nx5.7, db), 0.61% ash and 32% wet gluten. Other ingredients were supplied from Ülker Group (Ankara, Turkey). Only reagent-grade chemicals were used.
Methods
Moisture, ash, protein and wet gluten contents of the flour sample were determined according to AACC Methods (AACC 2000). Cookies were produced according to AACC Method No: 10.54 Baking Quality of Cookie Flour-Micro Wire-Cut Formulation (AACC 2000). Cookies were made of fine granulated sucrose (25.6 g), brownulated granulated sucrose (8.0 g), non-fat dry milk (0.8 g), salt (1.0 g), sodium bicarbonate (0.8 g), all purpose shortening (32.0 g), high fructose corn syrup (1.2 g), ammonium bicarbonate (0.4 g), flour ( 80.0 g, 13% moisture basis) and deionized water (variable amount) at 21 ± 1°C. Hunter color values (L, a, b) of the cookies were determined with a Gardner Colorview Spectrophotometer (U.S.A). Solid Fat Content (SFC) was measured by pulsed NMR using Maran SFC (Resonance Instrumental Ltd., Witney, UK) according to AOCS Official Methods Cd 16b-93. Measurements were carried out at 0, 10, 20, 30, 40°C and a constant resonance frequency of 20 MHz (AOCS 1989). In-es and non-in-es blends of CO, HO and OO with PO were prepared at different ratios (100, 75, 50, 25, 0%) and used in cookie formulations.
Interesterification
Interesterification reactions were done in a 1-L suction flask, using a hot plate-stirrer. Oil samples were dried by heating under vacuum for 20 min at 95°C to remove traces of moisture from the oil. NaOCH3 (0.5%) was added as a catalyst and the mixture was stirred at 80–90°C. After heating for a few seconds, the color of the mixture became brownish due to formation of active catalyst and the triacylglycerols. After 30-min reaction, the catalyst was inactivated by adding 2% of citric acid and stirring for 15 min at 90°C. The reaction mixture was washed three times with warm water (55°C, 250 ml) to remove the citric acid and sodium methoxide. Filter aid (2%) (Celite analytical filter aid 300 G, Fisher Scientific Co., Pittsburgh, PA) was added, mixed well, and the mixture was filtered through a Buchner filter (Haldenwanger, Berlin). The filtrate was dried under vacuum in a rotary evaporator. Residual water was removed with excess anhydrous sodium sulphate followed by filtration through a Whatman No. 2 filter [Rousseau and Marangoni 1999]. The in-es oils were kept at 4°C until used.
Fatty acid composition
For preparation of fatty acids methyl esters (FAME), 0.4 g fat was dissolved in 4 mL of isooctane and methylated in 0.2 mL 2 M methanolic KOH. Analysis of FAME was performed on a Hewlett Packard 6890 series gas-chromatograph with split injection 1:50, equipped with flame-ionization detector (FID) and a 30 m fused silica capillary column (ID = 0.25 mm) coated with 0.25 μm of CP-wax 52 CB (Chromopack, Supelco, Bellefonte, PA). The working temperatures of the injector, column and detector were 250, 190 and 275 °C, respectively. Nitrogen was used as the carrier gas. Flow rates of hydrogen and air were selected to attain the maximum FID signal response. Samples were injected into the column inlet using a Hewlett-Packard 7673 automatic injector. FAMEs were identified by comparison of their retention time and equivalent chain length with respect to standard FAMEs (47885-U: Supelco, Bellefonte, PA). FAMEs from samples were quantified according to their percentage area (AOAC 1990).
Statistical evaluation
The experimental design was carried out with four replication. Data were analyzed for variance using the TARIST statistical package. When significant differences were found (p ≤ 0.01), the Least Significant Difference (LSD) test was used to determine the differences among means.
Results and discussion
Fatty acids methyl esters
Interesterification does not affect the degree of unsaturation and does not cause isomerization in oils and fats (Basturk et al.2007); therefore, only the fatty acid compositions of the in-es experimental shortenings are given in Table 1. As expected HS contained TFA at high level (14.38%) because of conversion of some double bonds from -cis to -trans isomer. The Food and Agriculture Organization (FAO) and World Health Organization (WHO) recommended that intake of TFA should not exceed 4% and TFA content of food should be reduced below this level because of their adverse effects on health (WHO 1993). Due to these concerns, some European countries limited TFA in foods to a maximum of 2% of fat content (Stender et al.2006). Increasing the ratio of CO in PO considerably increased the linoleic acid content and decreased the SFA/UFA ratio of the blend. Incorporation of HO and OO in PO decreased the palmitic acid and increased the oleic acid contents of the blends. SFA/UFA ratios of blends decreased as the ratios of HO and OO increased in their blends with PO. Olive oil and HO had oleic acid as the most abundant fatty acid. Olive oil is known for its high oleic acid content however, HO shows higher oleic acid content than olive oil. Palm oil gave a typical fatty acid composition high in palmitic acid (42.08%) and oleic acid as the predominant unsaturated fatty acid. Linoleic acid was the most abundant fatty acid (56.56%) in CO. Reduction in SFA/UFA ratio due to incorporation of CO, OO and HO in PO indicated an improvement in nutritional content of product. HS with lower SFA/UFA ratio seems to be better than experimental shortenings used in this study from health point of view because lower SFA/UFA can be an indication of beneficial nutritional content (Tsanev et al.1998). However, the lower SFA/UFA ratio of HS is mainly due to accounting of TFAs as desired unsaturated fatty acids. TFAs are considered to be harmful more than SFAs (Stender et al. 2006). Therefore if TFAs were taken into account as SFA, the SFA/UFA ratio of HS would be higher than those of the most of the experimental shortenings used in this study.
Table 1.
Fatty acids contents of interesterified fats
| Sample % | Fatty acids % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 12:0 | 14:0 | 16:0 | 16:1 | 18:0 | 18:1c | 18:1t | 18:2c | 18:2t | SFA/UFA | |
| HS | 0.29 | 0.71 | 27.96 | 0.14 | 5.81 | 24.92 | 14.18 | 23.92 | 0.20 | 0.55 |
| PO | 0.22 | 0.92 | 42.08 | - | 7.28 | 43.08 | 6.42 | 1.02 | ||
| 25CO + 75PO | 0.18 | 0.90 | 37.31 | 0.12 | 5.97 | 36.29 | 19.20 | 0.80 | ||
| 50CO + 50PO | 0.19 | 0.85 | 32.50 | 0.23 | 4.86 | 29.82 | 31.54 | 0.62 | ||
| 75CO +25PO | 0.19 | 0.81 | 27.68 | 0.38 | 3.48 | 23.57 | 43.87 | 0.47 | ||
| CO | 0.1 | 0.69 | 23.74 | 0.49 | 0.98 | 17.31 | 56.56 | 0.34 | ||
| 25OO +75PO | 0.18 | 0.72 | 33.77 | - | 6.15 | 51.46 | 7.17 | 0.70 | ||
| 50OO + 50PO | 0.12 | 0.47 | 26.28 | - | 4.97 | 60.42 | 7.72 | 0.47 | ||
| 75OO + 25PO | 0.09 | 0.24 | 18.95 | - | 3.75 | 68.28 | 8.26 | 0.30 | ||
| OO | - | - | 11.67 | 0.68 | 2.46 | 76.03 | 8.83 | 0.17 | ||
| 25HO + 75PO | 0.20 | 0.74 | 32.21 | - | 5.94 | 53.47 | 7.43 | 0.64 | ||
| 50HO + 50PO | 0.13 | 0.48 | 23.09 | - | 4.51 | 63.44 | 8.28 | 0.39 | ||
| 75HO +25PO | - | - | 14.17 | - | 3.46 | 73.42 | 8.95 | 0.21 | ||
| HO | - | - | 5.46 | - | 2.35 | 82.46 | 9.73 | 0.08 | ||
HS Hydrogenated shortening, PO Palm oil; CO Cottonseed oil; OO Olive oil;
HO Hazelnut oil; UFA Unsaturated fatty acids; SFA Saturated fatty acids
Solid fat content
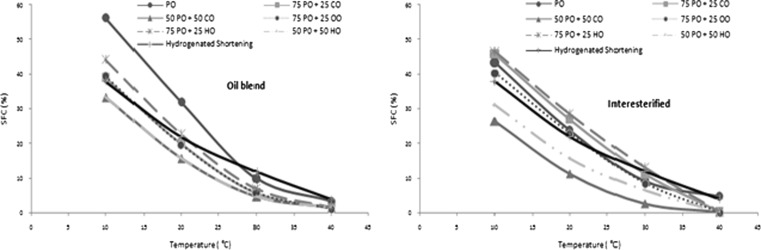
In all of the non-in-es and in-es samples SFC decreased as the ratio of CO, HO and OO increased in the blend. In general in-es samples showed lower SFC at measuring temperatures than their non-in-es counterparts (except CO). Some experimental shortenings showed comparable SFC profile with HS in spite of their modified fatty acid composition; high in unsaturated fatty acids with zero-TFA content (Fig. 1). Generally interesterification decreases the SFC of oils and this cause a flat SFC profile which has typically a wide plastic range whereas oil with steeper SFC profile typically has a narrow plastic range (Dogan et al. 2007). Oils and fats show different SFC and melting characteristics after interesterification due to their fatty acid composition and triacylglyceride arrangements. Location of SFA and UFA on the central (sn-2) or the terminal (sn-3) carbon atoms of glycerol interfere with the packing of triacylglyceride and affect crystal formation, consequently, their melting point. By interesterification the palmitic acid is randomly distributed on the triacylglyceride, therefore 2-oleoyl 1,3-dipalmitin (POP) in palm oil can be converted to β′ tending 2-stearoyldipalmitin (PSP). β′ crystals incorporate relatively large amounts of liquid oil in the crystal network due to their relatively small size and this leads to the production of smooth, continuous and homogenous products (DeMan and DeMan 1994). Nawar (1985) stated that β′ crystals that have relatively very small size, by trapping and holding air during creaming process contribute to good creaming properties. Blends of PO, palm olein and palm stearin with other oils and fats provide good plasticity. This is because more β′ crystals are formed in the in-es blend while β crystals are predominant in direct blends. In choosing oil sources for base stocks, necessity of having β′ crystals in the final product to promote optimum creaming should be taking into account (Stender et al. 2006; Baldwin et al. 1972). The lower SFC of in-es oils was well reflected to spread ratios of cookies.
Fig. 1.
SFC comparison of some shortening samples before and after interesterification with hydrogenated shortening. PO: Palm oil, CO: Cotton oil, OO: Oil oil, HO: Hazelnut oil
Spread ratio values
The effects of non-in-es and in-es CO, HO and OO and their blends with PO on spread ratios of cookies are presented in Table 2. Incorporation of non-in-es and in-es CO, HO and OO significantly (p < 0.05) affect the spread ratios of cookies. Among the 50% PO incorporated batches, in-es HO-PO and in-es OO-PO and non-in-es CO-PO contained shortenings caused higher spread ratios than the others. In 75% PO added batches, in-es CO:PO and non-in-es OO:PO added cookies showed significantly (p < 0.05) higher spread ratios than the other groups.
Table 2.
The effects of oil types and their ratios in palm oil blends on spread ratios
| Ratio | Interesterified | Non-Interesterified | ||||
|---|---|---|---|---|---|---|
| CO | HO | OO | CO | HO | OO | |
| 100 | 7.0 a | 7.1 a | 7.2 a | 8.0 a | 7.7 a | 6.9 b |
| 75 | 8.6 a | 7.4 b | 7.4 b | 7.1 b | 7.6 a | 7.2 b |
| 50 | 7.1 b | 7.8 a | 7.8 a | 8.3 a | 7.4 b | 7.0 b |
| 25 | 7.8 a | 6.8 c | 7.3 b | 7.5 b | 7.4 b | 8.0 a |
| LSD | 0.42 | 0.38 | ||||
Mean values of four determination within each column with different lower case letter (a–c) are significantly different (p < 0.05)
The results of two-way ANOVA showed that oil type, method, ratio, interaction of oil type x ratio, ratio x method, oil type x ratio x method (interesterfication or direct blend) significantly affected (p < 0.05) the spread ratios of cookies with the exception of the interaction of oil type x method. When the individual effects of oil type on spread ratio was examined, it was found that CO incorporated cookies gave significantly higher (p < 0.05) spread ratios than HO and OO added samples. A high palmitic acid content of CO (23.74%) probably makes it suitable for the interesterification. Wiedermann (1978) indicated that vegetable oils containing 10% of palmitic acid tend to crystallise in β form, whereas those having 20% of palmitic acid had the tendency to crystallize in β′ form. PO also crystallize in β′ form because of diversity of its fatty acids content. Therefore incorporation of PO in shortening formulation prevents or delay crystallization into undesired β form (Yap et al.1989; Ward 1988). In this study it was determined that cookies with interesterified fat gave significantly higher (p < 0.05) spread ratios than those with non interesterified fat.
Color values
The effects of in-es and non-in-es CO, HO and OO incorporation on the L, a and b values of cookies, regardless of their ratio in PO are presented in Table 3. The L value corresponds to the lightness. The +a value represents redness and the +b value represents yellowness. The L values of cookies with in-es HO were significantly higher (p < 0.05) than those with in-es CO and in-es OO. Cookies with in-es CO, HO and OO showed lower a and b values than their non-in-es counterparts. Generally, cookies with in-es shortenings showed higher L and lower a and b color values than their non-in-es counterparts (Table 4). Regardless of oil type, 25% CO, HO and OO containing PO blends gave the highest L values (Table 5). Higher L and lower a and b values indicated lighter surface color. Possible cause of lighter color of in-es shortenings added cookies is the bleaching and washing step of the oils after interesterification. Dogan et al. (2007) reported higher L values for crust and crumb colors of cakes prepared with in-es shortenings. The results of two-way ANOVA showed that oil type, method, ratio, interaction of oil type x ratio, oil type x method, ratio x method, oil type x ratio x method significantly affected (p < 0.05) the L, a and b color values of cookies however the oil type did not affect b color value.
Table 3.
The effect of vegetable oil type on color values
| Oils | Interesterified | Non-interesterified | ||||
|---|---|---|---|---|---|---|
| L | a | b | L | a | b | |
| Cottonseed | 63.4c | 9.6a | 24.4 | 62.0b | 9.9b | 24.8 |
| Hazelnut | 65.1a | 8.5c | 24.6 | 61.2c | 10.4a | 24.8 |
| Olive | 63.8b | 9.3b | 24.4 | 62.5a | 9.6c | 24.8 |
| LSD (p < 0.05) | 0.45 | 0.23 | 0.41 | 0.20 | ||
Mean values of four determination within each column with different lower case letters (a–c) are significantly different (p < 0.05)
Table 4.
The effect of non-interesterified and interesterified shortenings incorporation on L, a and b color values of cookies
| L | a | b | |
|---|---|---|---|
| Interesterified | 64.1a | 9.2b | 24.4b |
| Non-interesterified | 61.9b | 10.0a | 24.8a |
| LSD (p < 0.05) | 0.24 | 0.12 | 0.08 |
Mean values of four determination within each column with different lower case letters (a–b) are significantly different (p < 0.05)
Table 5.
The effects of ratio of vegetable oils in palm oil on color values
| Ratio (%) | Interesterified | Non-interesterified | ||||
|---|---|---|---|---|---|---|
| L | a | b | L | a | b | |
| 100 | 63.4c | 9.0c | 24.6c | 63.0b | 9.7c | 25.2a |
| 75 | 62.6d | 9.8a | 25.0b | 64.2a | 8.8d | 25.0b |
| 50 | 63.6c | 9.6ab | 25.5a | 61.5d | 10.0b | 24.9b |
| 25 | 66.1a | 7.9d | 23.6d | 62.7c | 10.0b | 24.7c |
| 0 | 64.8b | 9.5b | 23.6d | 59.0e | 11.4a | 24.2d |
| LSD (p < 0.05) | 0.59 | 0.29 | 0.24 | 0.52 | 0.26 | 0.10 |
Mean values of four determination within each column with different lower case letters (a–d) are significantly different (p < 0.05)
In conclusion, our results indicate that incorporation of cottonseed, hazelnut and olive oils in palm oil considerably increased the nutritional value of experimental shortenings by decreasing the SFA/UFA ratio. Some in-es shortenings showed comparable SFC contents to hydrogenated shortening and this was well reflected to spread ratios of in-es shortenings incorporated cookies. In-es shortening added cookies had higher L values, consequently lighter surface colors. This is probably due to bleaching and washing step of the oils after interesterification. It was concluded that in-es blends of CO, HO and OO with PO could be used for production of cookies with zero-trans and high in unsaturated fatty acids content.
Acknowledgement
This study was supported by Hacettepe University Research Fund (98 02 602 003). We thank Ülker Group and Etsun Inc.
References
- American Association of cereal chemists, approved methods of the AACC. 10. St. Paul: The Association; 2000. [Google Scholar]
- Aini IN, Miskandar MS. Utilization of palm oil and palm products in shortenings and margarines. Eur J Lipid Sci Technol. 2007;109:422–432. doi: 10.1002/ejlt.200600232. [DOI] [Google Scholar]
- Official methods of analysis. Washington: Association of Official Analytical Chemists; 1990. [Google Scholar]
- The official methods and recommended practices of the American oil chemists’ society. 4. Champagin: AOCS Press; 1989. [Google Scholar]
- Ascherio A, Willett WC. Health effects of trans fatty acids. Am J Clin Nutr. 1997;66:1006–1010. doi: 10.1093/ajcn/66.4.1006S. [DOI] [PubMed] [Google Scholar]
- Baldwin RR, Baldry RP, Johansen RG. Fat systems for bakery products. JAOCS. 1972;49:473–477. [Google Scholar]
- Basturk A, Javidipour I, Boyacı IH. Oxidative stability of natural and chemically interesterfied cottonseed, palm and soybean oils. J Food Lipids. 2007;14:170–188. doi: 10.1111/j.1745-4522.2007.00078.x. [DOI] [Google Scholar]
- Block CR, Harris WS, Reid KJ, Spertus JA. Omega-6 and trans fatty acids in blood cell membranes: a risk factor for acute coronary syndromes? Am Hear J. 2008;156(6):1117–1123. doi: 10.1016/j.ahj.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka V, Gulia N, Ahlawat KS, Khatkar BS. Trans fats – sources, health risks and alternative approach – a rewiev. J Food Sci Technol. 2011;48(5):534–541. doi: 10.1007/s13197-010-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMan L, DeMan J. Functionality of palm oil and palm kernel oil in margarine and shortening. PORIM Occasional Papers. 1994;32:1–16. [Google Scholar]
- Dogan IS, Javidipour I, Akan T. Effects of interesterified palm and cottonseed oil blends on cake quality. Int J Food Sci Technol. 2007;42:157–164. doi: 10.1111/j.1365-2621.2006.01178.x. [DOI] [Google Scholar]
- Jakobsen UM, Bysted A, Andersen NL, Berit L, Heitmann LB, Hartkopp HB, Leth T, Overvad K, Dyerberg J. Intake of ruminant trans fatty acids and risk of coronary heart disease–an overview. Atheroscler Suppl. 2006;7(2):9–11. doi: 10.1016/j.atherosclerosissup.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Javidipour I, Tuncturk Y. Effect of using interesterified and non-interesterified corn and palm oil blends on quality and fatty acid composition of Turkish White cheese. Int J Food Sci Technol. 2007;42:1465–1474. doi: 10.1111/j.1365-2621.2006.01366.x. [DOI] [Google Scholar]
- Javidipour I, Vural H. Effects of incorporation of interesterified plant oils on quality and fatty acid composition of Turkish-type salami. Nahrung. 2002;46:404–407. doi: 10.1002/1521-3803(20021101)46:6<404::AID-FOOD404>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Javidipour I, Vural H, Ozbas OO, Tekin A. Effects of interesterified vegetable oils and sugar beet fiber on the quality of Turkish-type salami. Int J Food Sci Technol. 2005;40:177–185. doi: 10.1111/j.1365-2621.2004.00928.x. [DOI] [Google Scholar]
- Manley D (2000) Fats and Oils. In: Technology of biscuits, crackers and cookies. 3rd edn. Woodhead Publishing Limited, Cambridge England, pp: 130–150
- Micha R, Mozaffarian D. Trans fatty acids: effects on cardiometabolic health and implications for policy. Prostaglandins, leukotrienes and essential fatty acids lipidomics to human health. 2008;79(3–5):147–152. doi: 10.1016/j.plefa.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawar WW. Lipids. In: Nawar WW, editor. Food chemistry. 2. New York: Marcel Dekker Inc; 1985. pp. 139–245. [Google Scholar]
- Öztürk S, Özboy ÖÖ, Javidipour I, Köksel H. Utilization of sugarbeet fiber and zero trans interesterified and non-interesterified shortenings in cookie production. Sugar Industry/Zuckerindustrie. 2008;133:704–709. [Google Scholar]
- Park DK, Terao J, Matsushita S. Influence of interesterification on the antioxidative stability of vegetable oils. Agric Biol Chem. 1983;47:121–123. doi: 10.1271/bbb1961.47.121. [DOI] [Google Scholar]
- Petrauskaite V, Greyt WD, Kellens M, Huyghebaert A. Physical and chemical properties of trans-free fats produced by chemical interesterification of vegetable oil blends. Journal American Oil Chemists Society. 1998;75:489–493. doi: 10.1007/s11746-998-0252-z. [DOI] [Google Scholar]
- Rodriguez A, Castro E, Salinas MC, Lopez R, Miranda M. Interesterification of tallow and sunflower oil. Journal American Oil Chemists Society. 2001;78:431–436. doi: 10.1007/s11746-001-0280-5. [DOI] [Google Scholar]
- Rousseau D, Marangoni AG. The effects of interesterification on physical and sensory attributes of butterfat and butterfat-canola oil spreads. Food Research International. 1999;31:381–388. doi: 10.1016/S0963-9969(98)00100-8. [DOI] [Google Scholar]
- Stender S, Dyerberg J, Bysted A, Leth T, Astrup A. A trans world journey. Atheroscler Suppl. 2006;7:47–52. doi: 10.1016/j.atherosclerosissup.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Tavella M, Peterson G, Espeche M, Cavallero E, Cipola L, Perego L, Caballero B. Trans fatty acid content of a selection of foods in Argentina. Food Chemistry. 2000;69:209–213. doi: 10.1016/S0308-8146(99)00257-5. [DOI] [Google Scholar]
- Tsanev R, Russeva A, Rizov T, Dontcheva I. Content of trans fatty acids in edible margarines. Journal American Oil Chemists Society. 1998;75:1731–1734. doi: 10.1007/s11746-998-0025-8. [DOI] [Google Scholar]
- Vural H, Javidipour I. Replacement of beef fat in frankfurters by interesterified palm, cottonseed and olive oils. European Food Research Technology. 2002;214:415–468. doi: 10.1007/s00217-002-0502-5. [DOI] [Google Scholar]
- Vural H, Javidipour I, Ozbas OO. Effects of interesterified vegetable oils and sugarbeet fiber on the quality of frankfurters. Meat Science. 2004;67:65–72. doi: 10.1016/j.meatsci.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Ward J. Processing canola oil products. JAOCS. 1988;65:1731–1734. [Google Scholar]
- WHO (1993) Fats and oils in human nutrition. Report of a joint consultation. Food and Agriculture Organization of the United Nations and the World Health Organization [PubMed]
- Wiedermann LH. Margarine and margarine oil: formulation and control. Journal American Oil Chemists Society. 1978;55:823–829. doi: 10.1007/BF02682655. [DOI] [Google Scholar]
- Willett WC. Trans fatty acids and cardiovascular disease – epidemi-ological data. Atheroscler Suppl. 2006;7(2):5–8. doi: 10.1016/j.atherosclerosissup.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Yap PH, Deman JM, Deman L. Polymorphic stability of hydrogenated canola oil as affected by addition of palm oil. JAOCS. 1989;66:1784–1791. [Google Scholar]
- Zoulias E, Oreopoulou V, Kounalaki E. Effect of fat and sugar replacement on cookie properties. Journal Science Food Agriculture. 2002;82(14):1637–1644. doi: 10.1002/jsfa.1230. [DOI] [Google Scholar]