Abstract
The bacteriocin susceptibility of Listeria monocytogenes MTCC 657, Enterococcus faecium DSMZ 20477, E. faecium VRE, and E. faecalis ATCC 29212 and their corresponding bacteriocin resistant variants was assessed. The single and combined effect of nisin and pediocin 34 and enterocin FH99 bacteriocins produced by Pediococcus pentosaceus 34, and E. faecium FH99, respectively, was determined. Pediocin34 proved to be more effective in inhibiting L. monocytogenes MTCC 657. A greater antibacterial effect was observed against E. faecium DSMZ 20477 and E. faecium (VRE) when the a combination of nisin, pediocin 34 and enterocin FH99 were used whereas in case of L. monocytogenes MTCC 657 a combination of pediocin 34 and enterocin FH99 was more effective in reducing the survival of pathogen. Bacteriocin cross-resistance and the antibiotic susceptibility of wild type and their corresponding resistant variants were assessed and results showed that resistance to a bacteriocin may extend to other bacteriocins within the same class and also the acquired resistance to bacteriocins can modify the antibiotic susceptibility/resistance profile of the bacterial species used in the study. According to the hydrophobicity nisin resistant variant of L. monocytogenes was more hydrophobic (p < 0.001), whereas the pediocin 34 and enterocin FH99 resistant variants were less hydrophobic than the wild type strain. Nisin, pediocin 34 and enterocin FH99 resistant variants of E. faecium DSMZ 20477 and E. faecium VRE were less hydrophobic than their wild type counterparts. Nisin resistant E. faecalis ATCC 29212 was less hydrophobic than its wild type counterpart.
Keywords: Nisin, Pediocin 34, Enterocin FH99, Enterococcus, Listeria
Introduction
For the past few decades, food safety has been an important issue globally due to increasing food-borne diseases and change in food habits. Illness caused due to the consumption of contaminated foods has a wide economic and public health impact worldwide. Therefore, the need to avoid economic losses due to microbial spoilage of raw materials and food products, the preservation of foods by natural, biological methods may be a satisfactory approach to solve many of the current food-related issues. Bacteriocins are ribosomally-synthesized peptides or proteins with antimicrobial activity, produced mainly by lactic acid bacteria (LAB). Several LAB bacteriocins with broad spectra of inhibitory activity offer potential applications in food biopreservation (Galvez et al. 2008). Nisin is a well known broad spectrum bacteriocin active against Gram-positive pathogens associated with food. Its use as food biopreservative is limited by its effectiveness against Gram-negative bacteria. Although the use of bacteriocins for preservation (biopreservation) is a novel approach to eliminating or controlling pathogens in food, the development of highly tolerant or resistant strains remains the main concern and decreases the efficiency of bacteriocins as biopreservatives. The various possible mechanisms involved in the development of resistance to nisin and Class IIa bacteriocins among the food-borne pathogens have been reviewed by Kaur et al. (2010).
Food application of pediocins and enterocins can provide a good alternative, besides nisin, in protecting food against food borne pathogens. As products of lactic acid bacteria, they provide natural means of preservation and can be accepted by the consumers in the way nisin became accepted. As the trend of consumption of minimal processed and preserved foods is increasing, use of pediocins by the food industry could offer solutions and provide alternatives to conventional preservation means. Importantly, enterocins also show a strong activity against Listeria, which can be of practical use in the food industry (Giraffa 1995; Galvez et al. 1998; Nunez et al. 1997). Application of enterococcal bacteriocins on dairy foods has been the focus of many investigations (Foulique Moreno et al. 2006; Giraffa 1995). Enterococcus strains displaying a limited inhibitory spectrum due to the production of enterocins targeted towards Listeria and/or Clostridium (Franz et al. 1996; Giraffa 1995; Torri Tarelli et al. 1994) would be interesting as protective cultures for cheese manufacture, given their very limited antagonistic activity towards dairy starter cultures such as Lactococcus and Streptococcus (Foulique Moreno et al. 2006; Sarantinopoulos et al. 2002). Recently, Lactobacillus brevis FPTLB3 isolated from freshwater fish has been reported to produce bacteriocin that had broad spectrum of inhibition (3200 AU/ml) against Escherichia coli MTCC 1563, Enterococcus faecalis MTCC 2729, Lactobacillus casei MTCC 1423, Lactobacillus sakei ATCC 15521 and Staphylococcus aureus ATCC 25923 (Banerjee et al. 2011).
The objective of our study was to evaluate the antibacterial efficacy of nisin, pediocin 34 (produced by Pediococcus pentosaceous 34) and enterocin FH99 (produced by Enterococcus faecium FH99) either alone or in combinations against Gram-positive bacteria i.e. E. faecium DSMZ 20477, E. faecium (VRE), E. faecalis ATCC 29212 and L. monocytogenes MTCC 657. The cross resistance of the bacteriocin resistant variants to various antibiotics and three bacteriocins viz, nisin, pediocin 34 and enterocin FH99, was investigated. Also in this study the surface properties such as surface hydrophobicity, which could impair the interaction of the antimicrobial peptides with the cytoplasmic membrane, were compared between the wild type and the resistant variants.
Materials and methods
Bacterial strains and culture conditions
E. faecium FH99, bacteriocinogenic strain was an isolate from human feces (Gupta et al. 2010). Pediococcus pentosaceous 34 (Rao and Malik 2003), a bacteriocinogenic strain was an isolate from cheddar cheese, Pediococcus acidilactici LB 42 (a sensitive strain used for detection of bacteriocin producers), was obtained from , Department of Animal Science, University of Wyoming, Laramie Wyoming, USA. E. faecalis DSMZ 20477 was obtained from , Institute of Microbiology and Toxicology, Federal Research Centre for Nutrition, Karlsruhe, Germany. E. faecium VRE (a vancomycin resistant strain isolated from human feces). E. faecalis 29212 was procured from American Type Culture Collection (ATCC) while L. monocytogenes MTCC 657 was procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology, Chandigarh, India. Table 1 shows the culture conditions and the culture medium used for the bacterial cultures.
Table 1.
Bacterial strains used in the study and culture conditions
| Bacteria | Strains | Culture Conditions |
|---|---|---|
| Pediococcus pentosaceous 34 | Bacteriocinogenic strain ; Lab. isolate | 37 °C, MRS |
| Enterococcus faecium FH99 | Bacteriocinogenic strain ; Lab. isolate | 37 °C, MRS |
| Pediococcus acidilactici | LB 42 (Indicator strain) | 37 °C, MRS |
| Enterococcus feacalis | ATCC 29212 | 37 °C, BHI |
| Listeria monocytogenes | MTCC 657 | 37 °C, BHI |
| Enterococcus faecium | DSMZ 20477 | 37 °C, BHI |
| Enterococcus faecium VRE | Vancomycin Resistant Strain (VRE) Lab. isolate | 37 °C, BHI |
DSMZ Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (German Collection of Microorganisms and Cell Cultures)
MTCC Microbial Type Culture Collection (Chandigarh, India)
ATCC American Type Culture Collection
BHI Brain Heart Infusion Broth
MRS de Man Rogosa and Shrape
Bacteriocin solutions preparation
One hundred milliliter aliquots of MRS broth (De Man et al. 1960) (pH 6.5) (HiMedia, Mumbai) were inoculated with active culture of E. faecium FH99 and Pediococcus pentosaceus 34 was inoculated (1%) and incubated at 37 °C for 24 h. Cell free culture supernatant (CFCS) were prepared by centrifugation of the cultures in refrigerated centrifuge at 10,000 rpm for 10 min. The supernatant was filter sterilized by passing through a 0.2 μm (Millipore), 45 mm diameter membrane filter and used for partial purification after neutralization. Crude enterocin FH99 and pediocin 34 were precipitated from broth media by 60% ammonium sulphate precipitation and the precipitates were dissolved in sterilized Milli Q water. The bacteriocins were purified by the method earlier described by Gupta et al. (2010). Nisin A (Nisaplin ®) was procured from Danisco (Gurgaon, India). Nisin stock solutions were prepared from pure nisin in 0.02 N HCl and autoclaved (Crandall and Montville 1998).
Antimicrobial activity assays
Measurement of activity units (AU/ml)
The antibacterial activity of nisin, pediocin 34 and enterocin FH99 was obtained using the spot on lawn assay as described by Ulhman et al. (1992), against Pediococcus acidilactici LB 42. Five microlitres of serial dilutions of the partially purified bacteriocin of Enterococcus faecium FH99 and Pediococcus pentosaceus 34 grown in MRS broth (De Man et al. 1960) were spotted on the Tryptone Glucose Yeast Extract (TGE) agar plates (Biswas et al. 1991) (1.5% agar). Before spotting, TGE agar plates were overlaid with TGE soft agar (0.75%) seeded with actively growing cells of the test organism. Plates were kept undisturbed for 3–4 h for diffusion of bacteriocin through agar and then incubated. The sensitivity of the strain in question was evaluated by checking for clear zones around the spots. Three independent replicates of experiment were done. The activity units of the culture broth were calculated using the following formula and expressed as activity units per ml:
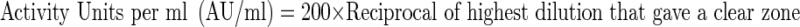 |
Bacteriocin susceptibility test
The bacteriocin susceptibility & MICs of the target strains i.e. Listeria monocytogenes MTCC 657, Enterococcus faecium DSMZ 20477, E. faecium VRE, and E. faecalis ATCC 29212 was tested by the spot on lawn assay. For MIC determinations, 5 μl of a 1:2 dilution series of a bacteriocin solution was placed in wells. The Minimum Inhibitory Concentration (MIC) value was interpreted as the lowest concentration of bacteriocin that resulted in a clear inhibition halo after 18 h incubation at 37 °C. The MIC was defined as the lowest concentration of bacteriocin that induced an inhibition zone.
Kinetics of cell growth inhibition by bacteriocins
Overnight cultures of E. faecium DSMZ 20477, E. faecium (VRE), E. faecalis ATCC 29212 and L. monocytogenes MTCC 657 were inoculated into fresh BHI broth tubes (1%) containing either pediocin 34 and enterocin FH99. These bacteriocins were used individually or in combination; the bacteriocin amounts used in the mixtures correspond to the calculated MICs of each bacteriocin. Additionally, the efficacy of nisin, pediocin 34 and enterocin FH99 in combination (half the concentration of MICs for each bacteriocin) was also evaluated. At different time intervals (1, 2, 4, 6 and 24 h) the survivors were enumerated on Brain heart infusion (BHI) agar medium after appropriate dilutions in saline solutions, and colonies were counted after 24–48 h of incubation at 37 °C. Three independent replicates of experiment were done.
Isolation of spontaneous bacteriocin resistant variants
Spontaneous resistant mutants of strains E. faecalis ATCC 29212, E. faecium DSMZ 20477, E. faecium VRE and L. monocytogenes MTCC 657 to nisin, pediocin 34 and enterocin FH99 were isolated after sequential exposure to a bacteriocin concentration 10-fold higher the MIC. Only nisin resistant variant for E. faecalis ATCC 29212 was developed since it was already resistant to pediocin 34 and enterocin FH99. The stability of these resistances in cultures without bacteriocins was checked and determined by MICs.
Bacteriocin cross-resistance by agar diffusion method
The sensitivity of E. faecium DSMZ 20477, E. faecium (VRE), E. faecalis ATCC 29212 and L. monocytogenes MTCC 657 and their resistant variants to nisin, pediocin 34 and enterocin FH99 were qualitatively determined by the agar well diffusion method (Barefoot and Klaenhammer 1983). Briefly, 5 ml of molten TGE agar (Biswas et al. 1991) containing 0.75% (w/v) agar medium were cooled at 47 °C and seeded with 1% (v/v) overnight culture of Listeria monocytogenes MTCC 657, E. faecium DSMZ 20477, E. faecium (VRE) and E. faecalis ATCC 29212 or their nisin, pediocin 34 or enterocin FH99 resistant variants. Seeded agar was then poured onto TGE agar plate and allowed to solidify at room temperature. Wells (8 mm) were cut in the solidified agar using a sterile metal cork borer and filled with 80 μl of sample. The plates were left at 5 °C for 2 h to allow diffusion of the tested aliquot and then incubated for 18 h at 37 °C. Absence or presence of inhibition zones as well as their diameters were recorded.
Antibiotic disk diffusion susceptibility test
Pattern of resistance/susceptibility to antibiotics of wild type and bacteriocin resistant variants isolates was studied by disc diffusion method as recommended by National Committee for Clinical Laboratory Standards (CLSI; Wayne, PA, USA). Antibiotic discs containing ampicillin (10 μg), chloramphenicol (30 μg), erythromycin (15 μg), gentamicin (10 μg), penicillin G (10 units), novobiocin (30 μg), bacitracin (10 μg), streptomycin (10 μg), tetracycline (30 μg), vancomycin (30 μg), rifampicin (5 μg), nalidixic acid and kanamycin (30 μg) were obtained from HiMedia. All the measurements were done in triplicate.
Cell surface hydrophobicity
Cell surface hydrophobicity of wild type E. faecium DSMZ 20477, E. faecium (VRE), E. faecalis ATCC 29212 and L. monocytogenes MTCC 657 and their bacteriocin resistant variants was determined according to the method described by Rosenberg et al. (1980) with slight modification using n-Hexadecane. Cultures of the strains were grown in BHI broth overnight at 37 °C. The cells (8 log10 cfu/ml) were harvested by centrifugation at 12,000 x g for 5 min at 5 °C, washed twice and resuspended in 5 ml phosphate urea magnesium (PUM) buffer (K2HPO4: 22.2 g; KH2PO4: 7.26 g; Urea: 1.8 g; MgSO4.7H2O: 0.2 g; in 1000 ml of distilled water; pH 7.1) and the cell suspension was adjusted to an absorbance value (A610) of approx. 0.8–1.0. Three ml of the bacterial suspension were put in contact with 1 ml of each of n-Hexadecane. The cells were pre-incubated at 37 °C for 10 min and then vortexed for 120 s. The suspension was then kept undisturbed at 37 °C for 1 h to allow phase separation and the hydrocarbon layer was allowed to rise completely. After 1 h, aqueous phase was removed carefully with a Pasteur pipette and the absorbance (A610) was measured using Spectrophotometer (Jenway Geneva, Jenway Ltd. Gransmore Green, Felsted, Dunmow, UK). The decrease in the absorbance was taken as a measure of the cell surface hydrophobicity (%Hydrophobicity) calculated with the equation:
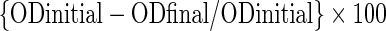 |
Where ODinitial and ODfinal are the absorbance (at 610 nm) before and after extraction with the n-Hexadecane. Three independent replicates of experiment were done.
Statistical analysis
Results were expressed as mean±SE of triplicates for each sample. Calculation of mean, standard Error (SE) was performed by subjecting data to various statistical analyses as and when needed, using SYSTAT 6.0.1., Statistical Software Package, 1996, ‘SPSS, Inc. (Richmond, CA, USA)’, Microsoft R excel 2000 Software Package, Microsoft Corporation,(Redmond, WA,USA). A two-way ANOVA was performed for the data on the evaluation of antibacterial efficacy of the bacteriocins individually and in combination against the indicator organisms and the significance (P < 0.05) was evaluated by Duncan’s multiple range test.
Results and discussion
Bacteriocin sensitivity profile of E. faecalis, E. faecium and L. monocytogenes strains
Antibacterial efficiency of three bacteriocins nisin, pediocin 34 (bacteriocin produced by Pediococcus pentosaceous 34) and enterocin FH99 (bacteriocin produced by Enterococcus faecium FH99), was evaluated against Gram positive food spoilage and pathogenic bacteria. Table 2 depicts the susceptibility of the target strains to nisin, pediocin 34 and enterocin FH99 bacteriocins. The Gram positive bacterial species studied in this work differed considerably in their sensitivity to nisin, pediocin 34 and enterocin FH99 as also reported by Bankerroum and Sandine (1988) and Ukuku and Shelef (1997) indicating important genus, species and strain differences in the degree of inhibition. E. faecalis ATCC 29212 was sensitive to nisin only. E. faecium DSMZ 20477, E. faecium (VRE) and L. monocytogenes MTCC 657 were sensitive to all the bacteriocins used in the study. Table 3 shows the MICs of the wild type strains E. faecalis ATCC 29212, E. faecalis DSMZ 20477, E. faecium (VRE) and L. monocytogenes MTCC 657 as determined by the spot-on-lawn assay. Pediocin 34 was more effective than nisin & enterocin FH99 in inhibiting L. monocytogenes MTCC 657. The MICs of the developed bacteriocin resistant variants are shown in Table 3.
Table 2.
Susceptibility of wild type strains and resistant variants to nisin, pediocin 34 and enterocin FH99 bacteriocins
| Culture | Strain | Nisin | Pedicin 34 | Enterocin FH99 |
|---|---|---|---|---|
| L. monocytogenes ATCC 53135 | WT | + | + | + |
| Nr | − | + | + | |
| Pr | + | − | − | |
| Er | + | + | − | |
| L. monocytogenes MTCC 657 | WT | + | + | + |
| Nr | − | + | + | |
| Pr | + | − | − | |
| Er | + | + | − | |
| E. faecium DSMZ 20477 | WT | + | + | + |
| Nr | − | − | − | |
| Pr | + | − | − | |
| Er | + | − | − | |
| E. faecium VRE | WT | + | + | + |
| Nr | − | − | − | |
| Pr | + | − | − | |
| Er | + | − | − | |
| E. feacalis ATCC 29212 | WT | + | − | − |
| Nr | − | − | − |
WT wild type, Nr nisin resistant, Pr Pedioicn 34 resistant, Er Enterocin FH99 resistant
With Halo production (+), without halo production (−)
Table 3.
MICs of nisin, pediocin 34 and enterocin FH99 bacteriocins
| Pathogen | Nisin (IU/ml) | P ediocin 34 (AU/ml) | Enterocin FH99 (AU/ml) |
|---|---|---|---|
| E. faecalis ATCC 29212 | 26.5 | ND | ND |
| Listeria monocytogenes MTCC 657 | 50 | 600 | 700 |
| E. faecium DSMZ 20477 | 53.5 | 70 | 937.5 |
| E. faecium VRE | 14 | 2187.5 | 3750 |
ND Not Determined
E.faecalis ATCC 29212 was inherently resistant to Pediocin 34 and Enterocin FH99
Kinetics of cell growth inhibition by bacteriocins
In the present study, the antibacterial efficacy of nisin, pediocin 34 and enterocin FH99 was evaluated singly as well as in different combinations against several Gram-positive bacteria i.e. E. faecalis ATCC 29212, E. faecium DSMZ 20477, E. faecium (VRE) and L. monocytogenes MTCC 657 in BHI broth. The calculated MICs of the bacteriocins were used to evaluate the antibacterial effect of bacteriocins alone (Table 3). In order to evaluate additive and synergistic effect of bacteriocins the different combinations and the concentrations of bacteriocins used against the target organisms are shown in Table 4. To evaluate the additive effect the concentrations of the bacteriocins in each combination used correspond to the MICs of the target organisms, whereas in order to evaluate the synergistic effect, the bacteriocins were used at half the concentrations of the MICs of the target organisms.
Table 4.
Concentrations of nisin, Pediocin 34 and Enterocin FH99 used alone and in different combinations to evaluate cell growth inhibition by bacteriocins
| ADDITIVEa | SYNERGISTICb | |||||||
|---|---|---|---|---|---|---|---|---|
| Pathogen | N+P | N+E | E+P | N+P+E | N+P | N+E | E+P | N+P+E |
| Listeria monocytogenes MTCC 657 | 50 + 600 | 50 + 700 | 600 + 700 | 50 + 600 + 700 | 25 + 300 | 25 + 350 | 300 + 350 | 25 + 300 + 350 |
| E. faecium DSMZ 20477 | 53.5 + 70 | 53.5 + 937.5 | 937.5 + 70 | 53.5 + 70 + 937.5 | 26.25 + 35 | 26.25 + 468.75 | 468.75 + 35 | 26.25 + 35 + 468.75 |
| E. faecium VRE | 14 + 2187.5 | 14 + 3750 | 2187.5 + 3750 | 14 + 2187.5 + 3750 | 7 + 1093 | 7 + 1875 | 1093 + 1875 | 7 + 1093 + 1875 |
N Nisin, P Pediocin 34, E Enterocin FH99
Units for concentrations: Nisin = IU/ml, Pediocin 34 = AU/ml, Enterocin FH99 = AU/ml
aCombination of bacteriocins corresponded to MICs of the target organisms
bCombination of bacteriocins correspond to Half the concentration of MICs of target organisms
The bactericidal effectiveness of nisin against E. faecalis, E. faecium DSMZ 20477, E. faecium (VRE) and L. monocytogenes MTCC 657 is shown in Table 5. In case of E. faecalis ATCC 29212, maximum viability loss of 4.3 log cycles was observed after 2 h incubation with nisin. Nisin was most effective in inhibiting the E. faecium DSMZ 20477 followed by enterocin FH99. Pediocin 34 was least effective in inhibiting E. faecium DSMZ 20477. In case of E. faecium (VRE), nisin followed by enterocin FH99 were observed to be more effective than pediocin 34. The viable cell count (log cfu/ml) of target organisms after treatment with nisin, pediocin 34 and enterocin FH99 alone and in different combinations is presented in Table 5. Pediocin 34 proved to be more effective than nisin and enterocin FH99 in inhibiting L. monocytogenes MTCC 657. Cintas et al. (1998) also reported pediocin to be more effective than nisin against some food borne pathogens such as L. monocytogenes. In case of all the bacterial strains it was observed that even when the bacteriocins displayed the most rapid inhibitory activity at 1 h, the survivors resumed growth, reaching the highest cell counts at 24 h. Similar observations were also made by Schillinger et al. (1998), who reported a regrowth of survivors of L. monocytogenes Scott A after exposure to nisin concentrations between 10 and 500 IU/ml as well as with those of Song and Richard (1997), who observed that survivors of L. innocua resumed growth after the addition of nisin, pediocin AcH, and enterococcin EFS2 into TSBYE broth. According to Muriana (1996), several studies indicated the immediate decrease of target cells by one to three log cycles cfu/ml when a bacteriocin was added, with none or little effect on future inoculations.
Table 5.
Viable cell count (log cfu/ml) of L. monocytogenes and Enterococcus strains after treatment with nisin (N), pediocin 34 (P) and enterocin FH99 (E) alone and in different combinations
| Culture | Antibacterial effect of Bacteriocins when used alone | ADDITIVE | SYNERGISTIC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incubation Time | Control | N | P | E | N+P | N+E | E+P | N+P+E | N+P | N+E | E+P | N+P+E | |
| E. faecalis ATCC 29212 | 1 h | 8.4 ± 0.01a,m | 4.6 ± 0.06a,n | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 2 h | 8.4 ± 0.03b,m | 4.0 ± 0.01b,n | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 4 h | 8.2 ± 0.07c,m | 5.9 ± 0.03c,n | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 6 h | 8.3 ± 0.01d,m | 5.8 ± 0.09d,n | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 24 h | 8.2 ± 0.07e,m | 2.3 ± 0.03e,n | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| L. monocytogenes MTCC 657 | 1 h | 7.6 ± 0.09a,m | 6.9 ± 0.09a,n | 5.7 ± 0.06a,o | 6.8 ± 0.07a,p | 5.3 ± 0.04a,q | 4.8 ± 0.11a,r | 3.0 ± 0.05a,s | 6.0 ± 0.10a,t | 3.8 ± 0.04a,u | 3.4 ± 0.05a,v | 2.3 ± 0.03a,w | 3.4 ± 0.05a,x |
| 2 h | 7.5 ± 0.07b,m | 6.9 ± 0.07b,n | 5.9 ± 0.04b,o | 6.5 ± 0.07b,p | 5.2 ± 0.03b,q | 5.2 ± 0.09b,r | 3.6 ± 0.22b,s | 5.0 ± 0.12b,t | 3.9 ± 0.08b,u | 4.3 ± 0.09b,v | 2.3 ± 0.09b,w | 3.5 ± 0.07b,x | |
| 4 h | 7.6 ± 0.07c,m | 7.0 ± 0.07c,n | 5.3 ± 0.03c,o | 7.3 ± 0.09c,p | 5.7 ± 0.11c,q | 5.3 ± 0.12c,r | 3.9 ± 0.05c,s | 7.0 ± 0.14c,t | 3.6 ± 0.09c,u | 4.0 ± 0.09c,v | 2.4 ± 0.09c,w | 4.1 ± 0.05c,x | |
| 6 h | 8.6 ± 0.09d,m | 6.8 ± 0.09d,n | 6.1 ± 0.03d,o | 7.5 ± 0.07d,p | 6.0 ± 0.10d,q | 4.0 ± 0.13d,r | 3.8 ± 0.09d,s | 7.8 ± 0.16d,t | 4.3 ± 0.03d,u | 2.9 ± 0.08d,v | 2.1 ± 0.05d,w | 4.5 ± 0.01d,x | |
| 24 h | 7.1 ± 0.15e,m | 6.8 ± 0.15e,m | 5.6 ± 0.03e,o | 6.4 ± 0.05e,p | 5.1 ± 0.15e,q | 4.2 ± 0.21e,r | 2.4 ± 0.18e,s | 4.3 ± 0.18e,t | 4.5 ± 0.04e,u | 4.5 ± 0.09e,v | 2.0 ± 0.02e,w | 3.9 ± 0.09e,x | |
| E. faecium DSMZ 20477 | 1 h | 6.9 ± 0.04a,m | 5.8 ± 0.09a,n | 6.7 ± 0.02a,m | 6.1 ± 0.04a,p | 6.8 ± 0.05a,m | 6.2 ± 0.09a,r | 6.5 ± 0.02a,s | 5.5 ± 0.015a,t | 3.0 ± 0.09a, u | 4.6 ± 0.09a,v | 4.6 ± 0.05a,w | 2.3 ± 0.03a,x |
| 2 h | 8.2 ± 0.01b,m | 6.5 ± 0.03b,n | 6.9 ± 0.09b,o | 7.3 ± 0.03b,p | 6.8 ± 0.09b,q | 6.1 ± 0.09b,r | 5.3 ± 0.03b,s | 4.9 ± 0.08b,t | 3.3 ± 0.03b,u | 3.4 ± 0.05b,v | 4.4 ± 0.05b,w | 2.3 ± 0.01b,x | |
| 4 h | 7.6 ± 0.08c,m | 7.4 ± 0.07c,m | 7.6 ± 0.09c,m | 7.3 ± 0.03c,m | 7.0 ± 0.02c,q | 6.9 ± 0.09c,r | 6.0 ± 0.09c,s | 5.3 ± 0.03c,t | 3.0 ± 0.05c,u | 3.3 ± 0.09c,v | 5.4 ± 0.09c,w | 2.2 ± 0.05c,x | |
| 6 h | 8.5 ± 0.04d,m | 5.7 ± 0.08d,n | 7.4 ± 0.06d,o | 8.3 ± 0.06d,p | 7.1 ± 0.04d,q | 6.1 ± 0.04d,r | 7.3 ± 0.03d,s | 6.2 ± 0.12d,t | 5.3 ± 0.03d,u | 4.0 ± 0.06d,v | 6.3 ± 0.04d,w | 3.0 ± 0.02d,x | |
| 24 h | 8.9 ± 0.09e,m | 8.0 ± 0.03e,n | 8.4 ± 0.03e,o | 8.5 ± 0.02e,p | 8.3 ± 0.03e,q | 8.3 ± 0.09e,r | 8.2 ± 0.02e,s | 7.3 ± 0.03e,t | 5.3 ± 0.03e,u | 5.0 ± 0.05e,v | 7.6 ± 0.05e,w | 2.3 ± 0.06e,x | |
| E. faecium VRE | 1 h | 5.1 ± 0.21a,m | 3.2 ± 0.04a,n | 3.4 ± 0.09a,o | 4.3 ± 0.06a,p | 2.4 ± 0.11a,q | 3.4 ± 0.22a,r | 5.3 ± 0.12a,s | 2.8 ± 0.11a,t | 2.3 ± 0.03a,u | 2.6 ± 0.09a,v | 3.6 ± 0.09a,w | 1.6 ± 0.06a,x |
| 2 h | 6.8 ± 0.03b,m | 2.4 ± 0.02b,n | 5.6 ± 0.03b,o | 5.3 ± 0.04b,p | 2.7 ± 0.22a,q | 2.1 ± 0.14b,r | 4.6 ± 0.08b,s | 1.6 ± 0.10b,t | 2.8 ± 0.09b,u | 2.3 ± 0.01b,v | 2.4 ± 0.09b,w | 1.4 ± 0.06b,x | |
| 4 h | 6.2 ± 0.12c,m | 2.9 ± 0.05c,n | 4.1 ± 0.05c,o | 4.9 ± 0.05c,p | 4.0 ± 0.23a,q | 2.3 ± 0.04c,r | 4.4 ± 0.07c,s | 2.6 ± 0.19c,t | 3.1 ± 0.01c,u | 2.0 ± 0.03c,v | 2.3 ± 0.09c,w | 2.0 ± 0.09c,x | |
| 6 h | 9.2 ± 0.02d,m | 5.0 ± 0.03d,n | 4.4 ± 0.09d,o | 7.0 ± 0.12d,p | 4.5 ± 0.31a,q | 4.1 ± 0.13d,r | 4.3 ± 0.12d,s | 3.1 ± 0.02d,t | 3.5 ± 0.02d,u | 2.2 ± 0.03d,v | 2.7 ± 0.02d,w | 2.1 ± 0.02d,x | |
| 24 h | 8.3 ± 0.07e,m | 5.4 ± 0.03e,n | 3.4 ± 0.07e,o | 3.6 ± 0.08e,p | 5.0 ± 0.12a,q | 4.1 ± 0.02d,r | 7.3 ± 0.03e,s | 3.0 ± 0.21e,t | 2.4 ± 0.12e,u | 2.1 ± 0.02e,v | 2.4 ± 0.05e,w | 1.6 ± 0.09e,x | |
Values are mean±SE of three independent determinations (n = 3); ND Not detected. Values with different alphaphets, (a,b,c.d,e for incubation time and m,n,o,p,q,r,s,t,u,v,w,x for different bacteriocin treatments) are statistically significant (P < 0.05)
The results of the present study indicate that combinations of different bacteriocins produce a more effective antibacterial effect against E. faecium DSMZ 20477, E. faecium (VRE) and L. monocytogenes MTCC 657 in comparison to the bacteriocins used alone. When the two non nisin bacteriocins were used together, a higher number of survivors were detected than with the pairs containing nisin for E. faecium VRE and E. faecium DSMZ 20477. Also, synergistic action was observed between different combinations of bacteriocins (Table 5) when tested against E. faecium DSMZ 20477, E. faecium (VRE) and L. monocytogenes MTCC 657. Though, a combination of nisin + pediocin 34 + enterocin F99 was most effective against E. faecium DSMZ 20477 and E. faecium (VRE), a combination of pediocin 34 + enterocin FH99 was most effective in inhibiting L. monocytogenes MTCC 657 (Table 5).
Similar results were reported by Hanlin et al. (1993) who while studying the antibacterial efficiency of pediocin AcH and nisin against several Gram-positive bacterial strains, assumed that a mixture containing more than one bacteriocin would have greater bactericidal effect to a sensitive population, since cells resistant to one bacteriocin might be killed by the other bacteriocin. Moreover synergistic effects were reported when the interactions between pairs of bacteriocins from lactic acid bacteria were tested which are in accordance with the results obtained by Mullet-Powell et al. (1998) One possible explanation for the different effectiveness of bacteriocin pairs would be that the bacteriocins used in this study belonged to different classes, which vary considerably in the nature and sequence of amino acid residues as earlier suggested by Moll et al. (1999). The synergistic action of combinations of two different bacteriocins with different structures produced by the same strain has also been reported in agar medium by Limonet et al. (2004). Similar results have been reported by Jamuna et al. (2005) who showed that the bacteriocins from L. acidophilus and L. casei have a better antibacterial activity in combination with Nisin than when used alone against food spoilage and pathogenic organisms in liquid and food systems. Vignolo et al. (2000) also reported that the combined effect of lactocin 705, enterocin CRL35, and nisin against L. monocytogenes FBUNT in meat slurry showed no viable counts after incubation for 3 h. Jamuna and Jeevaratnam (2009) have also reported the synergistic effect of nisin and bacteriocin from Pediococcus acidilactici to be more effective in inhibiting the growth of L. monocytogenes and S. aureus in sealed pouches of vegetable pulav. When pediocin 34 and enterocin FH99 were used alone or in combination, a higher number of survivors were detected than with the pairs containing nisin for the strains of E. faecium. Cross-resistance between bacteriocins has also been observed when the sensitivity of Listeria variants to lactocin 705, enterocin CRL35, and nisin was tested and insensitivity of a variant to lactocin 705 and enterocin CRL35 while retaining sensitivity to nisin, and vice versa, was associated with the mechanism by which a bacteriocin enters the cell following binding to the cell surface, as well as with the ability to form pores in bacterial membranes Vignolo et al. (2000).
Stability of developed resistance
The stability of nisin, pediocin 34 and enterocin FH99 resistance was determined for the resistant variants of E. faecium DSMZ 20477, E. faecium (VRE), E. faecalis ATCC 29212 and L. monocytogenes MTCC 657. We assessed the MICs (Table 3) of the developed bacteriocin resistant variants by the spot on lawn assay as described by Ulhman et al. (1992). Nisin resistant variants of E. faecium DSMZ 20477, E. faecium (VRE), E. faecalis ATCC 29212 and L. monocytogenes MTCC 657 were was 60, 300, 100 and 200 fold more resistant to nisin than their corresponding wild type strains, respectively (data not shown). Pediocin 34 resistant variants of E. faecium DSMZ 20477, E. faecium (VRE) and L. monocytogenes MTCC 657 were was 80, 2 and 6-fold more resistant to pediocin 34 than their corresponding wild type strains, respectively and enterocin FH99 resistant variants of E. faecium DSMZ 20477, E. faecium (VRE) and L. monocytogenes MTCC 657 were was 70, 2 and 5-fold more resistant to enterocin FH99 than their corresponding wild type strains. Harris et al. (1991) detected mutant strains of L. monocytogenes, at frequencies of 10−6 and 10−8, which were able to grow at nisin concentrations 5 to 10 times higher than was the original population.
The nisin resistance phenotype in L.monocytogenes MTCC 657, E. faecium DSMZ 20477, E. faecium VRE and E.faecalis ATCC 29212 was stable during at 50, 40, 20 and 30 successive cultures, respectively, without nisin. The pediocin resistance phenotype in L.monocytogenes MTCC 657, E. faecium DSMZ 20477 and E. faecium VRE was stable during at 20, 30 and 10 successive cultures, respectively, without pediocin 34. The enterocin resistance phenotype in L.monocytogenes MTCC 657, E. faecium DSMZ 20477 and E. faecium VRE was stable during at 30, 30 and 20 successive cultures, respectively, without pediocin 34.
Bacteriocins cross resistance
The bacteriocin cross resistance profiles of wild type and their corresponding nisin, pediocin 34 and enterocin FH99 resistant variants is shown in Table 2. Pediocin 34 resistant variant of L. monocytogenes MTCC 657 displayed cross resistance to enterocin FH99 but not to nisin. On the other hand its nisin resistant variant was sensitive to both pediocin 34 and enterocin FH99. Nisin resistance in E. faecium DSMZ 20477 conferred cross resistance to both pediocin 34 and enterocin FH99. Enterocin FH99 resistant variant displayed cross resistance to pediocin 34 bacteriocin. Pediocin 34 resistant variant of E. faecium DSMZ 20477 showed cross resistance to enterocin FH99. Similar results were observed in case of E. faecium (VRE). Nisin resistant E. faecalis ATCC 29212 was resistant to pediocin 34 and enterocin FH99.
Several reports suggest that resistance to a bacteriocin may extend to other bacteriocins within the same class or even in other classes. The nisin resistant strain of L. monocytogenes ATCC 700302 was observed to be showing cross resistance to the class IIa bacteriocin pediocin PA-1 and the class IV leuconocin S (Crandall and Montville 1998). L. monocytogenes mutants resistant to mesenterocin 52, curvaticin 13, and plantaricin were also reported to be cross-resistant to the other bacteriocins (Rekhif et al. 1994). In addition, piscicolin 126-resistant mutants of L. monocytogenes which emerged in cheese made from milk containing the bacteriocin were also resistant to pediocin P02 (Ukuku and Shelef 1997). These reports of cross-resistance indicate that the use of multiple bacteriocins to achieve greater antibacterial efficacy (Hanlin et al. 1993) might not be feasible. The development of resistance to one of the bacteriocins in the combination might render the organism resistant to the others too.
Antibiotic susceptibility
An undesirable consequence of an extended use of natural antimicrobials such as nisin in food might be cross-resistance to clinically used antibiotics in food-borne pathogens only few studies have comprehensively addressed this issue (Crandall and Montville 1998; Gravesen et al. 2001). In this work, we have analyzed the antibiotic susceptibility of L. monocytogenes MTCC 657, E. faecium DSMZ 20477, E. faecium (VRE) and E. faecalis ATCC 29212 along with their nisin, pediocin 34 and enterocin FH99 resistant counterparts as shown in Table 6.
Table 6.
Susceptibilitya of wild type strains and their bacteriocin resistant counterparts to antibiotics
| Bacterial culture & Strains | Antibiotics | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | V | B | S | K | E | G | Na | A | R | P | C | Nv | Pb | ||
| L. monocytogenes ATCC 53135 | WT | S | M | M | M | M | M | M | R | M | S | M | S | M | M |
| Nr | S | S | M | M | M | S | S | R | S | S | S | S | S | S | |
| Pr | S | M | M | M | M | M | M | R | M | S | M | S | M | M | |
| Er | S | M | M | M | M | M | M | S | M | S | M | S | M | R | |
| L. monocytogenes MTCC 657 | WT | S | S | S | S | S | S | S | R | S | S | S | S | R | S |
| Nr | S | S | S | S | S | S | S | S | S | S | S | S | M | S | |
| Pr | S | S | S | M | M | R | R | R | S | S | M | S | R | S | |
| Er | S | S | S | S | S | S | S | R | S | S | S | S | S | S | |
| E. faecium DSMZ 20477 | WT | S | S | S | R | R | M | R | R | R | R | R | M | R | S |
| Nr | S | S | S | R | R | M | R | R | S | S | S | S | S | S | |
| Pr | S | S | S | R | R | M | R | R | R | R | R | M | R | S | |
| Er | S | S | S | R | R | M | M | R | R | S | S | S | M | S | |
| E. faecium VRE | WT | M | R | S | R | R | R | R | R | R | S | R | S | S | S |
| Nr | S | S | S | R | R | R | R | R | R | S | S | S | S | R | |
| Pr | S | S | S | R | R | R | R | R | R | S | R | S | S | R | |
| Er | S | S | S | R | R | R | R | R | R | S | S | S | S | R | |
| E. faecalis ATCC 29212 | WT | S | S | S | R | M | R | S | S | S | S | S | S | S | R |
| Nr | S | S | S | R | R | R | R | R | S | S | S | S | S | M | |
WT wild type, Nr nisin resistant, Pr Pedioicn 34 resistant, Er Enterocin FH99 resistant
T Tetracycline, V Vancomycin, B Bacitracin, K Kanamycin, E Erythromycin, G Gentamycin, Na Nalidixic acid, A Ampicillin, R Rifampicin, P PenicillinG, C Chloramphenicol, Nv Novobiocin, Pb Polymyxin B
S sensitive; M moderately sensitive; R resistant
aZone of Inhibition calculated according to the table given by NCCLS (2001)
The nisin resistant strains of L. monocytogenes MTCC 657, E. faecium DSMZ 20477, E. faecium (VRE) were more susceptible to most of the antibiotics tested than their wild type counterparts. This could be related to the fitness cost commonly associated to the development of the nisin resistant phenotype i.e. the changes conferring bacteriocin resistance could possibly reduce the growth potential of the cells or render them more sensitive to preservation parameters such as salt, low pH, or low temperature (Dykes and Hastings 1998). Only cross-resistance to the membrane disturbing polymixin B was depicted by the three resistant variants of E. faecium (VRE). This result seems reasonable as nisin shares the same primary target with polymixin B: the cytoplasmic membrane. Also, the resistance has been partially correlated with changes in the membrane composition which potentially interfere with the pore forming ability of nisin in the cytoplasmic membrane (Crandall and Montville 1998; Mazzotta and Montville 1997). Moreover, changes in the cell envelope such as a thickened cell wall, polysaccharide production or a higher degree of D-alanine substitution in the teichoic acids were also described as resistance strategies to avoid killing by cationic antimicrobial peptides (Davies et al. 1996; Peschel et al. 1999). Basically, these mechanisms lower the net negative surface charge and restrict the accessibility of nisin and, hence, of other cationic drugs such as polymixin B and amino glycosides, to their targets.
Pediocin 34 resistant strain of L. monocytogenes MTCC 657 and nisin resistant strain of E. faecalis ATCC 29212 were observed to be resistant to gentamicin. Nisin resistant variant of E. faecalis ATCC 29212 was also resistant to nalidixic acid. The site of action of gentamicin is protein synthesis and nalidixic acid inhibits a subunit of DNA gyrase and induces formation of relaxation complex analogue. It also inhibits the nicking dosing activity on the subunit of DNA gyrase that release the positive binding stress on the supercoiled DNA. The increase in resistance to gentamicin and nalidixic acid might result from an alteration in the cell wall which prevents these compounds from reaching their targets (Crandall and Montville 1998).
Cell surface hydrophobicity
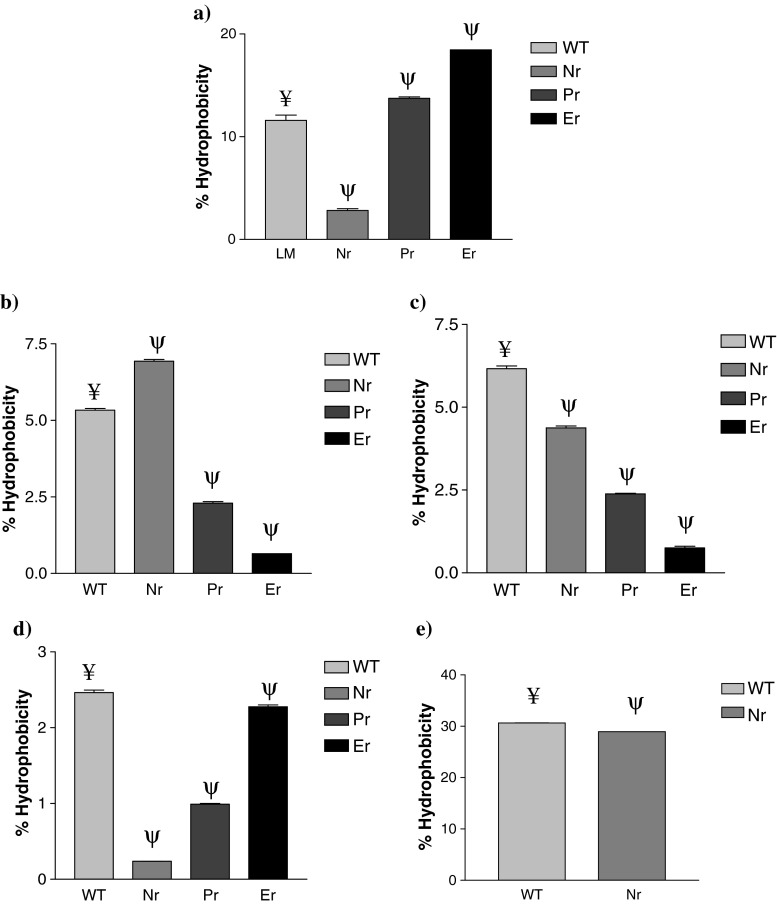
According to the hydrophobicity measurements significant differences (p < 0.001) were observed between wild type E. faecium DSMZ 20477, E. faecium (VRE), E. faecalis ATCC 29212 and L. monocytogenes MTCC 657 and their nisin resistant, pediocin 34 resistant and enterocin FH99 resistant counterparts, respectively (Fig. 1).
Fig. 1.
Surface hydrophobicity * of the wild type (WT) (a) Listeria monocytogenes ATCC 53135, (b) L. monocytogenes MTCC 657 (c) E. faecium DSMZ 20477, (d) E. faecium VRE, (e) E. faecalis ATCC 29212 and their nisin resistant (Nr), Pediocin 34 resistant (Pr) and Enterocin FH99 resistant (Er) variants. *Values are presented as mean±SEM; n = 3. ¥, ψ Values with different superscripts differ significantly at the level of p < 0.001 between wild type and bacteriocin resistant strains
Nisin resistant variant of L. monocytogenes was more hydrophobic than the corresponding wild type, whereas the pediocin 34 and enterocin FH99 resistant variants were less hydrophobic than the wild type strain Nisin, pediocin 34 and enterocin FH99 resistant variants of E. faecium DSMZ 20477 and E. faecium VRE were less hydrophobic than their wild type counterparts. Also, nisin resistant E. faecalis 29212 was less hydrophobic than its wild type counterpart. This may be due to a substantial change in the surface architecture of the resistant variants which might involve a different protein display at the surface. These results are in accordance with those reported by Martinez and Rodriguez (2005).
Conclusion
The results of the present study indicate that treatment with a combination of two or more different bacteriocins has an advantage of protection against many spoilage and pathogenic bacteria because of synergistic or additive effect. The results of this study also show that although the use of Nisin is permitted in a number of countries in a variety of foods, other bacteriocins viz, pediocin and enterocin with different and/or more effective antimicrobial activity may be considered as new biopreservatives. This study showed that resistance to a bacteriocin may extend to other bacteriocins within the same class. The development of bacteriocin resistance may hinder further application of bacteriocins in food preservation and it also raises concerns about the extensive use of bacteriocins in food regarding the cross resistance in food borne pathogens towards other bacteriocins and towards clinically used antibiotics. Since bacteriocins are considered as potential tools for biopreservation, more study is needed to determine the distribution of bacteriocin-resistance phenomena among microorganisms that cause food spoilage and among food borne pathogens
References
- Banerjee SP, Dora KC, Chowdhury S (2011) Detection, partial purification and characterization of bacteriocin produced by Lactobacillus brevis FPTLB3 isolated from freshwater fish Bacteriocin from Lb. brevis FPTLB3. J Food Sci Technol. doi:10.1007/s13197-011-0240-4 [DOI] [PMC free article] [PubMed]
- Bankerroum N, Sandine WE. Inhibitory action of nisin against Listeria monocytogenes. J Dairy Sci. 1988;71:3237–3245. doi: 10.3168/jds.S0022-0302(88)79929-4. [DOI] [PubMed] [Google Scholar]
- Barefoot SF, Klaenhammer TR. Detection and activity of Lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl Environ Microbiol. 1983;45:1808–1815. doi: 10.1128/aem.45.6.1808-1815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SR, Ray P, Johnson MC, Ray B. Influence of growth conditions on the production of a bacteriocin AcH by Pediococcus acidilactici H. Appl Environ Microbiol. 1991;5:1265–1267. doi: 10.1128/aem.57.4.1265-1267.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintas LM, Casaus P, Fernandez MF, Hernández PE. Comparative antimicrobial activity of enterocin L50, pediocin PA-1, nisin A, and lactocin S against spoilage and foodborne pathogenic bacteria. Food Microbiol. 1998;15:289–298. doi: 10.1006/fmic.1997.0160. [DOI] [Google Scholar]
- Crandall AD, Montville TJ. Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl Environ Microbiol. 1998;64(231):237. doi: 10.1128/aem.64.1.231-237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies EA, Falahee MB, Adams MR. Involvement of the cell envelope of Listeria monocytogenes in the acquisition of nisin resistance. J Appl Bacteriol. 1996;81:139–146. doi: 10.1111/j.1365-2672.1996.tb04491.x. [DOI] [PubMed] [Google Scholar]
- De Man JC, Rogosa M, Sharpe ME. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;2:130–135. doi: 10.1111/j.1365-2672.1960.tb00188.x. [DOI] [Google Scholar]
- Dykes GA, Hastings JW. Fitness costs associated with class IIa bacteriocin resistance in Listeria monocytogenes B73. Lett Appl Microbiol. 1998;26:5–8. doi: 10.1046/j.1472-765X.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- Foulique Moreno MR, Sarantinopoulos P, Tsakalidou E, De Vuyst L. The role and application of enterococci in food and health. Intl J Food Microbiol. 2006;106:1–24. doi: 10.1016/j.ijfoodmicro.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Franz CMAP, Schillinger U, Holzapfel WH. Production and characterization of enterocin 900, a bacteriocin produced by Enterococcus faecium BFE 900 from black olives. Intl J Food Microbiol. 1996;29:255–270. doi: 10.1016/0168-1605(95)00036-4. [DOI] [PubMed] [Google Scholar]
- Galvez A, Valdivia E, Abriouel H, Camafeita E, Mendez E, Martinez-Bueno M, Maqueda M. Isolation and characterization of enterocin EJ97, a bacteriocin produced by Enterococcus faecalis EJ97. Arch Microbiol. 1998;171:59–65. doi: 10.1007/s002030050678. [DOI] [PubMed] [Google Scholar]
- Galvez A, López RL, Abriouel H. Application of bacteriocins in the control of foodborne pathogenic and spoilage bacteria. Crit Rev Biotech. 2008;28:125–152. doi: 10.1080/07388550802107202. [DOI] [PubMed] [Google Scholar]
- Giraffa G. Enterococcal bacteriocins: their potential as anti- Listeria factors in dairy technology. Food Microbiol. 1995;12:291–299. doi: 10.1016/S0740-0020(95)80109-X. [DOI] [Google Scholar]
- Gravesen A, Sorensen K, Aarestrup FM, Knochel S. Spontaneous nisin resistant Listeria monocytogenes mutants with increased expression of a putative penicillin-binding protein and their sensitivity to various antibiotics. Microb Drug Resis. 2001;7:127–135. doi: 10.1089/10766290152045002. [DOI] [PubMed] [Google Scholar]
- Gupta H, Malik RK, De S, Kaushik JK. Purification and Characterization of Enterocin FH 99 Produced by a Faecal Isolate Enterococcus faecium FH 99. Indian J Microbiol. 2010;50:145–155. doi: 10.1007/s12088-010-0039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlin MB, Kalchayanand N, Ray P, Ray B. Bacteriocins of lactic acid bacteria in combination have greater antibacterial activity. J Food Protect. 1993;56:252–255. doi: 10.4315/0362-028X-56.3.252. [DOI] [PubMed] [Google Scholar]
- Harris LJ, Fleming HP, Klaenhammer TR. Sensitivity and resistance of Listeria monocytogenes ATCC 19115, Scott A and UAL500 to nisin. J Food Prot. 1991;54:836–840. doi: 10.4315/0362-028X-54.11.836. [DOI] [PubMed] [Google Scholar]
- Jamuna M, Jeevaratnam K. Antibacterial efficacy of nisin and pediocins against food spoilage and pathogenic organisms in broth and a vegetarian food. J Food Sci Technol. 2009;46(6):563–568. [Google Scholar]
- Jamuna M, Babusha ST, Jeevaratnam K. Inhibitory efficacy of nisin and bacteriocins from Lactobacillus isolates againast food spoilage and pathogenic organisms in model and food systems. Food Microbiol. 2005;22:449–454. doi: 10.1016/j.fm.2004.11.008. [DOI] [Google Scholar]
- Kaur G, Malik RK, Mishra SK, Singh TP, Bharadwaj A, Singroha G, Vij S, Kumar N. Nisin and class IIa bacteriocin resistance among listeria and other food-borne pathogens and spoilage bacteria. Microb Drug Resis. 2010;17(2):197–205. doi: 10.1089/mdr.2010.0054. [DOI] [PubMed] [Google Scholar]
- Limonet M, Revol-Junelles A, Cailliez-Grimal C, Milliere J. Synergistic mode of action of mesenterocins 52A and 52B produced by Leuconstoc mesenteroides subsp. mesenteroides FR 52. Curr Microbiol. 2004;48:204–207. doi: 10.1007/s00284-003-4165-7. [DOI] [PubMed] [Google Scholar]
- Martinez B, Rodriguez A. Antimicrobial susceptibility of nisin resistant Listeria monocytogenes of dairy origin. FEMS Microbiol Lett. 2005;252:67–72. doi: 10.1016/j.femsle.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Mazzotta AS, Montville TJ. Nisin induces changes in membrane fatty acid composition of Listeria monocytogenes nisin-resistant strains at 10 °C and 30 °C. J Appl Microbiol. 1997;82:32–38. doi: 10.1111/j.1365-2672.1997.tb03294.x. [DOI] [PubMed] [Google Scholar]
- Moll GN, Konings WN, Driessen AJM. Bacteriocins: mechanism of membrane insertion and pore formation. Anton Leeuwen. 1999;76:185–198. doi: 10.1023/A:1002002718501. [DOI] [PubMed] [Google Scholar]
- Mullet-Powell N, Lacoste-Armynot AM, Viñas M, Simeon de Buochberg M. Interactions between pairs of bacteriocins from lactic acid bacteria. J Food Prot. 1998;61:1210–1212. doi: 10.4315/0362-028x-61.9.1210. [DOI] [PubMed] [Google Scholar]
- Muriana PM (1996) Bacteriocins for control of Listeria spp. in food. J Food Prot (Suppl). 54–63 [DOI] [PubMed]
- National Committee for Clinical Laboratory Standards (2001) Performance standards for antimicrobial susceptibility testing; eleventh international supplement. NCCLS document M100–S11. National Committee for Clinical Laboratory Standards. Wayne, Pa.
- Nunez M, Rodrıguez JL, Garcıa E, Gaya P, Medina M. Inhibition of Listeria monocytogenes by enterocin 4 during the manufacture and ripening of Manchego cheese. J Appl Microbiol. 1997;83:671–677. doi: 10.1046/j.1365-2672.1997.00275.x. [DOI] [PubMed] [Google Scholar]
- Peschel A, Ott M, Jack RW, Kalbacher H, Jung G, Gotz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- Rao KN, Malik, RK (2003) Emerging biotechnologies in dairy and meat processing Chromosomal linked bacteriocin production in Pediococcus pentosaceus. In: Marwaha SS, Arora JK (eds.) Biotechnological Strategies in Agroprocessing. Asiatech Publications, New Delhi, pp. 230–257
- Rekhif N, Atrih A, Lefebvre G. Selection and properties of spontaneous mutants of Listeria monocytogenes ATCC 15313 resistant to different bacteriocins produced by lactic acid bacteria strains. Curr Microbiol. 1994;28:237–241. doi: 10.1007/BF01575967. [DOI] [Google Scholar]
- Rosenberg M, Gutnick D, Rosenberg E. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett. 1980;9:29–33. doi: 10.1111/j.1574-6968.1980.tb05599.x. [DOI] [Google Scholar]
- Sarantinopoulos P, Leroy F, Leontopoulou E, Georgalaki MD, Kalantzopoulos G, Tsakalidou E, De Vuyst L. Bacteriocin production by Enterococcus faecium FAIR-E 198 in view of its application as adjunct starter in Greek feta cheese making. Intl J Food Microbiol. 2002;72:125–136. doi: 10.1016/S0168-1605(01)00633-X. [DOI] [PubMed] [Google Scholar]
- Schillinger U, Chung HS, Keppler K, Holzapfel WH. Use of bacteriocinogenic lactic acid bacteria to inhibit spontaneous nisin-resistant mutants of Listeria monocytogenes Scott A. J Appl Microbiol. 1998;85:657–663. doi: 10.1111/j.1365-2672.1998.00573.x. [DOI] [PubMed] [Google Scholar]
- Song HJ, Richard J. Antilisterial activity of three bacteriocins used at sub-minimal inhibitory concentrations and cross-resistance of the survivors. Int J Food Microbiol. 1997;36:155–161. doi: 10.1016/S0168-1605(97)01254-3. [DOI] [PubMed] [Google Scholar]
- Torri Tarelli G, Carminati D, Giraffa G. Production of bacteriocins active against Listeria monocytogenes and Listeria innocua from dairy enterococci. Food Microbiol. 1994;11:243–252. doi: 10.1006/fmic.1994.1027. [DOI] [Google Scholar]
- Ukuku DO, Shelef LA. Sensitivity of six strains of L. monocytogenes to nisin. J Food Prot. 1997;60:867–869. doi: 10.4315/0362-028X-60.7.867. [DOI] [PubMed] [Google Scholar]
- Ulhman U, Schillinger U, Rupnow JR, Holzapfel WH. Identification and characterization of two bacteriocin-producing strains of Lactococcus lactis isolated from vegetables. Int J Food Microbiol. 1992;16:141–151. doi: 10.1016/0168-1605(92)90007-P. [DOI] [PubMed] [Google Scholar]
- Vignolo G, Palacios J, Farias ME, Sesma F, Schillinger U, Holzapfel W, Oliver G. Combined effect of bacteriocins on the survival of various Listeria species in broth and meat system. Curr Microbiol. 2000;41:410–416. doi: 10.1007/s002840010159. [DOI] [PubMed] [Google Scholar]