Abstract
Casein hydrolysate was prepared by hydrolyzing casein with Neutrase and then modified by a Neutrase-catalyzed plastein reaction. The prepared hydrolysate had a degree of hydrolysis of 13.0% and exhibited ACE inhibition in vitro with an IC50 value of 40.4 μg⋅mL−1. With the decreased amount of free amino groups of the modified hydrolysate as the response, some conditions of the plastein reaction including substrate concentration, enzyme to substrate ratio, reaction temperature and time were studied by single factor experiments and response surface methodology, and optimized finally as 62% (w/w), 3.0 kU⋅g−1 peptides, 30 °C and 6.3 h, respectively. The maximum decreased amount of free amino groups of the modified hydrolysate prepared under these optimized conditions was 210.0 μmol⋅g−1 peptides, while corresponding IC50 value was lowered to 14.7 μg⋅mL−1. The present result indicates that Neutrase-catalyzed plastein reaction was capable of enhancing ACE-inhibitory activity in vitro of casein hydrolysate, and also highlights the importance of a forthcoming study to investigate the peptide compositions of the modified hydrolysate and the role of protease used in the plastein reaction.
Keywords: Casein, Casein hydrolysate, ACE-inhibitory activity, Plastein reaction, Neutrase
Introduction
Proteolysis of food proteins might produce some peptides with antihypertensive or antioxidant activity, which could be served as functional food ingredients such as angiotensin I-converting enzyme (ACE) inhibitors (Ariyoshi 1993; Janitha et al. 2002; Meisel 1997; Robert et al. 2004; Yamamoto 1997) or antioxidants (Naqash and Nazeer 2011; Sun et al. 2011). Milk proteins are particularly good sources of ACE-inhibitory peptides and had been widely studied in the past (da Costa et al. 2007; López-Fandiño et al. 2006; Miguel et al. 2009; Ortiz-Chao et al. 2009). To obtain the peptides with high ACE-inhibitory activity, a lot of works have been carried out by in vitro enzymatic hydrolysis (Contreras et al. 2009) or fermentation (Pihlanto et al. 2010) of food proteins. For example, two powerful ACE-inhibitory peptides, IPP and VPP with IC50 values of 5 and 9 μmol⋅L−1, respectively, were obtained from the commercial fermented milk products or milk protein hydrolysates (FitzGerald et al. 2004; Nakamura et al. 1995).
Proteases used in protein hydrolysis show clear influence on ACE- inhibitory activity of the obtained protein hydrolysates. Jiang et al. (2007) hydrolyzed yak milk casein with six commercial proteases, and found that the hydrolysate prepared with Neutrase had the highest ACE-inhibitory activity (IC50 = 0.38 mg⋅mL−1). Hydrolysis conditions also exhibit impacts on ACE-inhibitory activity of protein hydrolysates. Optimization of hydrolysis conditions is another approach to prepare protein hydrolysates with higher activity (Guo et al. 2009). If ACE-inhibitory peptides are prepared just by enzymatic hydrolysis of protein substrates, the amino acid sequences of the generated peptides are coincident with the primary structure of the protein substrates. This means that it is impossible to obtain ACE-inhibitory peptides with different amino acid sequences from the protein substrates by enzymatic hydrolysis, i.e., ACE-inhibitory activities of protein hydrolysates or the sequences of the most active peptides are limited by the applied protein substrates.
Plastein reaction involves the formation of polypeptides by a reversal of the usual protein hydrolysis by proteases, and had been applied to prepare higher molecular, protein-like substances (Ymashita et al. 1971). Three different mechanisms of plastein reaction are suggested as condensation (Yamashita et al. 1976a), transpeptidation (Combes and Lozano 1992) and physical forces (Andrews and Alichanidis 1990). These mechanisms might take place simultaneously (Stevenson et al. 1999). Plastein reaction can be applied to enhance biological value and functional properties of some food proteins, improve flavor quality of protein hydrolysates, or provide a ways to synthesize new proteins (Ashley et al. 1983; Sukan and Andrews 1982a, b; Yamashita et al. 1976b). Some peptides with amino acid sequences different form protein substrates might be generated if peptide condensation or transpeptidation does exist during plastein reaction of protein hydrolysates, i.e., plastein reaction might lead to peptide junction between some peptides or one amino acid residue previously bound at the terminal of one peptide rebind into the terminal of other peptides. As the result, plastein reaction creates new amino acid sequences, and is thus capable of mediating the ACE-inhibitory activity of the protein hydrolysates treated by plastein reaction.
In the present work, Neutrase-catalyzed plastein reaction of casein hydrolysate prepared by Neutrase was studied. Casein hydrolysate with higher ACE-inhibitory activity was prepared by Neutrase-catalyzed hydrolysis of casein, and used as substrate of the plastein reaction. Single factor experiments and response surface methodology were applied to optimize some reaction conditions, including substrate concentration, enzyme to substrate (E/S) ratio, reaction temperature and reaction time. The ACE-inhibitory activities of some modified hydrolysates were evaluated in vitro and compared with the activity of the original casein hydrolysate, in order to investigate if the plastein reaction could enhance ACE-inhibitory activity of the casein hydrolysate.
Materials & methods
Materials and chemicals
Casein, rabbit lung acetone powder and N-(3-[2-furyl]acryloyl)-L-phenylalanylglycylglycine (FAPGG) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Neutrase with an actual activity of 30 kU⋅mL−1 was purchased from Novozyme China (Tianjing, China). Captopril (purity > 99.0%) was purchased from Fluka (Fluka Chemie AG, Buchs, Switzerland). Other reagents used were of analytical grades. Highly purified water was prepared with Milli-Q PLUS (Millipore Corporation, New York, NY, USA) and used to prepare all buffers and solutions.
Preparation of casein hydrolysate
Casein (10 g, on protein basis) was dissolved in 100 mL deionized water to give an original protein concentration of 10% (w/v). The pH of solution was adjusted to 6.5 by 2 mol⋅L−1 NaOH solution. Neutrase solution was prepared immediately prior to using. After withdrawal of casein solution of 5 mL (zero time sample), hydrolysis reaction was started by adding Neutrase solution to the remaining casein solution to give approximately E/S ratio of 2 kU⋅g−1 proteins, and kept at 45 °C with continuous stirring. During hydrolysis, no NaOH solution was added to the casein solution to adjust the pH. Aliquots of the remaining casein solution (about 5 mL) were withdrawn after 2, 4, 6, 8, 10 and 12 h of hydrolysis, respectively. The separated casein solutions were heated at 100 °C for 15 min to inactivate Neutrase, cooled to room temperature and centrifuged at 11,000 × g for 20 min. The obtained supernatants (containing casein hydrolysate) were analyzed as below to determine the corresponding degree of hydrolysis (DH) and ACE-inhibitory activity in vitro. Based on analysis results, casein hydrolysate with higher ACE-inhibitory activity was prepared in bulk, lyophilized and stored at −20 °C, and used as substrate of the plastein reaction.
Analysis of protease activity and protein or peptide content
The actual activity of Neutrase was assayed by a method described by Sarath et al. (1989). Nitrogen content of casein, casein hydrolysate or the modified hydrolysate was determined by the Kjeldahl procedure according to FIL-IDF 20B: 1993 (IDF 1993), and multiplied by 6.38 to give protein or peptide content.
Determination of amount of free amino groups and degree of hydrolysis of casein hydrolysate
The amount of free amino groups of casein, casein hydrolysate or the modified hydrolysate on protein or peptide basis was measured by o-phthaldialdehyde (OPA) assay (Church et al. 1983; Spellman et al. 2003) with some modifications. OPA reagent was prepared by combing following reagents and diluting them to a final volume of 100 mL with water: 75 mL 0.2 mol⋅L−1 sodium borate buffer (pH 9.5), 5 mL 400 g SDS⋅L−1, 80 mg OPA (in 1 mL methanol) and 0.4 mL β-mercaptoenthanol. The assay was carried out by adding the analysis sample (or standard) solution of 3 mL to the OPA reagent of same volume. The absorbance was measured at 340 nm with an UV spectrophotometer (UV-2401PC, Shimadzu, Kyoto, Japan), and the data were taken after 5 min.
L-Leucine standard solution was prepared as follows: L-leucine (0.3000 g) was dissolved in 1 mol⋅L−1 HCl solution, and then diluted to 600 μg⋅mL−1 with water. Diluted solutions (0 to 30 μg⋅mL−1) were prepared by further dilution, and used to determine the standard curve.
DH of the prepared casein hydrolysate was determined by assaying its amount of free amino groups and calculating from the Eq. 1 given by Adler-Nissen (1979).
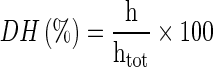 |
1 |
Where, h is the number of broken peptide bonds per unit weight, and htot is the total number of bonds per unit weight and equals 8.2 meq⋅g−1 proteins for casein (Adler-Nissen 1979).
Determination of ACE-inhibitory activity in vitro
ACE-inhibition assay was performed as per the method of Murray et al. (2004) with some modifications. ACE activity was measured with FAPGG as substrate and the extract of rabbit lung acetone powder as ACE source. The reaction mixture contained 100 μL of the casein hydrolysate dissolved in deionized water in a concentration range from 0 to 20 mg⋅mL−1, 500 μL of 1.6 mmol⋅L−1 FAPGG in 0.1 mol⋅L−1 sodium borate buffer (pH 8.3) with 0.3 mol⋅L−1 NaCl, and 300 μL 10 × diluted ACE extract in 0.1 mol⋅L−1 sodium borate buffer (pH 8.3) containing 5% (v/v) glycerol. ACE extract was added to initiate the reaction. After 30 min incubation at 37 °C, the reaction was terminated by adding 0.1 mol⋅L−1 EDTA solution 100 μL, and 3.0 mL water was added to dilute the reaction mixture. EDTA solution was added immediately before ACE extract in zero-time control assays. The decreased absorbance at 340 nm was determined in the spectrophotometer and taken as a measure of ACE activity. A control sample containing 100 μL of deionized water instead of casein hydrolysate was also assayed. ACE inhibition (%) was calculated as 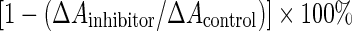 , where ΔAinhibitor and ΔAcontrol were the decreased absorbance at 340 nm of the analysis sample and control, respectively.
, where ΔAinhibitor and ΔAcontrol were the decreased absorbance at 340 nm of the analysis sample and control, respectively.
The concentration of the analysis sample needed to inhibit ACE by 50% (IC50) under these conditions was obtained by assaying various diluted sample solutions and plotting ACE inhibition percentage as a function of peptide concentration (Shalaby et al. 2006). The synthetic ACE inhibitor captopril was used as positive control with a practical IC50 of 5.2 nmol⋅L−1 in the present work.
Modification of casein hydrolysate by Neutrase-catalyzed plastein reaction
Different amount of casein hydrolysate was dissolved in deionized water together with different amount of Neutrase to give a fixed E/S ratio of 1.0 kU⋅g−1 peptides and a final peptide concentration of 20, 30, 40, 50 and 60% (w/w), respectively. The pH of the mixture was adjusted to 6.8. All prepared mixtures were kept at 30 °C for 6 h to carry out the plastein reaction with continuous stirring. No NaOH solution was added during the plastein reaction. After the plastein reaction, all mixtures were heated at 100 °C for 15 min to inactivate Neutrase. All modified hydrolysates prepared were stored at −20 °C until analysis of the amount of free amino groups and ACE-inhibitory activities in vitro. The decreased amount of free amino groups (μmol⋅g−1 peptides) of the modified hydrolysate was calculated by subtracting the amount of free amino groups of the modified hydrolysate after the plastein reaction from that of the original casein hydrolysate before the plastein reaction.
Similar approach was used to study the influences of E/S ratio (by changing the addition level of Neutrase), reaction temperature (by keeping the mixture at different temperature) and reaction time (by selecting different reaction times) on the decreased amount of free amino groups of the modified hydrolysate by single factor experiments.
Optimal conditions of Neutrase-catalyzed plastein reaction
Some conditions of the plastein reaction of casein hydrolysate were optimized by response surface methodology (RSM) with a central composite design (CCD). Based on the previous results from single factor experiments, E/S ratio (kU⋅g−1 peptides) (X1), substrate concentration (w/w,%) (X2) and reaction time (h) (X3) were chosen as independent variables, and reaction temperature was fixed at 30 °C. An experimental design consisting of 20 runs and three independent variables at five different levels was applied (Table 1). The decreased amount of free amino groups of the modified hydrolysate was taken as the dependent variable or response (Y). The second-order polynomial coefficients were calculated and analyzed with Design Expert software Version 7.0 (Stat-Ease Inc., Minneapolis, MN, US). Equation 2, a second-degree polynomial, was used to predict response.
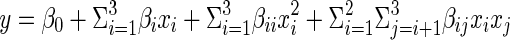 |
2 |
Table 1.
Independent variables and levels used in the study of response surface methodology
| Independent variables | Levels | ||||
|---|---|---|---|---|---|
| –α | −1 | 0 | +1 | α | |
| Substrate concentration (%) | 28 | 35 | 45 | 55 | 62 |
| E/S ratio (kU⋅g−1 peptides) | 0.5 | 1.5 | 3.0 | 4.5 | 5.5 |
| Reaction time (h) | 3.3 | 4.0 | 5.0 | 6.0 | 6.7 |
Where, y was dependent variable (decreased amount of free amino groups of modified hydrolysate); β0, βi, βii and βij were the coefficients estimated by the model to represent linear, quadratic and cross product effects of X1, X2 and X3 factors on the response, respectively, and xi, xj were the levels of independent variables. The fitted polynomial was expressed as three-dimensional surface plots to visualize the relationship between the response and experimental levels of each variable and to deduce optimal reaction conditions. The combination of different optimized variables, which produced the maximum response, was determined to verify the validity of the model obtained. Also, confirmation experiments were carried out to verify the validity of statistical experimental strategies.
Statistical analysis
All data were expressed as means ± standard deviation from three independent experiments (n = 3). Differences between the mean values of multiple groups were analyzed by one-way analysis of variance (ANOVA) with Duncan’s multiple comparison test. All the tests were considered statistically significantly at P < 0.05. RSM was analyzed by Design Expert software Version 7.0 (Stat-Ease Inc., Minneapolis, MN, US). SPSS software version 13.0 (SPSS Inc., Chicago, IL, US) and Microsoft Excel version 2003 software (Microsoft Corporation, Redmond, WA, US) were used to analyze or report the data.
Results & discussion
Preparation of casein hydrolysate by Neutrase
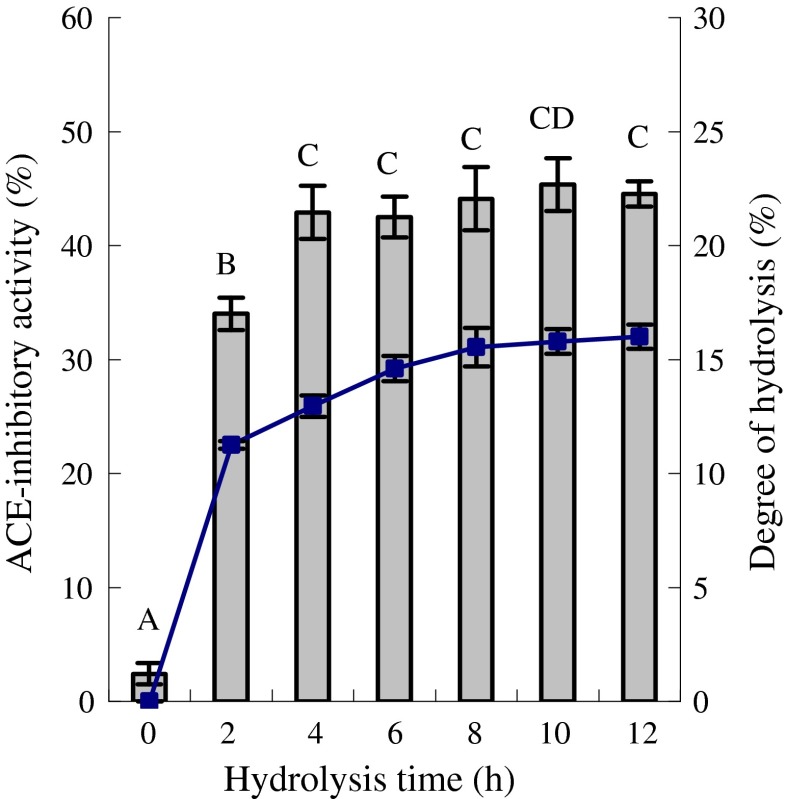
Neutrase was used in the present study to prepared casein hydrolysate. Original casein showed ACE inhibition about 2.4% under evaluation conditions. ACE-inhibitory activities in vitro of six casein hydrolysates prepared at different hydrolysis intervals, together with DH, were evaluated (Fig. 1). The data show that as hydrolysis time of casein progressed from 0 to 4 h, the DH of the prepared hydrolysate increased from 0% to 13.0% and the ACE-inhibitory activity increased from 2.4% to 42.9%. Longer hydrolysis time of casein did not impact the activity of the prepared hydrolysate significantly (P > 0.05). Casein hydrolysate with DH of 13.0% had an evaluated IC50 value of 40.4 μg⋅mL−1, was thus prepared in bulk and served as the substrate of the plastein reaction.
Fig. 1.
Angiotensin-converting enzyme (ACE) inhibitory activity (%, means) and degree of hydrolysis (DH) of six casein hydrolysates prepared at different hydrolysis times. The column chart was for ACE-inhibitory activity and the graph chart was for DH. Each experiment was carried out in triplicate. Different capital letters above the column indicate that one-way ANOVA of the means is significantly different (P <0.05)
Marambe et al. (2008) hydrolyzed flaxseed proteins with Flavourzyme for 12 h to obtain a hydrolysate with DH of 11.94% and an IC50 value of 70 μg⋅mL−1. Miguel et al. (2009) had prepared casein hydrolysate with IC50 value of 52.8 μg⋅mL−1 by pepsin. Otte et al. (2007) hydrolyzed sodium caseinate with thermolysin to obtain a hydrolysate with an IC50 value of 95 μg⋅mL−1. Contreras et al. (2009) prepared some casein hydrolysates with pepsin, and the IC50 values of the prepared hydrolysates were from 22.19 to 60.85 μg⋅mL−1. In another reported work (Zhao and Li 2009), casein hydrolysate prepared with Alcalase to a DH of 11.2% exhibited IC50 value of 47 μg⋅mL−1. The hydrolysate prepared in the present study had similar or somewhat higher activity than the hydrolysates in these mentioned studies, except for the hydrolysates of Contreras et al. Mao et al. (2007) reported that when yak milk casein was hydrolyzed for 6 h, the most active hydrolysate showed ACE inhibition of 79.5%, but longer hydrolysis time led the peptides lost their ability to inhibit ACE. The present study result shared similar to the Mao’s result in the time profiles of the activity and DH.
Impacts of some reaction conditions on plastein reaction of casein hydrolysate
The impact of substrate concentration on the plastein reaction of casein hydrolysate is shown in Fig. 2a. Higher substrate concentration led the modified hydrolysate higher decreased amount of free amino group. As substrate concentration increased from 20% to 60% (w/w), the decreased amount of free amino groups of the modified hydrolysate increased from 7.3 to 168.5 μmol⋅g−1 peptides. When substrate concentration was more than 50% (w/w), reaction mixture was viscous which might hinder the efficiency of Neutrase. If substrate concentration was lowered, the decreased amount of free amino groups of the modified hydrolysate was in a lower level. Substrate concentration of 45% was chosen as centre point with 10% as a step change in CCD (as shown in Table 1).
Fig. 2.
Effects of substrate concentration (a), E/S ratios (b), reaction temperature (c) and reaction time (d) on the decreased amount of free amino groups of the casein hydrolysate modified by Neutrase-catalyzed plastein reaction. Each experiment was carried out in triplicate
The impact of E/S ratio on the plastein reaction of casein hydrolysate is given in Fig. 2b. The results indicated that the more Neutrase added the more deceased amount of free amino groups of the modified hydrolysate. Consideration of the cost of reaction and the potential of further hydrolysis, the centre point for E/S ratio was selected at 3.0 kU⋅g−1 peptides with a step change of 1.5 kU⋅g−1 peptides in CCD (Table 1).
The impact of reaction temperature on the plastein reaction of casein hydrolysate is shown in Fig. 2c. The decreased amount of free amino groups of the modified hydrolysate prepared at 40 °C was 143.8 μmol⋅g−1 peptides, while that prepared at 20, 30 or 50 °C was 134.8, 139.6 or 139.6 μmol⋅g−1 peptides, respectively. Reaction temperature did not show much impact on the reaction. Consideration of heat stability of Neutrase and reaction rate of the plastein reaction, reaction temperature was fixed at 30 °C in later work.
The impact of reaction time on the plastein reaction of casein hydrolysate is shown in Fig. 2d. The decreased amount of free amino groups of the modified hydrolysate increased rapidly as the plastein reaction progressed from the beginning to 6 h, but showed a decreased trend after then. Reaction time of 5.0 h was chosen as centre point with 1.0 h as a step change in CCD (Table 1).
Plastein reaction undertakes usually at higher substrate concentration (Lozano and Combes 1991; Pallavicini et al. 1980). Sukan and Andrews (1982a) reported that the formation of plastein products was maximal when substrate concentration ranged from 20% to 40% (w/w), and fell sharply both above and below this range. The present result shares similarity to this conclusion. An active protease is important to catalyze plastein reaction (Andrews and Alichanidis 1990), and reaction temperature must be a key factor. Lower temperature is beneficial as plastein reaction is an exothermic reaction (Fujimaki et al. 1971). If reaction temperature is too high, although the initial rate of the plastein reaction is rapid, but the reaction is soon stopped and overall result is much lower than at low temperature for longer time (Sukan and Andrews 1982a). Unfortunately, the present result shows that reaction temperature only had a little impact on the plastein reaction of casein hydrolysate. The decreased amount of free amino groups of the modified hydrolysate in the present study increased rapidly at initial stage of reaction (from 0 to 6 h), and then showed a decrease trend (from 6 to 12 h), which was similar to the result of Williams et al. (2001).
Model fitting and optimal conditions of plastein reaction of casein hydrolysate
RSM had been used in some studies to optimize the hydrolysis or extraction conditions of some food proteins, such as for the preparation of shrimp protein hydrolysate (Dey and Dora 2011) or chicken gelatin (Rafieian et al. 2011). The present study also employed the RSM with a CCD to select the suitable E/S ratio, substrate concentration and reaction time for the plastein reaction of the casein hydrolysate. The response results from CCD were analyzed, and the analysis results are given in Tables 2 and 3. All independent variables gave significant impacts (P < 0.05) on the response. The probability P-value is very low (P < 0.0001), indicating the model is significant. The ANOVA shows that there is an insignificant (P > 0.05) lack of fit, which validated the model. The value of determination coefficient (R2) is 0.9686, indicating a higher correlation between the experimental and predicted values. The adjusted determination coefficient (R2Adj) is 0.9403, high enough to advocate the significance of the model. A quadratic mathematical model is then given as Eq. 3.
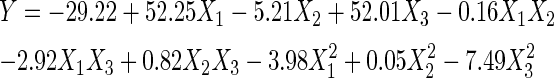 |
3 |
Table 2.
ANOVA response for linear, quadratic and interactive effects of variables used in the modela
| Model term | Coefficient estimated | P-valuesb |
|---|---|---|
| X1 | 52.25 | 0.0004 |
| X 2 | −5.21 | <0.0001 |
| X3 | 52.01 | 0.0138 |
| X1X2 | −0.16 | 0.3303 |
| X1X3 | −2.92 | 0.0974 |
| X2X3 | 0.82 | 0.0063 |
| X21 | −3.98 | 0.0005 |
| X22 | 0.05 | 0.0258 |
| X23 | −7.49 | 0.0018 |
aX1, X 2 and X3 represent E/S ratio, substrate concentration and reaction time, respectively
b P < 0.05 indicates a statistical significance
Table 3.
ANOVA for response surface quadratic model
| Source | Sum of squares | Degree of freedom | Mean squares | F-values | P-valuesa |
|---|---|---|---|---|---|
| Model | 14175.30 | 9 | 1575.03 | 34.28 | <0.0001 |
| Residual | 459.52 | 10 | 45.95 | ||
| Lack of fit | 297.79 | 5 | 59.56 | 1.84 | 0.2596 |
| Pure error | 161.74 | 5 | 32.35 | ||
| Corrected total | 14634.83 | 19 |
a P < 0.05 indicates a statistical significance
To assess further the effects of independent variables on the decreased amount of free amino groups of the modified hydrolysate, three-dimensional response surfaces plots (Fig. 3) are generated by keeping one variable at zero level and changing other two variables with different combinations. Figure 3a shows the effects of E/S ratio and substrate concentration on the decreased amount of free amino groups of the modified hydrolysate (reaction time at its zero level). Quadratic effect for two variables existed but substrate concentration had greater influence on the response. The decreased amount of free amino groups of the modified hydrolysate increased as substrate concentration increased. A larger decreased amount of free amino groups occurred when E/S ratio was in 1.5 to 4.9 kU⋅g−1 peptides and substrate concentration was from 50% to 55% (w/w). Figure 3b reveals the effects of E/S ratio and reaction time on the decreased amount of free amino groups of the modified hydrolysate (substrate concentration at its zero level). Quadratic effect of E/S ratio and reaction time on the response was also noticed. When E/S ratio was in 2.4 to 4.1 kU⋅g−1 peptides and reaction time was from 4.3 to 6.0 h, a larger decreased amount of free amino groups was obtained. In Fig. 3c, quadratic effect of substrate concentration and reaction time (E/S ratio at its zero level) is also observed.
Fig. 3.
Response surface graphs for the decreased amount of free amino groups of the modified casein hydrolysate as a function of: a E/S ratio and substrate concentration (reaction time at its central level), b E/S ratio and reaction time (substrate concentration at its central level), c substrate concentration and reaction time (E/S ratio at its central level)
These results mentioned above indicate that the response surface might have a maximum point within the studied range of independent variables. The maximum point predicted by the model was 215.1 μmol⋅g−1 peptides, and the optimal conditions given by the software were E/S ratio of 3.0 kU⋅g−1 peptides, substrate concentration of 62% and reaction time of 6.3 h at a fixed reaction temperature of 30 °C. The practical result was 210.0 μmol⋅g−1 peptides (mean value of three experiments). This result declares that the obtained model, Eq. 3, could predict the decreased amount of free amino groups of the modified hydrolysate during the plastein reaction.
ACE-inhibitory activity in vitro of the modified casein hydrolysate
Five modified casein hydrolysates (i.e., MCH 1–5) were prepared at the optimized conditions mentioned above but with a reaction time of 2, 3, 4, 5 and 6.3 h, respectively. The decreased amount of free amino groups of five MCHs ranged from 89.8 to 210.0 μmol⋅g−1 peptides, as Table 4 listed. The IC50 values of these MCHs ranging from 14.7 to 31.1 μg⋅mL−1 (Table 4) were lower than that of original casein hydrolysate (40.4 μg⋅mL−1), showing that the carried out plastein reaction enhanced the ACE-inhibitory activity of the MCH. The data in Table 4 also declare that some condensation occurred during plastein reaction of casein hydrolysate, for the amount of free amino groups of the MCH was decreased (i.e., the MCH had a lower DH than the original hydrolysate). The data also show that the higher decrease of free amino groups of the MCH, the higher activity.
Table 4.
ACE-inhibitory activities in vitro of five modified casein hydrolysates subjected to Neutrase- catalyzed plastein reaction
| Samplea | Decreased amount of free amino groups (μmol⋅g−1 peptides) | ICb50 (μg⋅mL−1) |
|---|---|---|
| CH | 0 | 40.4 ± 2.0 A |
| MCH 1 | 89.8 | 31.1 ± 1.5 B |
| MCH 2 | 102.1 | 22.0 ± 0.5 C |
| MCH 3 | 137.1 | 18.2 ± 0.7 D |
| MCH 4 | 176.4 | 16.0 ± 1.0 DE |
| MCH 5 | 210.0 | 14.7 ± 0.2 E |
aCH, casein hydrolysate; MCH 1–5, modified casein hydrolysate prepared with reaction time of 2, 3, 4, 5 and 6.3 h, respectively
bThe values represent the solution concentration of the sample needed to inhibit ACE by 50% under assaying conditions. Different capital letters after the values indicate that one-way ANOVA of the means is significantly different (P <0.05)
It is revealed in Fig. 1 that insufficient or excess hydrolysis of casein would lead to the casein hydrolysate a lower or higher DH and a lower or similar ACE-inhibitory activity. The data in Table 4 indicate that the plastein reaction improved the activity of the MCH although it had a lowered DH. In the previous work (Zhao and Li 2009), the modified casein hydrolysates prepared by Alcalase-catalyzed plastein reaction also had an improved activity (IC50 = 0.5 μg⋅mL−1), which supported the present result. Alcalase has specificity mainly for hydrophobic amino acids while Neutrase has specificity mainly for leucine and phenylalanine (Kunst 2003). This fact highlights that the protease used in the plastein reaction should be a key factor to mediate ACE-inhibitory activity of the modified hydrolysate, and the possible impacts of various proteases are needed to be investigated in the later study. One speculation is provided here to explain why the carried out plastein reaction enhanced the activity of the MCH. Due to the specify of Neutrase used in the present study, some hydrophobic amino acids such as leucine and phenylalanine in some peptides might be attached covalently into other peptides by transpeptidation, or some peptides with hydrophobic amino acids were conjugated covalently into other peptides by condensation. All these reactions might lead to these newly formed peptides higher content of hydrophobic amino acids and thus higher ACE-inhibitory activity, as it had been revealed that most naturally occurring ACE-inhibitory peptides are rich in hydrophobic amino acids (Cheung et al. 1980; Suetsuna and Nakano 2000). More studies are thus needed to investigate the peptide compositions in the MCH in later work to reveal the most active peptides.
Conclusion
Hydrolysis of casein by Neutrase to a DH of 13.0% could give casein hydrolysate ACE inhibition in vitro with an IC50 value of 40.4 μg⋅mL−1. With the decreased amount of free amino groups of the modified casein hydrolysate as the response, Neutrase-catalyzed plastein reaction of casein hydrolysate was optimized with single factor experiments and response surface methodology to obtain some suitable conditions as following: E/S ratio of 3.0 kU⋅g−1 peptides, substrate concentration of 62% (w/w), reaction temperature of 30 °C and reaction time of 6.3 h. Under these optimized conditions, the decreased amount of free amino groups of the modified hydrolysate was 210.0 μmol⋅g−1 peptides, while the corresponding IC50 value was lowered to 14.7 μg⋅mL−1. The result revealed that the Neutrase-catalyzed plastein reaction could be served as an effective ways to enhance the ACE-inhibitory activity of casein hydrolysate. It is highlighted that the specificity of protease used in the plastein reaction and the peptides formed during the plastein reaction should be studied in later work.
Acknowledgement
This work was funded by the National Natural Science Foundation of China (No. 30972132) and the Innovative Research Team of Higher Education of Heilongjiang Province (No. 2010td11). The authors also thank anonymous reviewers and the editors for their constructive and valuable works to our paper.
References
- Adler-Nissen J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J Agric Food Chem. 1979;27:1256–1261. doi: 10.1021/jf60226a042. [DOI] [PubMed] [Google Scholar]
- Andrews AT, Alichanidis E. The plastein reaction revisited: evidence for a purely aggregation reaction mechanism. Food Chem. 1990;35:243–261. doi: 10.1016/0308-8146(90)90015-V. [DOI] [Google Scholar]
- Ariyoshi Y. Angiotensin-converting enzyme inhibitors derived from food proteins. Trends Food Sci Tech. 1993;4:139–144. doi: 10.1016/0924-2244(93)90033-7. [DOI] [Google Scholar]
- Ashley DVM, Temler R, Barclay D, Dormond CA, Jost R. Amino acid-enriched plasteins: a source of limiting amino acids for the weanling rat. J Nutr. 1983;113:21–27. doi: 10.1093/jn/113.1.21. [DOI] [PubMed] [Google Scholar]
- Cheung HS, Wang FL, Ondetti MA, Sabo E, Cushman DW. Binding of peptide substrates and inhibitors of angiotensin converting enzyme. J Biol Chem. 1980;255:401–407. [PubMed] [Google Scholar]
- Church FC, Swaisgood HE, Porter DH, Catignani GL. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J Dairy Sci. 1983;66:1219–1227. doi: 10.3168/jds.S0022-0302(83)81926-2. [DOI] [Google Scholar]
- Combes D, Lozano P. α-Chymotrypsin in plastein synthesis. Influence of water activity. Ann NY Acad Sci. 1992;672:409–414. doi: 10.1111/j.1749-6632.1992.tb32706.x. [DOI] [Google Scholar]
- Contreras MM, Carrón R, Montero MJ, Ramos M, Recio I. Novel casein-derived peptides with antihypertensive activity. Int Dairy J. 2009;19:566–573. doi: 10.1016/j.idairyj.2009.05.004. [DOI] [Google Scholar]
- da Costa EL, Gontijo JAD, Netto FM. Effect of heat and enzymatic treatment on the antihypertensive activity of whey protein hydrolysates. Int Dairy J. 2007;17:632–640. doi: 10.1016/j.idairyj.2006.09.003. [DOI] [Google Scholar]
- Dey SS, Dora KC (2011) Optimization of the production of shrimp waste protein hydrolysate using microbial proteases adopting response surface methodology. J Food Sci Technol, available online (DOI: 10.1007/s13197-011-0455-4) [DOI] [PMC free article] [PubMed]
- FitzGerald RJ, Murray BA, Walsh DJ. Hypotensive peptides from milk proteins. J Nutr. 2004;134:980S–988S. doi: 10.1093/jn/134.4.980S. [DOI] [PubMed] [Google Scholar]
- Fujimaki M, Kato M, Aria S, Yamashita M. Application of microbial proteinase to soybean and other materials to improve acceptability. J Appl Bacteriol. 1971;34:119–131. doi: 10.1111/j.1365-2672.1971.tb02272.x. [DOI] [PubMed] [Google Scholar]
- Guo Y, Pan D, Tanokura M. Optimisation of hydrolysis conditions for the production of the angiotensin-I-converting enzyme (ACE) inhibitory peptides from whey protein using response surface methodology. Food Chem. 2009;114:328–333. doi: 10.1016/j.foodchem.2008.09.041. [DOI] [Google Scholar]
- IDF (1993) Determination of the nitrogen (Kjeldahl method) and calculation of the crude protein content. In: IDF Standard 20B. International Dairy Federation, Brussels, Belgium
- Janitha PK, Wanasundara PD, Ross ARS, Amarowicz R, Ambrose SJ, Pegg RB, Shand PJ. Peptides with angiotensin I-converting enzyme (ACE) inhibitory activity from defibrinated, hydrolyzed bovine plasma. J Agric Food Chem. 2002;50:6981–6988. doi: 10.1021/jf025592e. [DOI] [PubMed] [Google Scholar]
- Jiang J, Chen S, Ren F, Luo Z, Zeng SS. Yak milk casein as a functional ingredient: preparation and identification of angiotensin-I-converting enzyme inhibitory peptides. J Dairy Res. 2007;74:18–25. doi: 10.1017/S0022029906002056. [DOI] [PubMed] [Google Scholar]
- Kunst T. Protein modification to optimize functionality: protein hydrolysates. In: Whitaker JR, Voragen AFJ, Wong DWS, editors. Handbook of food enzymology. Now York: Marcel Dekker; 2003. pp. 221–236. [Google Scholar]
- López-Fandiño R, Otte J, van Camp J. Physiological, chemical and technological aspects of milk-protein-derived peptides with antihypertensive and ACE-inhibitory activity. Int Dairy J. 2006;16:1277–1293. doi: 10.1016/j.idairyj.2006.06.004. [DOI] [Google Scholar]
- Lozano P, Combes D. α-Chymotrypsin in plastein synthesis: influence of substrate concentration on enzyme activity. Biotechnol Appl Biochem. 1991;14:212–221. [PubMed] [Google Scholar]
- Mao XY, Ni JR, Sun WL, Hao PP, Fan L. Value-added utilization of yak milk casein for the production of angiotensin-I-converting enzyme inhibitory peptides. Food Chem. 2007;103:1282–1287. doi: 10.1016/j.foodchem.2006.10.041. [DOI] [Google Scholar]
- Marambe PWMLHK, Shand PJ, Wanasundara JPD. An in-vitro investigation of selected biological activities of hydrolysed flaxseed (Linum usitatissimum L.) proteins. J Am Oil Chem Soc. 2008;85:1155–1164. doi: 10.1007/s11746-008-1293-z. [DOI] [Google Scholar]
- Meisel H. Biochemical properties of bioactive peptides derived from milk proteins: potential nutraceuticals for food and pharmaceutical applications. Livest Prod Sci. 1997;50:125–138. doi: 10.1016/S0301-6226(97)00083-3. [DOI] [Google Scholar]
- Miguel M, Contreras MM, Recio I, Aleixandre A. ACE—inhibitory and antihypertensive properties of a bovine casein hydrolysate. Food Chem. 2009;112:211–214. doi: 10.1016/j.foodchem.2008.05.041. [DOI] [Google Scholar]
- Murray BA, Walsh DJ, FitzGerald RJ. Modification of the furanacryloyl-L– phenylalanyl-glycylglycine assay for determination of angiotensin-I-converting enzyme inhibitory activity. J Biochem Biophys Meth. 2004;59:127–137. doi: 10.1016/j.jbbm.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Yamamoto N, Sakai K, Okubo A, Yamazaki S, Takano T. Purification and characterization of angiotensin-I converting enzyme inhibitors from a sour milk. J Dairy Sci. 1995;78:777–783. doi: 10.3168/jds.S0022-0302(95)76689-9. [DOI] [PubMed] [Google Scholar]
- Naqash SY, Nazeer RA (2011) Antioxidant and functional properties of protein hydrolysates from pink perch (Nemipterus japonicus) muscle. J Food Sci Technol, available online (DOI: 10.1007/s13197-011-0416-y) [DOI] [PMC free article] [PubMed]
- Ortiz-Chao P, Gómez-Ruiz JA, Rastall RA, Mills D, Cramer R, Pihlanto A, Korhonen H, Jauregi P. Production of novel ACE inhibitory peptides from β-lactoglobulin using Protease N Amano. Int Dairy J. 2009;19:69–76. doi: 10.1016/j.idairyj.2008.07.011. [DOI] [Google Scholar]
- Otte J, Shalaby SM, Zakora M, Pripp AH, El-Shabrawy SA. Angiotensin- converting enzyme inhibitory activity of milk protein hydrolysates: effect of substrate, enzyme and time of hydrolysis. Int Dairy J. 2007;17:488–503. doi: 10.1016/j.idairyj.2006.05.011. [DOI] [Google Scholar]
- Pallavicini C, Finley JW, Stanley WL, Watters GG. Plastein synthesis with α-chymotrypsin immobilised on chitin. J Sci Food Agric. 1980;31:273–278. doi: 10.1002/jsfa.2740310311. [DOI] [Google Scholar]
- Pihlanto A, Virtanen T, Korhonen H. Angiotensin I converting enzyme (ACE) inhibitory activity and antihypertensive effect of fermented milk. Int Dairy J. 2010;20:3–10. doi: 10.1016/j.idairyj.2009.07.003. [DOI] [Google Scholar]
- Rafieian F, Keramat J, Kadivar M (2011) Optimization of gelatin extraction from chicken deboner residue using RSM method. J Food Sci Technol, available online (DOI: 10.1007/s13197-011-0355-7) [DOI] [PMC free article] [PubMed]
- Robert MC, Razaname A, Mutter M, Juillerat MA. Identification of angiotensin- I-converting enzyme inhibitory peptides derived from sodium caseinate hydrolysates produced by Lactobacillus helveticus NCC 2765. J Agric Food Chem. 2004;52:6923–6931. doi: 10.1021/jf049510t. [DOI] [PubMed] [Google Scholar]
- Sarath G, De La Motte RS, Wagner FW. Protease assay methods. In: Beynon RJ, Bond JS, editors. Proteolytic enzymes, a practical approach. Oxford: IRL Press; 1989. pp. 25–55. [Google Scholar]
- Shalaby SM, Zakora M, Otte J. Performance of two commonly used angiotensin- converting enzyme inhibition assays using FA-PGG and HHL as substrates. J Dairy Res. 2006;73:178–186. doi: 10.1017/S0022029905001639. [DOI] [PubMed] [Google Scholar]
- Spellman D, McEvoy E, O’Cuinn G, FitzGerald RJ. Proteinase and exopeptidase hydrolysis of whey protein: comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis. Int Dairy J. 2003;13:447–453. doi: 10.1016/S0958-6946(03)00053-0. [DOI] [Google Scholar]
- Stevenson DE, Morgan KR, Fenton GA, Moraes G. Use of NMR and mass spectrometry to detect and quantify protease-catalyzed peptide bond formation in complex mixtures. Enzyme Microb Technol. 1999;25:357–363. doi: 10.1016/S0141-0229(99)00053-8. [DOI] [Google Scholar]
- Suetsuna K, Nakano T. Identification of an antihypertensive peptide from peptic digest of wakame (Undaria pinnatifida) J Nutr Biochem. 2000;11:450–454. doi: 10.1016/S0955-2863(00)00110-8. [DOI] [PubMed] [Google Scholar]
- Sukan G, Andrews AT. Application of the plastein reaction to caseins and to skim milk powder I. Protein hydrolysis and plastein formation. J Dairy Res. 1982a;49:265–278. doi: 10.1017/S0022029900022366. [DOI] [Google Scholar]
- Sukan G, Andrews AT. Application of the plastein reaction to caseins and to skim milk powder II. Chemical and physical properties of the plasteins. J Dairy Res. 1982b;49:279–293. doi: 10.1017/S0022029900022378. [DOI] [Google Scholar]
- Sun Q, Shen H, Luo Y. Antioxidant activity of hydrolysates and peptide fractions derived from porcine hemoglobin. J Food Sci Tech. 2011;48:53–60. doi: 10.1007/s13197-010-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RJH, Brownsell VL, Andrews AT. Application of the plastein reaction to mycoprotein: I. Plastein synthesis. Food Chem. 2001;72:329–335. doi: 10.1016/S0308-8146(00)00233-8. [DOI] [Google Scholar]
- Yamamoto N. Antihypertensive peptides derived from food proteins. Biopolymers. 1997;43:129–134. doi: 10.1002/(SICI)1097-0282(1997)43:2<129::AID-BIP5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Arai S, Fujimaki M. Plastein reaction for food protein improvement. J Agric Food Chem. 1976;24:1100–1104. doi: 10.1021/jf60208a038. [DOI] [Google Scholar]
- Yamashita M, Arai S, Fujimaki M. A low-phenylalenine, high-tyrosine plastein as an acceptable dietetic food. J Food Sci. 1976;41:1029–1032. doi: 10.1111/j.1365-2621.1976.tb14382.x. [DOI] [Google Scholar]
- Ymashita M, Arai S, Tsai SJ, Fujimaki M. Plastein reaction as a method for enhancing the sulfur-containing amino acid level of soybean protein. J Agric Food Chem. 1971;19:1151–1154. doi: 10.1021/jf60178a029. [DOI] [PubMed] [Google Scholar]
- Zhao XH, Li YY. An approach to improve ACE-inhibitory activity of casein hydrolysates with plastein reaction catalyzed by Alcalase. Euro Food Res Tech. 2009;229:795–805. doi: 10.1007/s00217-009-1110-4. [DOI] [Google Scholar]