Abstract
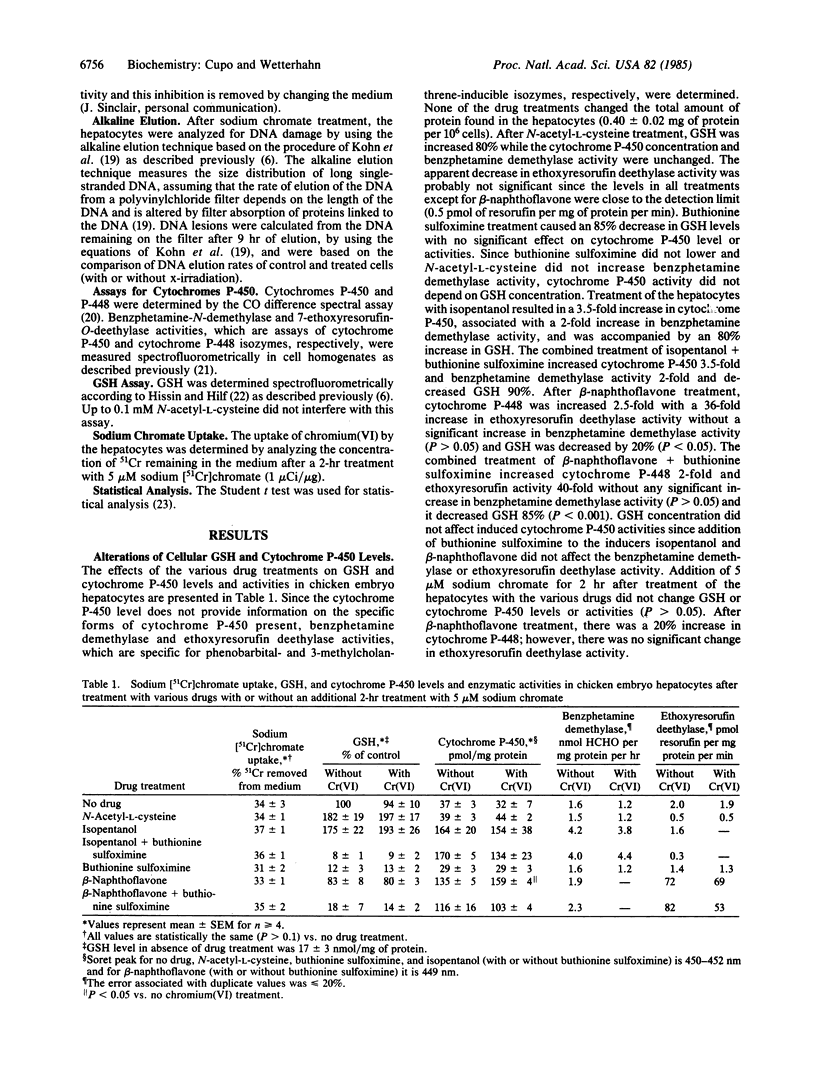
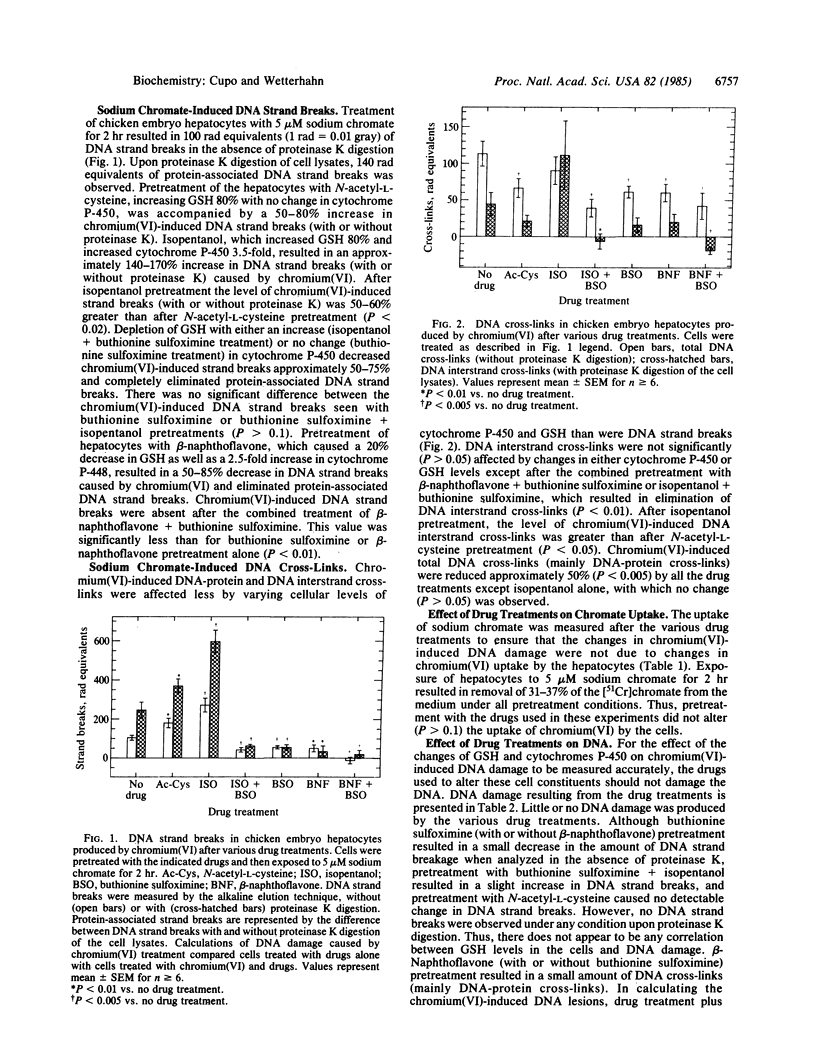
The role of glutathione and cytochrome P-450 in the production of DNA damage by chromium(VI) was examined in chicken embryo hepatocytes by the alkaline elution technique. Cellular levels of glutathione and cytochrome P-450 were altered by treating the hepatocytes with N-acetyl-L-cysteine, buthionine sulfoximine, isopentanol, or beta-naphthoflavone. A dramatic increase in chromium(VI)-induced DNA strand breaks was observed after increasing glutathione levels in the cells. Chromium(VI)-induced DNA strand breaks were even more numerous when the level of cytochrome P-450 was also increased. Upon depletion of glutathione levels and induction of cytochrome P-450 or cytochrome P-448, little or no DNA strand breaks or DNA interstrand cross-links were observed after chromium(VI) treatment. Chromium(VI)-induced DNA-protein cross-links generally decreased after either increases or decreases in cellular levels of glutathione or cytochrome P-450 or P-448. These results suggest that glutathione enhances chromium(VI)-induced DNA damage through metabolic activation of chromium(VI). The possible production of reactive chromium species upon metabolism by glutathione and cytochrome P-450 or P-448 and their involvement in DNA damage is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus F. R., Sinclair J. F., Sinclair P., Meyers U. A. Drug-mediated induction of cytochrome(s) P-450 and drug metabolism in cultured hepatocytes maintained in chemically defined medium. J Biol Chem. 1979 Mar 25;254(6):2148–2153. [PubMed] [Google Scholar]
- Appel K. E., Schwarz M., Rickart R., Kunz W. Influences of inducers and inhibitors of the microsomal monooxygenase system on the alkylating intensity of dimethylnitrosamine in mice. J Cancer Res Clin Oncol. 1979 May 14;94(1):47–61. doi: 10.1007/BF00405349. [DOI] [PubMed] [Google Scholar]
- Bianchi V., Celotti L., Lanfranchi G., Majone F., Marin G., Montaldi A., Sponza G., Tamino G., Venier P., Zantedeschi A. Genetic effects of chromium compounds. Mutat Res. 1983 May-Jun;117(3-4):279–300. doi: 10.1016/0165-1218(83)90128-3. [DOI] [PubMed] [Google Scholar]
- Brooker J. D., Srivastava G., Borthwick I. A., May B. K., Elliott W. H. Evidence that 2-allyl-2-isopropylacetamide, phenobarbital and 3,5-diethoxycarbonyl-1,4-dihydrocollidine induce the same cytochrome P450 mRNA in chick embryo liver. Eur J Biochem. 1983 Nov 2;136(2):327–332. doi: 10.1111/j.1432-1033.1983.tb07745.x. [DOI] [PubMed] [Google Scholar]
- Cresteil T., Lesca P. Enhancement of DNA binding, mutagenicity and carcinogenicity of polycyclic aromatic hydrocarbons after induction of cytochrome P-450 by ellipticines in rats and mice. Chem Biol Interact. 1983 Nov;47(2):145–156. doi: 10.1016/0009-2797(83)90154-0. [DOI] [PubMed] [Google Scholar]
- Cupo D. Y., Wetterhahn K. E. Repair of chromate-induced DNA damage in chick embryo hepatocytes. Carcinogenesis. 1984 Dec;5(12):1705–1708. doi: 10.1093/carcin/5.12.1705. [DOI] [PubMed] [Google Scholar]
- Dawson J. R., Norbeck K., Anundi I., Moldéus P. The effectiveness of N-acetylcysteine in isolated hepatocytes, against the toxicity of paracetamol, acrolein, and paraquat. Arch Toxicol. 1984 Mar;55(1):11–15. doi: 10.1007/BF00316579. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Seres D. S., Lechner J. F., Harris C. C. DNA-protein cross-linking by chromium salts. Chem Biol Interact. 1981 Sep;36(3):345–354. doi: 10.1016/0009-2797(81)90077-6. [DOI] [PubMed] [Google Scholar]
- Garcia J. D., Jennette K. W. Electron-transport cytochrome P-450 system is involved in the microsomal metabolism of the carcinogen chromate. J Inorg Biochem. 1981 Jul;14(4):281–295. doi: 10.1016/s0162-0134(00)80286-x. [DOI] [PubMed] [Google Scholar]
- Gruber J. E., Jennette K. W. Metabolism of the carcinogen chromate by rat liver microsomes. Biochem Biophys Res Commun. 1978 May 30;82(2):700–706. doi: 10.1016/0006-291x(78)90931-2. [DOI] [PubMed] [Google Scholar]
- Hissin P. J., Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976 Jul;74(1):214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- Ho D., Fahl W. E. Modification of glutathione levels in C3H/10T1/2 cells and its relationship to benzo(a)pyrene anti-7,8-dihydrodiol 9,10-epoxide-induced cytotoxicity. J Biol Chem. 1984 Sep 25;259(18):11231–11235. [PubMed] [Google Scholar]
- Inskeep P. B., Guengerich F. P. Glutathione-mediated binding of dibromoalkanes to DNA: specificity of rat glutathione-S-transferases and dibromoalkane structure. Carcinogenesis. 1984 Jun;5(6):805–808. doi: 10.1093/carcin/5.6.805. [DOI] [PubMed] [Google Scholar]
- Kerklaan P., Bouter S., Mohn G. Isolation of a mutant of Salmonella typhimurium strain TA1535 with decreased levels of glutathione (GSH-). Primary characterization and chemical mutagenesis studies. Mutat Res. 1983 Dec;122(3-4):257–266. doi: 10.1016/0165-7992(83)90004-0. [DOI] [PubMed] [Google Scholar]
- Miller E. C., Miller J. A. Searches for ultimate chemical carcinogens and their reactions with cellular macromolecules. Cancer. 1981 May 15;47(10):2327–2345. doi: 10.1002/1097-0142(19810515)47:10<2327::aid-cncr2820471003>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Norseth T., Alexander J., Aaseth J., Langård S. Biliary excretion of chromium in the rat: a role of glutathione. Acta Pharmacol Toxicol (Copenh) 1982 Nov;51(5):450–455. doi: 10.1111/j.1600-0773.1982.tb01052.x. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Rannug U., Sundvall A., Ramel C. The mutagenic effect of 1,2-dichloroethane on Salmonella typhimurium I. Activation through conjugation with glutathion in vitro. Chem Biol Interact. 1978 Jan;20(1):1–16. doi: 10.1016/0009-2797(78)90076-5. [DOI] [PubMed] [Google Scholar]
- Révész L., Edgren M. Glutathione-dependent yield and repair of single-strand DNA breaks in irradiated cells. Br J Cancer Suppl. 1984;6:55–60. [PMC free article] [PubMed] [Google Scholar]
- Saito K., Yamazoe Y., Kamataki T., Kato R. Activation and detoxication of N-hydroxy-Trp-P-2 by glutathione and glutathione transferases. Carcinogenesis. 1983 Dec;4(12):1551–1557. doi: 10.1093/carcin/4.12.1551. [DOI] [PubMed] [Google Scholar]
- Shedlofsky S. I., Sinclair P. R., Sinclair J. F., Bonkovsky H. L. Increased glutathione in cultured hepatocytes associated with induction of cytochrome P-450. Lack of effect of glutathione depletion on induction of cytochrome P-450 and delta-aminolevulinate synthase. Biochem Pharmacol. 1984 May 1;33(9):1487–1491. doi: 10.1016/0006-2952(84)90417-9. [DOI] [PubMed] [Google Scholar]
- Sinclair J. F., Sinclair P. R., Smith E. L., Bement W. J., Pomeroy J., Bonkowsky H. Ethanol-mediated increase in cytochrome P-450 in cultured hepatocytes. Biochem Pharmacol. 1981 Oct;30(20):2805–2809. doi: 10.1016/0006-2952(81)90418-4. [DOI] [PubMed] [Google Scholar]
- Sinclair J. F., Wiebkin P., Zaitlin L. M., Smith E. L., Sinclair P. R. Aminopyrine and biphenyl metabolism in cultured hepatocytes. Induction by alcohols. Biochem Pharmacol. 1984 Jan 15;33(2):187–190. doi: 10.1016/0006-2952(84)90474-x. [DOI] [PubMed] [Google Scholar]
- Tsapakos M. J., Hampton T. H., Sinclair P. R., Sinclair J. F., Bement W. J., Wetterhahn K. E. The carcinogen chromate causes DNA damage and inhibits drug-mediated induction of porphyrin accumulation and glucuronidation in chick embryo hepatocytes. Carcinogenesis. 1983 Aug;4(8):959–966. doi: 10.1093/carcin/4.8.959. [DOI] [PubMed] [Google Scholar]
- Tsapakos M. J., Hampton T. H., Wetterhahn K. E. Chromium(VI)-induced DNA lesions and chromium distribution in rat kidney, liver, and lung. Cancer Res. 1983 Dec;43(12 Pt 1):5662–5667. [PubMed] [Google Scholar]
- Tsapakos M. J., Wetterhahn K. E. The interaction of chromium with nucleic acids. Chem Biol Interact. 1983 Sep 1;46(2):265–277. doi: 10.1016/0009-2797(83)90034-0. [DOI] [PubMed] [Google Scholar]
- Ueng T. H., Moore L., Elves R. G., Alvares A. P. Isopropanol enhancement of cytochrome P-450-dependent monooxygenase activities and its effects on carbon tetrachloride intoxication. Toxicol Appl Pharmacol. 1983 Nov;71(2):204–214. doi: 10.1016/0041-008x(83)90337-x. [DOI] [PubMed] [Google Scholar]



