Abstract
Lapatinib is approved in combination with capecitabine for treatment of patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (MBC) who have progressed on prior trastuzumab in the metastatic setting. Vinorelbine is an important chemotherapy option for MBC. We evaluated efficacy and safety of lapatinib plus vinorelbine, compared with lapatinib plus capecitabine, in women with HER2-positive MBC. In this open-label, multicenter, phase II study, eligible patients (N = 112) were randomized 2:1 to lapatinib plus vinorelbine [(N = 75) 1,250 mg orally once daily (QD) continuously plus 20 mg/m2/day intravenously] or lapatinib plus capecitabine [(N = 37) 1,250 mg orally QD continuously plus 2,000 mg/m2/day orally, 2 doses]. The primary endpoint was progression-free survival (PFS). Other endpoints included overall survival (OS) and safety. Patients progressing within the study were given the option of crossover to the other treatment arm; time to second progression was an exploratory endpoint. Patient demographics, stratification, and prognostic factors were well balanced between treatments. Median PFS in both arms was 6.2 months [95 % confidence interval (CI) 4.2, 8.8 (lapatinib plus vinorelbine); 4.4, 8.3 (lapatinib plus capecitabine)]. Median OS on lapatinib plus vinorelbine was 24.3 months (95 % CI 16.4, NE) and 19.4 months (95 % CI 16.4, 27.2) on lapatinib plus capecitabine. In total, 42 patients opted to cross over; median PFS was 3.2 months (95 % CI 1.7, 5.1) on lapatinib plus vinorelbine and 4.0 months (95 % CI 2.1, 5.8) on lapatinib plus capecitabine. Lapatinib plus vinorelbine offers an effective treatment option for patients with HER2-overexpressing MBC, having displayed comparable efficacy and tolerability rates to lapatinib plus capecitabine.
Electronic supplementary material
The online version of this article (doi:10.1007/s10549-013-2828-z) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, HER2, Lapatinib, Vinorelbine, Capecitabine
Introduction
The human epidermal growth factor receptor 2 (HER2) is frequently overexpressed in human cancers [1]. HER2-positive breast cancers are associated with more aggressive disease and poorer prognosis, leading to shorter overall survival (OS) and disease-free survival intervals [2], if not adequately addressed by targeted treatment. However, prognosis has improved considerably since the introduction of single- or even dual-targeted treatments [3, 4]. Trastuzumab is used as an adjuvant therapy for patients with early-stage disease in combination with chemotherapy, and either as monotherapy or in combination with cytotoxic agents for patients with metastatic disease. Lately, different dual HER2-targeted treatments have been established in order to increase treatment efficacy. Statistically significant benefits in OS, progression-free survival (PFS) and clinical benefit response rate (CBR) were observed following combination treatment with lapatinib and trastuzumab, compared with lapatinib alone, in women with heavily pretreated, HER2-positive metastatic breast cancer (MBC) [5–7]. Similarly, a combination of pertuzumab, trastuzumab, and docetaxel resulted in significant improvements in OS, compared with placebo, trastuzumab, and docetaxel, in patients with HER2-positive breast cancer [4]. However, even more, subsequent treatment options are needed, particularly for those patients who have previously received or progressed on trastuzumab, or for whom trastuzumab is not clinically appropriate.
Lapatinib, a small-molecule dual kinase inhibitor of HER2, is effective in combination with capecitabine for the treatment for HER2-overexpressing MBC in patients who have progressed on prior therapy including an anthracycline, a taxane, and trastuzumab in the metastatic setting [8]. There is a clinical need for additional cytotoxic combinations in multiple-line treatment, as progression is common in HER2-positive disease.
Vinorelbine, a semi-synthetic, antimitotic, microtubule destabilizing drug, is an emerging chemotherapy option for HER2-overexpressing MBC, with overall response rates (ORRs; also described as objective response rates) of 34–47 % [9–11] as monotherapy for breast cancer. The combination of lapatinib and vinorelbine may be of clinical value, having been studied in two phase I studies and two non-randomized phase II studies; however, no randomized studies have been reported to date [12–15].
The aim of this study was to evaluate the efficacy and safety of lapatinib plus vinorelbine, compared with lapatinib plus capecitabine, in women with HER2-positive MBC.
Materials and methods
Study design
This randomized, open-label, multicenter, phase II study (Clinical Trials.gov: NCT01013740, GSK protocol number: LAP112620) included women with HER2-positive MBC who had received no more than one chemotherapeutic regimen in the metastatic setting. Patients were enrolled between November 2009 and February 2012 from 40 sites in 10 countries. The study was performed in accordance with the Declaration of Helsinki and approved by local ethics committees.
Sample size was based on feasibility. A 2:1 randomization scheme was used targeting 70 subjects in the lapatinib plus vinorelbine group and 35 subjects in the lapatinib plus capecitabine group. Patients were randomized to receive either lapatinib 1,250 mg orally once daily (QD) continuously plus vinorelbine 20 mg/m2−/day intravenously on days 1 and 8 every 3 weeks, or lapatinib 1,250 mg orally QD continuously plus capecitabine 2,000 mg/m2/day orally in 2 doses (12 h apart on days 1–14 every 3 weeks). Patients were stratified by prior receipt of therapy for MBC (yes or no) and site of metastatic disease (visceral/soft tissue or bone only). Patients received randomized study treatment until disease progression or discontinuation of study treatment due to unacceptable toxicity, withdrawal of consent, loss to follow-up, or death. Patients had disease assessments at screening and then every 9 weeks until progression. Following progression patients were contacted every 12 weeks to collect survival data. An interim safety review was conducted after enrollment of the first 30 patients (20 randomized to lapatinib plus vinorelbine, 10 randomized to lapatinib plus capecitabine) [16]. The safety review was conducted by core members of the lapatinib safety review team, leading to the conclusion that the trial be continued.
The primary focus was to evaluate PFS in the lapatinib plus vinorelbine group with a descriptive intent only. The control arm of lapatinib plus capecitabine was used to validate the patient population and to lend support to the activity of the combination of lapatinib with vinorelbine. The study was not powered to detect differences between the combinations; hence, no hypothesis testing was performed. No interim analyses for efficacy were performed for this study.
The analysis of PFS was performed when all patients had been followed up for a minimum of 6 months, or had otherwise progressed, died, or withdrew consent (if sooner). OS data were analyzed at the time of the PFS analysis; an updated OS analysis is planned when all patients have a minimum of 18 months follow-up (to be reported separately).
Following disease progression, patients were given the option of crossing over to the alternative treatment arm, and continuing in a post-progression crossover phase. Patients who crossed over to the other treatment arm received treatment until second disease progression, discontinuation of study treatment due to unacceptable toxicity, withdrawal of consent, loss to follow-up, or death.
Patient population
Eligible patients were women ≥18 years of age with histologically or cytologically confirmed HER2-positive (3+ immunohistochemistry, or a positive score by fluorescence in situ hybridization or chromogenic in situ hybridization) stage IV breast cancer who had received no more than one prior chemotherapy regimen in the metastatic setting. Prior therapy may have included anthracyclines and taxanes; prior therapy with trastuzumab was permitted but not required. Patients who had not received prior treatment for MBC were required to fulfill one or more of the following conditions (as determined by the study investigator): (1) relapse following receipt of trastuzumab-based therapy in the adjuvant setting; (2) contraindication to receiving trastuzumab; (3) documented medical reason for trastuzumab not being appropriate, or unsuitability for taxane-based chemotherapy. Patients were required to have adequate organ and bone marrow function, a European Cooperative Oncology Group performance status of 0–1, and a cardiac ejection fraction of at least 50 % (measured by echocardiogram or multigated acquisition scan). Patients with stable central nervous system (CNS) metastasis (for at least 3 months) were permitted. Bisphosphonate therapy for bone metastases was allowed, but treatment must have been initiated prior to the first dose of study medication.
Exclusion criteria included patients with active cardiac, hepatic, or biliary diseases and diseases or surgeries affecting gastrointestinal function. Patients undergoing concurrent treatment with anticancer or investigational agents, females pregnant or lactating at any time during the study, and those with peripheral neuropathy of grade 2 or greater were also excluded.
All patients provided written informed consent prior to study entry.
Study endpoints
Tumor response data were assessed by the investigator according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines [17]. The primary endpoint was PFS, defined as the time from randomization to the time of first documented disease progression at any site, or death due to any cause.
Secondary endpoints included: OS, defined as the time from randomization until death due to any cause; ORR, defined as the percentage of patients experiencing confirmed complete response (CR) and partial response (PR); CBR, defined as the percentage of patients achieving either a confirmed CR or PR, or having stable disease (SD) for at least 24 weeks (patients with unknown or missing response were treated as non-responders and included in the denominator when calculating the CBR percentage); for the subset of patients who showed a confirmed response (CR or PR), duration of response (DoR) is defined as time from first documented evidence of CR or PR until the first documented sign of disease progression or death due to any cause; and time to response (TTR) is the time from randomization until the first documented evidence of CR or PR (whichever is recorded first). The time to second progression following crossover was included as an exploratory outcome measure. Data regarding the number of patients with CNS metastases were collected post hoc as an exploratory outcome measure.
Toxicities were also measured by recording the incidence and grading of adverse events (AEs) and serious AEs (SAEs; the study protocol extended the definition of an SAE to include any grade 4 laboratory abnormality in order to expedite reporting of grade 4 neutropenia).
Statistical analysis
All efficacy analyses were performed using SAS version 9.1 and conducted on the intent-to-treat population, which comprised all patients who were randomized to study treatment, regardless of whether they actually received study medication. Clinical safety and tolerability were assessed in the safety population, which comprised all patients who took at least one dose of study medication. PFS, OS, DoR, and TTR were summarized using Kaplan–Meier survival curves, from which the median and 95 % confidence intervals (CIs) were calculated. Greenwood’s formula was used to calculate the standard error of the estimates from the Kaplan–Meier curve. The treatment hazard ratio (HR) and 95 % CI were based on the stratified log-rank test (the Pike estimator [18] ), stratifying for prior receipt of therapy for MBC (yes or no), and site of metastatic disease (visceral/soft tissue or bone only) was calculated for PFS and OS. Exact 95 % CI for ORR and CBR in each arm were calculated.
Results
Study population
A total of 112 patients with HER2-positive MBC were enrolled; N = 75 were randomized to receive lapatinib plus vinorelbine, and N = 37 were randomized to receive lapatinib plus capecitabine.
Patient demographics (Table 1) were similar between treatment arms after randomization, with the exception of median time since initial diagnosis, which was shorter for patients treated with lapatinib plus capecitabine than patients treated with lapatinib plus vinorelbine. Stratification and prognostic factors (Table 1) were also well balanced across the treatment arms. According to groups of treatment (lapatinib plus vinorelbine or lapatinib plus capecitabine), 48 (64 %) and 25 (68 %) patients received the study treatment as second-line therapy. In total, 38 (51 %) and 15 (41 %) patients had been treated with trastuzumab in the (neo)adjuvant setting; while 32 (43 %) and 20 (54 %) patients had received it in the metastatic setting, respectively. The patient flow is summarized in Fig. 1.
Table 1.
Patient demographics and stratification and prognostic factors
| Lapatinib 1,250 mg QD plus vinorelbine 20 mg/m2 (N = 75) | Lapatinib 1,250 mg QD plus capecitabine 2,000 mg/m2 (N = 37) | |
|---|---|---|
| Patient demographics | ||
| Median age, years (range) | 57 (32–79) | 58 (36–83) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 11 (15) | 3 (8) |
| Not Hispanic or Latino | 64 (85) | 34 (92) |
| Median time since initial diagnosis, months (range) | 36.6 (1–178) | 24.3 (8–240) |
| ErbB2 (HER/neu) IHC status, n (%) | ||
| 0–1+ | 0 | 0 |
| 2+ | 6 (8) | 6 (16) |
| 3+ | 67 (89) | 31 (84) |
| Unknown | 2 (3)a | 0 |
| ErbB2 (HER/neu) FISH status, n (%) | ||
| Positive | 10 (13) | 7 (19) |
| Negative or borderline | 0 | 0 |
| Unknown | 65 (87) | 30 (81) |
| ErbB2 (HER/neu) CISH status, n (%) | ||
| Positive | 9 (12) | 4 (11) |
| Negative or borderline | 0 | 0 |
| Unknown | 66 (88) | 33 (89) |
| Estrogen receptor status, n (%) | ||
| Positive | 37 (49) | 19 (51) |
| Negative | 38 (51) | 18 (49) |
| Not available/unknown | 0 | 0 |
| Progesterone receptor status, n (%) | ||
| Positive | 25 (33) | 14 (38) |
| Negative | 50 (67) | 23 (62) |
| Not available/unknown | 0 | 0 |
| Stratification and prognostic factors | ||
| Receipt of prior therapy for advanced or MBC, n (%) | ||
| No | 27 (36) | 12 (32) |
| Yes | 48 (64) | 25 (68) |
| Visceral or non-visceral disease, n (%) | ||
| Visceral only | 29 (39) | 17 (46) |
| Bone only | 5 (7) | 1 (3) |
| Visceral and non-visceral | 41 (55) | 19 (51) |
| Stage at initial diagnosis, n (%) | ||
| 0–I | 7 (9) | 4 (11) |
| II (a–b) | 23 (31) | 12 (33) |
| III (a–c) | 31 (41) | 15 (40) |
| IV | 12 (16) | 6 (16) |
| Unknown | 2 (3) | 0 |
| Measureable disease, n (%) | ||
| Yes | 65 (87) | 31 (84) |
| No | 10 (13) | 6 (16) |
| Prior anticancer therapy, n (%) | ||
| Any therapy | 73 (97) | 37 (100) |
| Chemotherapy | 72 (96) | 35 (95) |
| Hormonal therapy | 29 (39) | 15 (41) |
| Immunotherapy | 0 | 0 |
| Biological therapy | 63 (84) | 32 (86) |
| Small-molecule targeted therapy | 2 (3) | 0 |
| Radiotherapy | 14 (19) | 5 (14) |
| Surgery | 70 (93) | 34 (92) |
| Unknown | 1 (1) | 0 |
aErbB2 (HER/neu) status was confirmed as positive by FISH in both cases. CISH chromogenic in situ hybridization; FISH fluorescence in situ hybridization; HER human epidermal growth factor receptor; IHC immunohistochemistry; MBC metastatic breast cancer; QD once daily
Fig. 1.
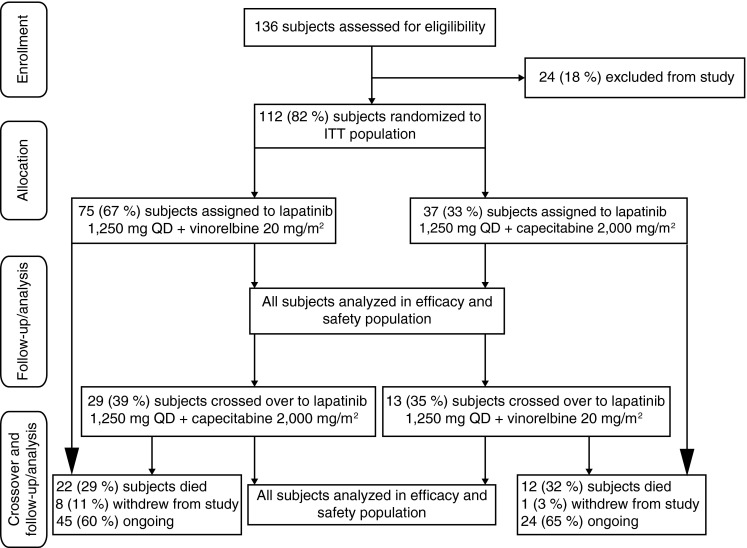
Patient flow ITT intent-to-treat; QD once daily
Compliance with lapatinib was similar between lapatinib plus vinorelbine and lapatinib plus capecitabine arms. Median compliance to lapatinib was 86.4 versus 98.2 %, and the median daily dose was 1,231.8 versus 1,236.0 mg, respectively.
A total of thirty-four patients discontinued study treatment prior to disease progression; 24 (33 %) patients in the lapatinib plus vinorelbine arm, and 10 (27 %) patients in the lapatinib plus capecitabine arm. The most common reasons were an AE [11 (15 %) patients in the lapatinib plus vinorelbine arm and 6 (16 %) patients in the lapatinib plus capecitabine arm], and decision by patient [6 (8 %) patients in the lapatinib plus vinorelbine arm, 4 (11 %) patients in the lapatinib plus capecitabine arm]. Five (7 %) and 2 (3 %) patients in the lapatinib plus vinorelbine arm withdrew due to investigator decision or protocol deviation, respectively.
In total, 42 patients crossed over to the other treatment arm; 29 from lapatinib plus vinorelbine to lapatinib plus capecitabine and 13 from lapatinib plus capecitabine to lapatinib plus vinorelbine. Patient demographics and baseline characteristics in the crossover population were well balanced, as observed in the randomized phase.
Efficacy
Primary endpoint
The median PFS after randomization was 6.2 months in both arms (95 % CI 4.2, 8.8 months in the lapatinib plus vinorelbine arm; 95 % CI 4.4, 8.3 months in the lapatinib plus capecitabine arm). In the lapatinib plus vinorelbine arm, 52 (69 %) disease progressions or deaths were recorded, compared with 24 (65 %) in the lapatinib plus capecitabine arm. The HR was 0.84 (95 % CI 0.53, 1.35; Fig. 2a).
Fig. 2.
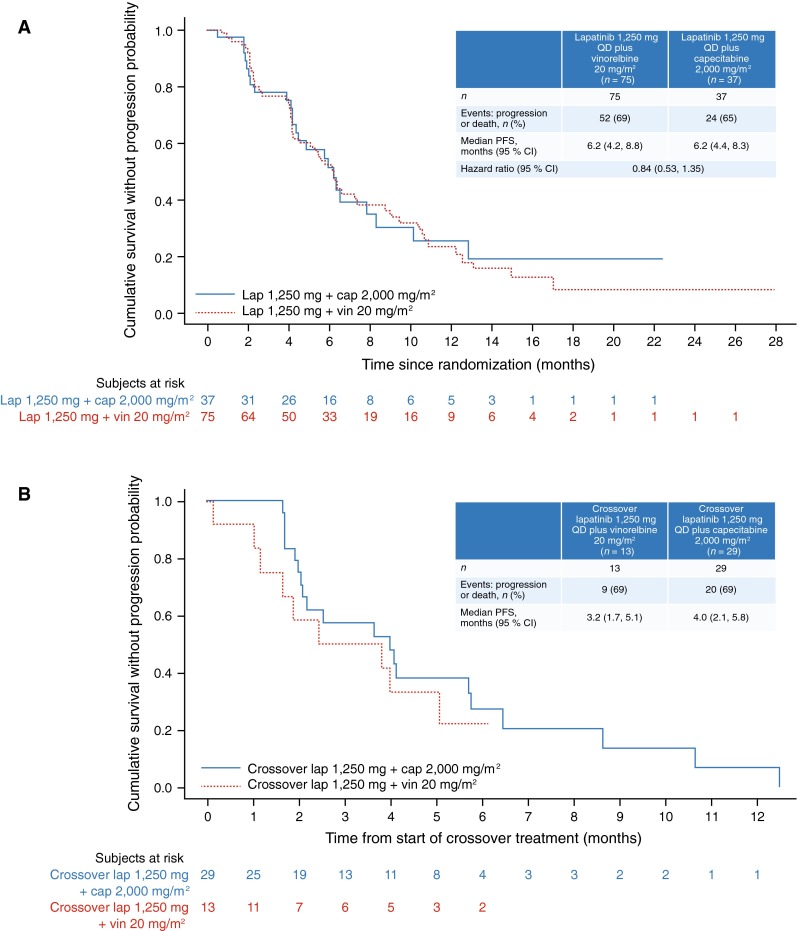
Kaplan–Meier plot: progression-free survival after (a) randomization (intent-to-treat population) and (b) start of crossover treatment (crossover population) QD once daily; PFS progression-free survival; CI confidence intervals; lap lapatinib; cap capecitabine; vin vinorelbine
Secondary endpoints
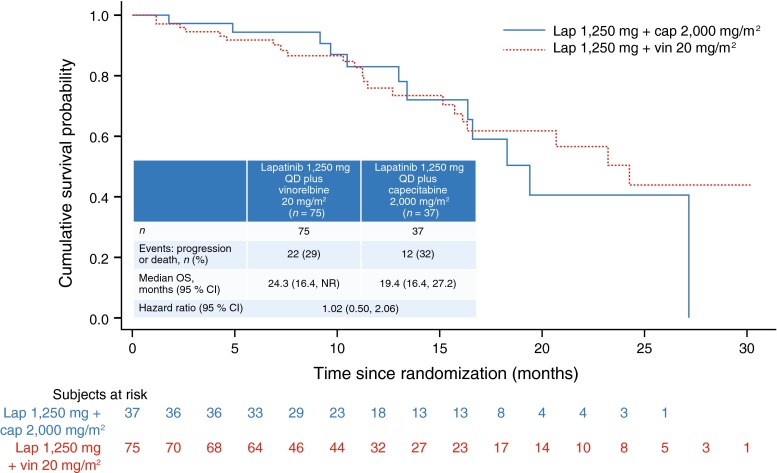
The median OS in the lapatinib plus vinorelbine arm was 24.3 months (95 % CI 16.4, NR months) which included 22 deaths (29 % patients), compared with 19.4 months in the lapatinib plus capecitabine arm (95 % CI 16.4, 27.2 months), including 12 deaths (32 % patients; Fig. 3).
Fig. 3.
Kaplan–Meier plot: overall survival after randomization (intent-to-treat population) QD once daily; OS overall survival; CI confidence intervals; lap lapatinib; cap capecitabine; vin vinorelbine
In the lapatinib plus vinorelbine arm, 15 (20 %) patients experienced a CR or PR, compared with 13 (35 %) patients in the lapatinib plus capecitabine arm.
In the lapatinib plus vinorelbine arm, 29 (39 %) patients matched the CBR criteria, compared with 18 (49 %) patients in the lapatinib plus capecitabine arm (Table 2).
Table 2.
Efficacy results; summary of survival and response rates in each treatment arm (ITT population)
| Lapatinib 1,250 mg QD plus vinorelbine 20 mg/m2 (N = 75) | Lapatinib 1,250 mg QD plus capecitabine 2,000 mg/m2 (N = 37) | |
|---|---|---|
| Overall response rate, n (%) | ||
| CR | 1 (1) | 2 (5) |
| PR | 14 (19) | 11 (30) |
| CR + PR (95 %CI) | 15 (20) (11.6, 30.8) | 13 (35) (20.2, 52.5) |
| SD | 35 (47) | 15 (41) |
| PD | 15 (20) | 7 (19) |
| Unknowna | 10 (13) | 2 (5) |
| Duration of response, months | ||
| Median duration of response (95 % CI) | 6.7 (4.6, 8.3) | 10.8 (4.3, NE) |
| Clinical benefit response rate, n (%) | ||
| CR | 1 (1) | 2 (5) |
| PR | 14 (19) | 11 (30) |
| SD < 24 weeks | 21 (28) | 10 (27) |
| SD ≥ 24 weeks | 14 (19) | 5 (14) |
| PD | 15 (20) | 7 (19) |
| Unknown | 10 (13) | 2 (5) |
| Total CBR (95 % CI) | 29 (39) (27.6, 50.6) | 18 (49) (31.9, 65.6) |
| Time to response | ||
| Response events, n (%) | 15 (20) | 13 (35) |
| Median time to response, weeks (95 % CI) | 9.4 (9.0, 10.1) | 9.3 (9.1, 10.0) |
aPatients did not have their responses assessed at the 9-week timepoint for the following reasons: lapatinib plus vinorelbine arm: AE (5 patients), patient’s choice (1 patient), disease progression (2 patients), investigator discretion (1 patient), and protocol deviation (1 patient); lapatinib plus capecitabine arm: patient’s choice (1 patient), and disease progression (1 patient). AE adverse event; CI confidence interval; CBR clinical benefit rate; CR complete response; ITT intent-to-treat; NE not evaluable; PD progressive disease; PR partial response; QD once daily; SD stable disease
Of those with a confirmed response, the median DoR was 6.7 months in the lapatinib plus vinorelbine arm and 10.8 months in the lapatinib plus capecitabine arm (Table 2).
The median TTR was 9.4 months in the lapatinib plus vinorelbine arm, compared with 9.3 months in the lapatinib plus capecitabine arm (Table 2).
Exploratory endpoints
Median PFS after start of crossover was 3.2 months for patients who crossed over from lapatinib plus capecitabine to lapatinib plus vinorelbine (95 % CI 1.7, 5.1 months), and 4.0 months for patients who crossed over from lapatinib plus vinorelbine to lapatinib plus capecitabine (95 % CI 2.1, 5.8 months). In the crossover lapatinib plus vinorelbine arm, 9 (69 %) disease progressions or deaths were recorded, compared with 20 (69 %) in the crossover lapatinib plus capecitabine arm (Fig. 2b).
The median time to second progression from randomization was 8.9 months for patients crossing over from lapatinib plus capecitabine to lapatinib plus vinorelbine (95 % CI 6.3, 10.6 months), and 10.3 months in patients crossing over from lapatinib plus vinorelbine to lapatinib plus capecitabine (95 % CI 8.5, 15.5 months) (Online Resource 1).
Four (5 %) patients in the lapatinib plus vinorelbine arm had CNS metastases at baseline and 3 (75 %) of these had a relapse at first progression; 8 (11 %) patients had new CNS metastases at first progression. In the lapatinib plus capecitabine arm, 4 (11 %) patients had metastases at baseline, with 2 (50 %) of these having a relapse at first progression; 5 (14 %) patients had new CNS metastases at first progression.
Safety and toxicity
The most commonly observed AEs occurring during the randomized phase were diarrhea, neutropenia, palmar-plantar erythrodysesthesia (PPE), rash, nausea, and fatigue (Table 3). The majority of AEs were grade 1 or 2 in severity. Grade 3 PPE was recorded in 6 (16 %) patients treated with lapatinib plus capecitabine and 2 (3 %) patients treated with lapatinib plus vinorelbine. Grade 3–4 neutropenia occurred in 23 (31 %) patients in the lapatinib plus vinorelbine arm, and 1 (3 %) patient in the lapatinib plus capecitabine arm. Neutropenia was the only recorded AE of toxicity grade 4 occurring in more than one patient.
Table 3.
All adverse events (of toxicity grades 1–4) occurring in ≥10 % patients (ITT and crossover populations)
| Number of patients (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lapatinib 1,250 mg QD plus vinorelbine 20 mg/m2 | Lapatinib 1,250 mg QD plus capecitabine 2,000 mg/m2 | |||||||||
| ITT population (N = 75) | ITT population (N = 37) | |||||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total | |
| Palmar-plantar erythrodysesthesia syndrome | 3 (4) | 1 (1) | 2 (3) | 0 | 6 (8) | 10 (27) | 8 (22) | 6 (16) | 0 | 24 (65) |
| Diarrhea | 15 (20) | 15 (20) | 5 (7) | 0 | 35 (47) | 9 (24) | 8 (22) | 5 (14) | 0 | 22 (59) |
| Neutropenia | 2 (3) | 12 (16) | 14 (19) | 9 (12) | 37 (49) | 1 (3) | 1 (3) | 1 (3) | 0 | 3 (8) |
| Rash | 13 (17) | 5 (7) | 0 | 0 | 18 (24) | 6 (16) | 3 (8) | 0 | 0 | 9 (24) |
| Nausea | 10 (13) | 6 (8) | 2 (3) | 0 | 18 (24) | 2 (5) | 0 | 1 (3) | 0 | 3 (8) |
| Fatigue | 10 (13) | 5 (7) | 2 (3) | 0 | 17 (23) | 2 (5) | 1 (3) | 0 | 0 | 3 (8) |
| Anemia | 7 (9) | 6 (8) | 1 (1) | 0 | 14 (19) | 1 (3) | 1 (3) | 0 | 0 | 2 (5) |
| Vomiting | 4 (5) | 4 (5) | 5 (7) | 0 | 13 (17) | 2 (5) | 1 (3) | 1 (3) | 0 | 4 (11) |
| Hyperbilirubinemia | 0 | 1 (1) | 0 | 0 | 1 (1) | 0 | 5 (14) | 1 (3) | 0 | 6 (16) |
| Leukopenia | 5 (7) | 1 (1) | 5 (7) | 1 (1) | 12 (16) | 2 (5) | 0 | 0 | 0 | 2 (5) |
| Decreased appetite | 7 (9) | 4 (5) | 1 (1) | 0 | 12 (16) | 2 (5) | 0 | 0 | 0 | 2 (5) |
| Pyrexia | 9 (12) | 2 (3) | 0 | 0 | 11 (15) | 0 | 1 (3) | 0 | 0 | 1 (3) |
| Asthenia | 6 (8) | 3 (4) | 1 (1) | 0 | 10 (13) | 2 (5) | 1 (3) | 2 (5) | 0 | 5 (14) |
| Cough | 7 (9) | 3 (4) | 0 | 0 | 10 (13) | 1 (3) | 0 | 0 | 0 | 1 (3) |
| Peripheral sensory neuropathy | 4 (5) | 4 (5) | 1 (1) | 0 | 9 (12) | 0 | 0 | 0 | 0 | 0 |
| Mucosal inflammation | 4 (5) | 3 (4) | 1 (1) | 1 (1) | 9 (12) | 0 | 0 | 0 | 0 | 0 |
| Headache | 3 (4) | 5 (7) | 0 | 0 | 8 (11) | 2 (5) | 0 | 0 | 0 | 2 (5) |
| Arthralgia | 6 (8) | 2 (3) | 0 | 0 | 8 (11) | 1 (3) | 2 (5) | 0 | 0 | 3 (8) |
| Aspartate aminotransferase increased | 5 (7) | 3 (4) | 0 | 0 | 8 (11) | 2 (5) | 1 (3) | 1 (3) | 0 | 4 (11) |
| Alanine aminotransferase increased | 4 (5) | 3 (4) | 1 (1) | 0 | 8 (11) | 0 | 2 (5) | 1 (3) | 0 | 3 (8) |
| Blood bilirubin increased | 0 | 0 | 0 | 0 | 0 | 0 | 3 (8) | 1 (3) | 0 | 4 (11) |
| Paronychia | 0 | 2 (3) | 0 | 0 | 2 (3) | 1 (3) | 2 (5) | 1 (3) | 0 | 4 (11) |
| Number of patients (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lapatinib 1,250 mg QD plus vinorelbine 20 mg/m2 | Lapatinib 1,250 mg QD plus capecitabine 2,000 mg/m2 | |||||||||
| Crossover population (N = 13) | Crossover population (N = 29) | |||||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total | |
| Neutropenia | 1 (8) | 2 (15) | 1 (8) | 1 (8) | 5 (38) | 0 | 0 | 0 | 0 | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 0 | 0 | 0 | 0 | 0 | 4 (14) | 1 (3) | 2 (7) | 0 | 7 (24) |
| Diarrhea | 1 (8) | 0 | 1 (8) | 0 | 2 (15) | 1 (3) | 2 (7) | 2 (7) | 0 | 5 (17) |
| Fatigue | 1 (8) | 1 (8) | 0 | 0 | 2 (15) | 1 (3) | 0 | 0 | 0 | 1 (3) |
| Febrile neutropenia | 0 | 0 | 0 | 2 (15) | 2 (15) | 0 | 0 | 0 | 0 | 0 |
| Pyrexia | 2 (15) | 0 | 0 | 0 | 2 (15) | 1 (3) | 1 (3) | 0 | 0 | 2 (7) |
| Alanine aminotransferase increased | 0 | 0 | 1 (8) | 0 | 1 (8) | 0 | 3 (10) | 0 | 0 | 3 (10) |
| Aspartate aminotransferase increased | 0 | 0 | 1 (8) | 0 | 1 (8) | 1 (3) | 2 (7) | 0 | 0 | 3 (10) |
ITT intent-to-treat; QD once daily
There were more SAEs in the lapatinib plus vinorelbine arm, compared with the lapatinib plus capecitabine arm [25 (33 %) vs. 4 (11 %), respectively]. The most common SAE was neutropenia [10 (13 %) patients in the lapatinib plus vinorelbine arm and no patients in the lapatinib plus capecitabine arm]; all cases were considered by the investigators to be related to study treatment. There was 1 fatal AE (intestinal obstruction), but this was not considered by the investigator as related to study treatment.
In the randomized phase, 14 (19 %) deaths occurred in lapatinib plus vinorelbine arm [12 (16 %) due to disease under study and 2 (3 %) due to other causes]. Seven (19 %) deaths were recorded in the lapatinib plus capecitabine arm; all due to disease under study.
The AE profile in the crossover population followed the same pattern as in the randomized population (Table 3). Grade 3 and 4 neutropenia were each observed in 1 (8 %) patient who crossed over from lapatinib plus capecitabine to lapatinib plus vinorelbine; also observed in this population were two cases (15 %) of grade 4 febrile neutropenia, and one case each of grade 3 diarrhea, aspartate aminotransferase increased, and alanine aminotransferase increased. In patients crossing over from lapatinib plus vinorelbine to lapatinib plus capecitabine, two cases each of grade 3 PPE, and grade 3 diarrhea were observed.
Three SAEs were observed in each arm after crossover (23 % in those who crossed over from lapatinib plus capecitabine to lapatinib plus vinorelbine versus 10 % in those who crossed over from lapatinib plus vinorelbine to lapatinib plus capecitabine). The most common SAE was febrile neutropenia, observed in 2 (15 %) patients who crossed over from lapatinib plus capecitabine to lapatinib plus vinorelbine. One fatal AE was observed after crossover; this was due to liver injury and occurred in a patient who crossed over from lapatinib plus vinorelbine to lapatinib plus capecitabine. In patients who crossed over from lapatinib plus capecitabine to lapatinib plus vinorelbine, 5 (38 %) deaths were recorded (all due to disease under study), compared with 8 (28 %) deaths in patients with the reverse crossover (7 due to disease under study, and 1 due to other causes). No fatal SAEs related to study treatment occurred in patients who crossed over to the other treatment arm.
Discussion
This is the first prospective, randomized study to evaluate the efficacy and safety of lapatinib plus vinorelbine in context with lapatinib plus capecitabine in this patient population. The median PFS, the primary endpoint, was 6.2 months in both treatment arms. Median OS was 24.3 months in patients treated with lapatinib plus vinorelbine and 19.4 months in patients treated with lapatinib plus capecitabine. These PFS and OS rates are well matched with those from other studies of lapatinib plus capecitabine [8, 19–21], supporting the use of lapatinib plus vinorelbine in the target population.
Adverse events in this study were consistent with the known lapatinib, vinorelbine, and capecitabine safety profiles, and no new relevant safety signals were detected [12–14, 19, 20]. As expected, PPE occurred in a larger proportion of patients in the lapatinib plus capecitabine arm, and neutropenia occurred in a larger proportion of patients treated with lapatinib plus vinorelbine. Of the neutropenia cases observed with lapatinib plus vinorelbine, 10 AEs (13 %) were extended to SAEs; all being considered related to study treatment. A similar incidence of grade 3–4 neutropenia has been reported in other studies using lapatinib plus vinorelbine [12–14]. However, further investigation is needed to establish how this treatment contributes to neutropenia and which doses of each compound are best tolerated while still remaining efficacious. This is especially important given the potential for a pharmacokinetic interaction between lapatinib and vinorelbine. This would explain the comparable hematological toxicity profile observed in both the VITAL and the GEP 01 studies, despite difference of doses: lapatinib 1,250 versus 1,000 mg daily, and vinorelbine 20 versus 22.5 mg/m2 on days 1 and 8 every 3 weeks. The toxicity profiles of both regimens appear to justify their routine clinical use, given that routine clinical use of this combination has a favorable benefit risk (the AEs are predictable and can be managed with standard monitoring and intervention).
For more than 9 years, trastuzumab was the only treatment option in HER2-overexpressing advanced breast cancer. In 2007, lapatinib was FDA approved in combination with capecitabine and in 2010, with an aromatase inhibitor [22]. In a meta-analysis, a significant benefit in PFS and OS was demonstrated with a lapatinib-containing regimen for patients with locally advanced or MBC [23]. In both the primary and the advanced setting, heterogeneous data have been observed in studies comparing the efficacy of lapatinib with trastuzumab. In the first-line metastatic setting, the COMPLETE study, which compared trastuzumab and taxane chemotherapy with lapatinib and taxane chemotherapy as first-line treatment, showed that PFS but not OS was superior in the trastuzumab patient group [24]. The Neo-ALTTO phase III study showed similar pathologic complete response (pCR) rates between the treatment groups administered with lapatinib (24.7 %) and with trastuzumab (29.5 %) [25]. Neo-ALTTO had more conservative protocol reporting requirements and stopping rules for specified AEs than other lapatinib studies, making comparisons difficult. Different toxicity and compliance profiles in these studies indicate that compliance might significantly influence the efficacy of HER2-targeted treatment. Therefore, a variety of treatment options, accounting for individual patient conditions may contribute to increased patient compliance.
Increasing data suggest that dual-targeted treatment, combining either trastuzumab with lapatinib or with pertuzumab might significantly increase efficacy of HER2-targeted treatment in a clinically relevant setting. In the randomized phase II CHERLOB trial, which evaluated preoperative taxane–anthracycline chemotherapy in combination with trastuzumab, lapatinib, or combined trastuzumab and lapatinib in patients with HER2-positive, stage II–IIIA breast cancer, the pCR rate was 25 % in the trastuzumab arm, 26.3 % in the lapatinib arm, and 46.7 % in the combination (trastuzumab–lapatinib) arm [26]. No patient had symptomatic cardiac events, including congestive heart failure. The Neo-ALTTO phase III study also showed that pCR was significantly higher in the treatment group administered with lapatinib plus trastuzumab (51.3 %) than in the group administered trastuzumab alone (29.5 %) [25]. In the NSABP B-41 study, the treatment arm combining trastuzumab and lapatinib produced a numerically higher pCR percentage (62 %) than single-agent HER2-directed therapy; however, the difference was not statistically significant [27].
In the metastatic setting, the Cleopatra study randomized 808 HER2-positive patients to receive placebo plus trastuzumab plus docetaxel, or pertuzumab plus trastuzumab plus docetaxel as first-line treatment until disease progression) [28]. Results showed a significant increase in median PFS in the pertuzumab group. In a recent second interim analysis, a significant OS benefit was shown for the dual blockade treatment [29]. However, these findings from first-line treatment may not be applicable to patients in a further treatment line situation with resistance to HER2-targeted treatment. In a phase III study, comparing monotherapy with lapatinib with a combination of lapatinib and trastuzumab in heavily pretreated patients, dual-targeted treatment showed a significant OS benefit [7]. Currently, however, the optimal timing of dual-targeted treatment remains unclear. Single HER2-targeted treatment with trastuzumab or lapatinib, therefore, remains the standard of care in most patients pretreated with HER2-directed agents, warranting a greater variety of treatment combinations for these two agents.
The continuation of targeted treatment in HER2-positive patients who suffer progressive disease on previous HER2-directed therapy has been well established. Four retrospective cohort studies, as well as prospective randomized controlled trials, demonstrated a significant benefit in PFS and OS when HER2-targeted treatment was applied beyond disease progression, in combination with alternative cytostatic or endocrine agents [5, 6, 29, 30]. In a recent pooled analysis comprising the data of 2,618 patients, Petrelli et al. [31] calculated a benefit in (weighted median) OS of 24 months in patients who received continued trastuzumab beyond disease progression. Treatment with lapatinib using an alternative combination for patients undergoing progression on trastuzumab treatment also can be effective [8, 13]. Here, patients progressing within the study were also given the option of crossing over to the other treatment arm, which enabled continued HER2-targeted treatment to be evaluated for efficacy after progression. Efficacy results showed that treatment with lapatinib was still effective after progression on lapatinib; median PFS was 3.2–4.0 months for patients crossing over to the other treatment arm and the time to second progression from randomization was 8.9–10.3 months. Our study indicates that efficacy and safety can be achieved for treatment with lapatinib after progression; presenting the first evidence which shows that treatment with lapatinib for patients progressing on lapatinib can be effective. Of course, these results must be put into context with the results of the Emilia Study, which compared treatment with trastuzumab emtansine with lapatinib and capecitabine, in patients with progressive disease after trastuzumab treatment [32]. The study showed an impressive benefit in PFS, with a HR of 0.65. The optimal timing of trastuzumab emtansine treatment and therapy of choice after subsequent progression, however, remains to be defined. It appears likely that combinations of chemotherapy and single HER2-neu-targeted treatment will also have to be used before or after treatment with trastuzumab emtansine.
Limitations of this study included the non-blinded study design. However, it should be noted that in this study, a blinded design was not feasible, due to differences in intravenous versus oral administration of vinorelbine and capecitabine, respectively. Furthermore, this study included both first- and second-line patients and not all patients had received previous treatment with trastuzumab. Here, a significant proportion of patients had not received trastuzumab as first-line treatment for MBC (57 % patients randomized to lapatinib plus vinorelbine and 46 % of patients randomized to lapatinib plus capecitabine). Due to the small number of patients in this study, it is not possible to assess any difference in effect in those patients who had received first-line therapy with trastuzumab. Finally, while most patient characteristics were well balanced between the two treatment arms, the median time from initial diagnosis until randomization differed (36.6 vs. 24.3 months, respectively), it is unlikely that this difference had a relevant impact on the outcome of the study.
In summary, lapatinib plus vinorelbine offers an effective treatment option for patients with HER2-overexpressing MBC, having demonstrated acceptable rates of efficacy and tolerability, validated by the control arm of lapatinib plus capecitabine.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1 Kaplan–Meier plot: time to second progression following crossover, from randomization (crossover population) QD, once daily; PFS, progression-free survival; lap, lapatinib; cap, capecitabine; vin, vinorelbine (EPS 901 kb)
Acknowledgments
The authors thank the patients who participated in this study and the following study site staff, for study management oversight: Dimitar Kalev, Constanta Timcheva, Maya Velikova (Bulgaria); Alejandro Acevedo, Christian Caglevic, Alejandro Majlis (Chile); Etienne Brain, Robert Herve, Eric Legouffe (France); Lars Hanker, Jens Huober, Agnieszka Korfel, Arnd Nusch, Christoph Salat, Wolfgang Wiest (Germany); Vassilis Georgoulias, Georgios Koumakis, Christos Papadimitriou (Greece); Michele Caruso, Stefano Cascinu, Lucia Del Mastro, Annamaria Molino, Alberto Zaniboni (Italy); Carlos Alberto Hernández-Hernández, Francisco Alejo Medina Soto (Mexico); Boguslawa Karaszewska, Elzbieta Nowara, Joanna Pikiel, Piotr Potemski, Pawel Rozanowski, Tomasz Sarosiek, Elzbieta Staroslawska (Poland); Vladimir Kovcin, Zora Neskovic-Konstantinovic (Serbia); and Kepa Amillano Párraga, Paula Gonzalez Villarroel, Jose Juan Illarramendi, José Valero Alvarez Gallego, Pilar Zamora Aunon (Spain). The work presented here, including the conduct of the study, data analysis, and interpretation, was supported by GlaxoSmithKline. Editorial assistance in the preparation of the manuscript was provided by David Griffiths, PhD, of Fishawack Indicia Ltd and funded by GlaxoSmithKline.
Conflict of interest
WJ currently receives consultancy/advisory fees from GlaxoSmithKline; WJ and PP have received grant funding from GlaxoSmithKline (this conflict is current for WJ and was last active for PP 3 years ago). KB, LM, and MDS are presently employed by GlaxoSmithKline and hold stocks/shares in GlaxoSmithKline. BK, CC, CP, CS, EB, ES, JP, and TS have no conflicts to disclose.
Ethical standards
The study was conducted according to current laws of the countries in which the study was performed.
References
- 1.Hung MC, Lau YK. Basic science of HER-2/neu: a review. Semin Oncol. 1999;26:51–59. [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Weigel MT, Dowsett M. Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocr Relat Cancer. 2010;17:R245–R262. doi: 10.1677/ERC-10-0136. [DOI] [PubMed] [Google Scholar]
- 4.Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackwell KL, Burstein HJ, Sledge G, Stein S, Ellis C, Casey M, Baselga J, O’Shaughnessy J. Updated survival analysis of a randomized study of lapatinib alone or in combination with tastuzumab in women with HER2-positive metastatic breast cancer progressing on trastuzumab therapy. Cancer Res. 2009;69:3. doi: 10.1158/0008-5472.SABCS-09-61. [DOI] [Google Scholar]
- 6.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 7.Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol. 2012;30:2585–2592. doi: 10.1200/JCO.2011.35.6725. [DOI] [PubMed] [Google Scholar]
- 8.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 9.Weber BL, Vogel C, Jones S, Harvey H, Hutchins L, Bigley J, Hohneker J. Intravenous vinorelbine as first-line and second-line therapy in advanced breast cancer. J Clin Oncol. 1995;13:2722–2730. doi: 10.1200/JCO.1995.13.11.2722. [DOI] [PubMed] [Google Scholar]
- 10.Terenziani M, Demicheli R, Brambilla C, Ferrari L, Moliterni A, Zambetti M, Caraceni A, Martini C, Bonadonna G. Vinorelbine: an active, non cross-resistant drug in advanced breast cancer. Results from a phase II study. Breast Cancer Res Treat. 1996;39:285–291. doi: 10.1007/BF01806156. [DOI] [PubMed] [Google Scholar]
- 11.Fumoleau P, Delgado FM, Delozier T, et al. Phase II trial of weekly intravenous vinorelbine in first-line advanced breast cancer chemotherapy. J Clin Oncol. 1993;11:1245–1252. doi: 10.1200/JCO.1993.11.7.1245. [DOI] [PubMed] [Google Scholar]
- 12.Chew HK, Somlo G, Mack PC, Gitlitz B, Gandour-Edwards R, Christensen S, Linden H, Solis LJ, Yang X, Davies AM. Phase I study of continuous and intermittent schedules of lapatinib in combination with vinorelbine in solid tumors. Ann Oncol. 2012;23:1023–1029. doi: 10.1093/annonc/mdr328. [DOI] [PubMed] [Google Scholar]
- 13.Brain E, Isambert N, Dalenc F, et al. Phase I study of lapatinib plus vinorelbine in patients with locally advanced or metastatic breast cancer overexpressing HER2. Br J Cancer. 2012;106:673–677. doi: 10.1038/bjc.2011.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saip P, Erlap Y, Ozkan M, Karaca H, Benekli M, Cetin B, Isikdogan A, Kucukoner M, Basaran G (2011) Phase II study of lapatinib in combination with vinorelbine in patients with ErbB2-amplified recurrent or metastatic breast cancer. J Clin Oncol 29 (suppl; abstr e13079) [DOI] [PubMed]
- 15.Chew HK, Schwartzberg LS, Bandarinath S, Rubin P, Schumaker G, Daugherty JP, DeSilvio M, Mahoney JM (2013) Phase II study of lapatinib in combination with vinorelbine, as first- or second-line therapy in women with HER2-overexpressing metastatic breast cancer. Presented at the American Society of Clinical Oncology Symposium 2013; May 30–June 3 2013; Chicago, Illinois (Abstract 621) [DOI] [PMC free article] [PubMed]
- 16.Janni W, Sarosiek T, Papadimitriou C, Álvarez Gallego J, Caruso M, Wiest W, Lim M, Andersson H, Das-Gupta A (2011) A phase II randomized trial of lapatinib with either vinorelbine or capecitabine as first- and second-line therapy for ErbB2-overexpressing metastatic breast cancer (MBC): safety results. J Clin Oncol 29 (suppl; abstr e11097)
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Berry G, Kitchin RM, Mock PA. A comparison of two simple hazard ratio estimators based on the logrank test. Stat Med. 1991;10:749–755. doi: 10.1002/sim.4780100510. [DOI] [PubMed] [Google Scholar]
- 19.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 20.Xu BH, Jiang ZF, Chua D, et al. Lapatinib plus capecitabine in treating HER2-positive advanced breast cancer: efficacy, safety, and biomarker results from Chinese patients. Chin J Cancer. 2011;30:327–335. doi: 10.5732/cjc.010.10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cameron D, Casey M, Oliva C, Newstat B, Imwalle B, Geyer CE. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist. 2010;15:924–934. doi: 10.1634/theoncologist.2009-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.FDA (2011) FDA Approval for Lapatinib Ditosylate. http://www.cancer.gov/cancertopics/druginfo/fda-lapatinib/. Acessed April 2013
- 23.Yip AY, Tse LA, Ong EY, Chow LW. Survival benefits from lapatinib therapy in women with HER2-overexpressing breast cancer: a systematic review. Anticancer Drugs. 2010;21:487–493. doi: 10.1097/CAD.0b013e3283388eaf. [DOI] [PubMed] [Google Scholar]
- 24.Gelmon KA, Boyle F, Kaufman B, Huntsman D, Manikhas A, Di Leo A, Martin M (2012) Open-label phase III randomized controlled trial comparing taxane-based chemotherapy (Tax) with lapatinib (L) or trastuzumab (T) as first-line therapy for women with HER2+ metastatic breast cancer: interim analysis (IA) of NCIC CTG. MA.31/GSK EGF 108919. J Clin Oncol 30 (suppl; abstr LBA671)
- 25.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guarneri V, Frassoldati A, Bottini A, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol. 2012;30:1989–1995. doi: 10.1200/JCO.2011.39.0823. [DOI] [PubMed] [Google Scholar]
- 27.Robidoux A, Tang G, Rastogi P, Geyer CE, Azar C, Norman Atkins J, Fehrenbacher L, Douglas Bear H, Baez-Diaz L (2012) Evaluation of lapatinib as a component of neoadjuvant therapy for HER2+ operable breast cancer: NSABP protocol B-41. J Clin Oncol 30 (suppl; abstr LBA506) [DOI] [PubMed]
- 28.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Extra JM, Antoine EC, Vincent-Salomon A, et al. Efficacy of trastuzumab in routine clinical practice and after progression for metastatic breast cancer patients: the observational Hermine study. Oncologist. 2010;15:799–809. doi: 10.1634/theoncologist.2009-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German Breast Group 26/Breast International Group 03-05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 31.Petrelli F, Barni S. A pooled analysis of 2,618 patients treated with trastuzumab beyond progression for advanced breast cancer. Clin Breast Cancer. 2013;13:81–87. doi: 10.1016/j.clbc.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Verma S, Miles D, Gianni L, et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1 Kaplan–Meier plot: time to second progression following crossover, from randomization (crossover population) QD, once daily; PFS, progression-free survival; lap, lapatinib; cap, capecitabine; vin, vinorelbine (EPS 901 kb)