Abstract
We show that neural crest stem cells affect mouse hair follicle development. During embryogenesis hair follicle induction is regulated by complex reciprocal and functionally redundant signals between epidermis and dermis, which remain to be fully understood. Canonical Wnt signalling is a hallmark of neural crest cells and also a prerequisite for hair follicle induction prior to hair placode formation in the epidermis. As neural crest stem cells invade the epidermis during early embryonic development we aimed at determining whether neural crest cells affect hair follicle development. To attenuate, but not silence, canonical Wnt signalling specifically in neural crest cells, we analyzed Wnt1-cre(+/−)::Pygo2(−/−) mice in which the β-catenin co-activator gene, Pygopus 2 (Pygo2), is deleted specifically in neural crest cells. Both, hair density and hair thickness were reduced in mutant mice. Furthermore, hair development was delayed and the relative ratio of hair types was affected. There was a decrease in zig-zag hairs and an increase in awl hairs. Mouse neural crest stem cells expressed ectodysplasin, an essential effector in the formation of zig-zag hair. Taken together, our data support the novel notion that neural crest cells are involved in the earliest stages of hair follicle development.
Keywords: Neural crest, Hair follicle, Pygopus2, Wnt, Zig‐zag hair, Awl hair
Introduction
This study addresses aspects of hair follicle formation. Prior to morphological changes in the ectoderm, the first phase of hair induction (hair stage 0 and earlier) is regulated by epidermal Wnt signalling, which is an absolute requirement for hair induction [1, 46]. In the absence of activated β-catenin, epidermal stem cells fail to differentiate into follicular keratinocytes, but instead adopt an epidermal fate [18]. Epidermal Wnt proteins expressed early in development are essential for subsequent uniform dermal Wnt signalling [3]. The stage of placode formation (hair stage 1) is primarily characterized by Eda-A1 (ectodysplasin) signalling through its cognate receptor Edar, which is expressed in epidermal cells and upon activation leads to downstream NFκB and Wnt10a/b signalling [38].
Neural crest stem cells transiently express Wnt1 during early emigration from the neural tube. The only other cells that express Wnt1 during embryonic development are dorsal neural tube cells of the future spinal cord [5]. Wnt1-driven Cre recombinase permanently excises loxP-flanked genes of interest. In order to delete Pygo2 early in development and specifically in neural crest stem cells, we therefore crossed Wnt1-cre mice with transgenic mice that have a loxP-flanked Pypogus2 (Pygo2) gene.
Pygo2 is one of the genes that comprise the neural crest stem cell molecular signature [17] but was not detected in epidermal stem cells [33]. Functionally, Pygo2 is an enhancer of β-catenin function in canonical Wnt signalling [19, 23]. In the developing skin, Pygo2 is expressed in scattered cells within the one-layered undifferentiated epidermis before it is expressed in hair placodes and then more widely in the epidermis [22].
In addition to Pygo2, embryonic neural crest cells express Wnt proteins [8], the Wnt receptors, Frizzled (Fzd) and LRP [43, 46, 47, 49], and they actively signal through the canonical Wnt pathway [5, 6, 44]. A subset of multipotent neural crest stem cells migrate laterally from the neural tube below the ectoderm, invade the ectoderm [34] and eventually settle in the bulge of hair follicles [4, 17, 40, 41]. As global Pygo2 knockout mice have a reduced hair density [23] these observations raised the question whether neural crest cells are involved in hair follicle formation at the pre-placode stage.
Materials and Methods
Animals and Genotyping
Work with mice was approved by the Newcastle University review board under license numbers 60/3621 and 60/3876. The following transgenic mouse lines were used: Wnt1-cre (gift of A McMahon; [5]), Rosa26-LacZ (R26R; gift of P Soriano; [42]) and Pygo2 (gift of S Potter; [39]). Wnt1-cre (+/−) mice were crossed with Pygo2(+/−) mice to obtain Wnt1-cre(+/−)::Pygo2(−/−) double transgenic mice (“Wnt1-cre(+/−)::Pygo(−/−)”) in which Pygo2 is deleted exclusively in neural crest cells as well as Wnt1-cre(+/−)::Pygo (+/+) control littermates. For visualizing neural crest cells in mutant mice, Wnt1-cre(+/−)::Pygo2(+/−) mice were bred and then crossed with R26R(+/−)::Pygo(+/−) mice in order to obtain Wnt1-cre(+/−)::R26R(+/−)::Pygo2(−/−) triple transgenic mice in which Pygo2 is deleted in a neural crest-specific way and neural crest cells can be visualized by their expression of β-glactosidase. Control littermates were Wnt1-cre(+/−)::R26R(+/−)::Pygo2(+/+). Double and triple transgenic animals were born with the expected Mendelian ratio, were indistinguishable from normal littermates and fed normally. Most mutant mice, however, died within the first 24–48 h postnatally. The cause of death might be connected to a significant decrease of neural crest cells in the enteric nervous system (unpublished data). This deficiency could lead to Hirschsprung disease-like problems, which are lethal. Therefore, P0 back skin from neonates was studied. For genotyping, tail biopsies were boiled in 25 mM NaOH/0.2 mM EDTA (Sigma-Aldrich) for 20 min and then re-buffered with an equal volume of 40 mM Tris-Cl (Sigma-Aldrich). Up to 5 μl of buffered extract was used per PCR reaction. For detection of Wnt1-cre and R26R, the primers and PCR conditions used were exactly as described previously [45]. For detection of the Pygo2(flox/−) allele the following primers (Integrated DNA Technologies) Wnt1-cre(+/−)::Pygo(−/−) allele, forward (F) CCTGGATTCTTGTTGCTGGTATG; reverse (R) AAGGTATTTGGTGCTCCGAGGG; Pygo2 WT or floxed allele, (F) TGTCTTGATGACAGCGTTTAGCC, (R) AGATTCAGTAAGCTGAGCCTGGTG [39]. PCR conditions were as follows: 1 cycle, 94 °C for 3 min, followed by 35 cycles consisting of 30 s denaturation at 94 °C, 30 s annealing at 62 °C, and 45 s extension at 72 °C and a final 10 min extension at 72 °C. PCR products were resolved on 1.5 % agarose gels.
Neural Crest Cultures
E9.5 mouse embryos were obtained from timed pregnant C57BL/6J females (The Jackson Laboratory). Neural tubes were dissected and cultured exactly as we have described [18]. Briefly, dissected trunk tissue was treated with trypsin and then triturated to remove somites and the notochord. Subsequently, neural tubes were placed into Cell-Start coated culture plates. Large numbers of neural crest cells emigrated overnight from the neural tubes and the cultures were fixed 18 h post-explantation.
Immunohistochemistry
Neural crest cultures were fixed with 4 % paraformaldehyde (Sigma) for 30 min, rinsed, and incubated overnight in the cold with FITC-conjugated Ectodermal Dysplasia 1 antibody at 1:100 (orb15540; biorbyt, Cambridge). The next day the cultures were rinsed exhaustively and mounted with Vectashield plus DAPI (H1200; Vector Laboratories) and a coverslip.
Paraffin Tissue Processing
Tissue used for haematoxylin/eosin (H&E) staining, and X-gal staining was processed as follows. After dissection in PBS, fixation with 4 % PFA at 4 °C overnight following by PBS rinse, tissue was trimmed and dehydrated in a 70 %, 95 %, 100 % × 2 ethanol series for 1 h each. They were then incubated 2 × 20 min in xylene, followed by a 50 %, 75 %, 100 % exchange with liquid paraffin for 20 min each in paraffin wells. Tissue was incubated in liquid paraffin overnight at 60 °C. The next day liquid paraffin was exchanged, the tissue embedded, paraffin blocks trimmed, mounted, and 7 μm sections prepared. Sections were collected on SuperFrost Plus microscope slides (Menzel-Gläser; VWR) and dried overnight at 42 °C.
Haematoxylin and Eosin Staining
For H&E staining slides were first deparaffined in xylene (2 × 5 min) and rehydrated through ethanol series (100 % × 2, 90 %, 70 %, 50 %, and 30 %) for 5 min each at RT. After a 5 min water rinse they were stained in haematoxylin (Thermo Scientific) for 20 s, rinsed with water, blued in Scott’s tap water (Sigma-Aldrich; 10 s), rinsed, stained with eosin (Thermo Scientific) for 20 s, rinsed, dehydrated (50 %, 70 %, 100 % ethanol for 1 min each) and processed through an equal xylene series (3 min each). Slides were mounted with DPX mounting medium (VWR), coverslipped, dried, and observed under bright field optics.
Morphometrical Analysis
Hair follicle density analysis was performed as described previously [30]. Cross-sections of the back skin of 3 mutant and 3 wild type mice were taken at and parallel to the skin surface and H&E stained. Images were captured and using Adobe Photoshop, 1 × 104 μm square grids were applied to the images. Hair follicles were counted within gridded field areas to calculate the number of hair follicles per 1 × 104 μm2. In total, 260 areas (260 × 1 × 104 μm2) from mutant skin and 252 areas from wild type control littermates were scored and statistically analysed.
To determine the cross sectional area of hair follicles, images were processed with AxioVision software (Carl Zeiss Microscopy). Overall, 676 hair follicles were measured from wild type animals and 575 hair from mutant animals from three animals of each genotype.
Morphometrical staging analysis was performed as described by Glotzer et al. [11], using criteria according to Paus et al. [31]. Longitudinal sections of back skin from 3 mutant and 3 wild type animals were prepared and H&E stained. To avoid staging of the same follicle twice every tenth section was analysed. Three hundred two mutant and 304 wild type hair follicles were staged from three animals for each genotype.
X-gal Staining
For X-gal staining back skin was dissected in PBS (Sigma-Aldrich), and processed in cushioned histological cassettes to avoid skin curling. X-gal staining was performed as described previously by Pennisi et al. [30] with modifications. After 1 h fixation in ice cold 4 % PFA at room temperature on a shaker, samples were washed 3 × 10 min in wash buffer consisting of 0.01 % sodium deoxycholate (Sigma-Aldrich), 0.02 % NP-40 (Sigma-Aldrich), 2 mM MgCI2 in PBS) followed by incubation in X-gal staining solution consisting of 5 mM Potassium ferricyanide (Sigma-Aldrich), 5 mM Potassium ferrocyanide (Sigma-Aldrich), 2 mM MgCI2, 0.01 % Sodium deoxycholate , 0.02 % NP-40, 20 mg/ml X-gal (Sigma-Aldrich) in DMF (Sigma-Aldrich) overnight at RT on the shaker in the dark. Then samples were washed in wash buffer 2 × 10 min at RT, fixed in 4 % PFA at 4 °C overnight and rinsed with PBS. For histochemical analyses samples were dehydrated through an ethanol series, cleared and infused with paraffin as described above. Embedding, mounting and sectioning were performed exactly as described above. Sections were counterstained with nuclear fast red using the protocol for H&E staining; haematoxylin, Scott’s tap water and eosin were replaced, however, by 5 min of staining with nuclear fast red, then dehydrated and mounted with DPX medium, coverslipped, dried, and analyzed under bright field optics. Images from every tenth section were acquired to quantify the amount of hair follicles with positive X-gal cells. Data were derived from two mice of each genotype; 256 hair follicles from 18 skin sections from Wnt1-cre(+/−)::Pygo(−/−) mice as well as 311 hair follicles were scored from 17 skin sections from wild type newborns.
Hair Count
Hair from the back skin of two Wnt1-cre(+/−)::Pygo2(−/−) rare survivors and from two Wnt1-cre(+/−)::Pygo2(+/+) control littermates were plucked, scored and categorized according to criteria described by Schlake [36]. A total of 490 hair from two Wnt1-cre(+/−)::Pygo(−/−) and 459 hair from two wild type animals each were analyzed.
Statistical Analysis
Statistical significance was determined using the two-tailed unpaired Student’s t-test (for hair density, hair follicle thickness, and neural crest cell positive hair follicles). For developmental staging, logistic regression analysis was performed using STATA software.
Results
We analyzed mutant back skin sections taken superficially and parallel to the skin surface and found that the average hair density was reduced by approximately 19 % (Fig. 1). Furthermore, the average cross-section area of hair from Wnt1-cre(+/−)::Pygo(−/−) newborns was significantly smaller compared to control littermates (Fig. 1). These results showed that Wnt1-cre(+/−)::Pygo(−/−) newborn mice have fewer and thinner hair follicles.
Fig. 1.
Decreased hair thickness and hair density in Wnt1-cre(+/−)::Pygo(−/−) newborn mice. a, a’ Tangential sections of back skin of Wint1-cre(+/−)::Pygo2(−/−) mice. Hair thickness was significantly reduced in Wnt1-cre(+/−)::Pygo(−/−) mice by ∼7 %. Number mice analysed, three animals per genotype. Number visual fields analysed, 31 (Wnt1-cre(+/−)::Pygo(−/−), and 33 (Pygo2). Number hair measured, 575 (Wnt1-cre(+/−)::Pygo(−/−) and 676 (Pygo2). b, b’ Sections from Wnt1-cre(+/−)::Pygo2(+/+) littermates. Average hair density was reduced by ∼19 % in Wnt1-cre(+/−)::Pygo(−/−) mice. Number mice analysed, three animals per genotype. Number visual fields analysed, 260 (Wnt1-cre(+/−)::Pygo(−/−) and 252 (Pygo2). Bars, 100 μm
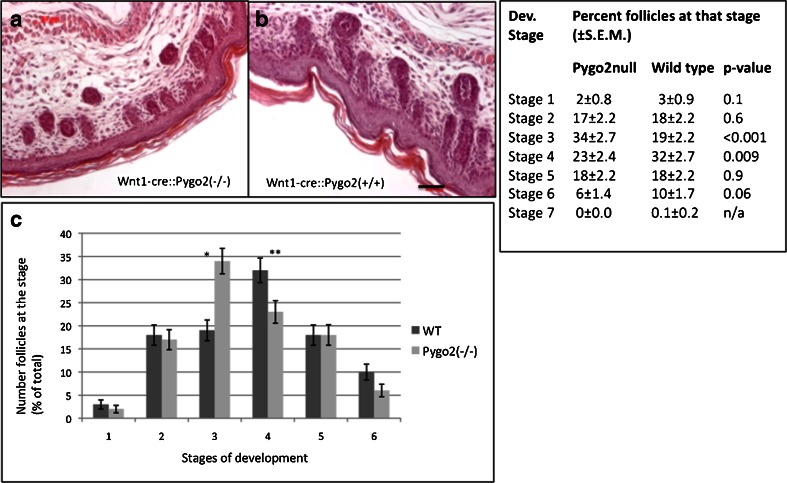
Hair development during embryogenesis and perinatally can be divided into 6 stages according to morphology (see e.g., [36]). Thus, to analyze hair development in more detail, hair in newborns was staged. Hair stages analyzed ranged from stage 1 to 6 in both wild type and mutant mice. While in control mice 32 % of follicles resided in stage 4, 23 % of follicles were in stage 4 in Wnt1-cre(+/−)::Pygo(−/−) mice (Fig. 2). Conversely, in Wnt1-cre(+/−)::Pygo(−/−) newborns 34 % of follicles and 19 % of control hair follicles remained in stage 3. This observation indicated that there is a delay in hair follicle development in Wnt1-cre(+/−)::Pygo(−/−) newborn mice (Fig. 2).
Fig. 2.
Delay in development of hair follicles in Wnt1-cre(+/−)::Pygo(−/−) newborns. Hair follicles in skin sections from Wnt1-cre(+/−)::Pygo2 (−/−) and Wnt1-cre(+/−)::Pygo2(+/+). Significantly more hair follicles from Wnt1-cre(+/−)::Pygo(−/−) mice were at stage 3 at birth, whereas significantly more follicles were at stage 4 in Pygo2 newborn mice. Number animals analysed, three per genotype. Number hair staged, 302 (Pygo null) and 304 (Pygo). Bar, 50 μm
Next we asked whether hair types are affected in Wnt1-cre(+/−)::Pygo(−/−) mice, and if so which ones. Wnt1-cre(+/−)::Pygo(−/−) mice that did not die perinatally developed a coat. Hair from these mice and from Wnt1-cre(+/−)::Pygo(+/+) littermates were plucked and scored according to hair type, that is awl, auchen and zig-zag hairs. Guard hairs were disregarded, as there were too few of them to produce meaningful data and there was guard hair breakage, which produced artefacts. There was a 38.6 % decrease in zig-zag hairs, a 73.3 % increase in awl hairs, and a 2.5-fold increase in auchen hairs in Wnt1-cre(+/−)::Pygo(−/−) mice compared to control littermates (Fig. 3). The morphology of zig-zag hair was altered in Wnt1-cre(+/−)::Pygo(−/−) mice. Zig-zag hair were shorter by about one third in Wnt1-cre(+/−)::Pygo(−/−) mice with only two to three bends, rather than the three to four bends observed in wild type mice [36]. These results show that neural crest cell-specific deletion of the Pygo2 gene early in development also affected the relative proportion of these hair types as well as the morphology of zig-zag hair.
Fig. 3.
Hair follicle formation is perturbed in Wnt1-cre(+/−)::Pygo(−/−) mice. Left panel, morphology of mutant hair. Right panel, three of the four types of hair follicles, awl, auchen and zig-zag hair were analysed in rare surviving Wnt1-cre::Pygo2(−/−) mice. There were too few guard hairs to collect meaningful data. In Wnt1-cre::Pygo2(−/−) the number of zig-zag hairs was significantly reduced by ∼39 %. As they should have the length of awl hair, zig-zag hairs were shorter by approximately one third compared to wild type zig-zag hairs. There was a 73 % significant increase in Awl hair, and a 2.5-fold increase in Auchen hair in Wnt1-cre(+/−)::Pygo(−/−) mice. At total of 490 hair from two Pygo null and 459 hair from two wild type animals were scored
In order to determine whether the number of neural crest cells present in hair follicles of mutant newborns was affected by the deletion of the Pygo2 gene, we analyzed Xgal stained sections of triple transgenic Wnt1-cre(+/−)::R26R(+/−)::Pygo2(−/−) mice and Wnt1-cre(+/−)::R26R(+/−)::Pygo2(+/+) control littermates. The portion of hair follicles that contained Xgal-positive cells was scored. There was a 10 % decrease in hair follicle sections that contained neural crest cells in Wnt1-cre(+/−)::Pygo(−/−) mice compared to control littermates, indicating a decrease in the number of neural crest cells present in hair follicles of mutant neonates (Fig. 4).
Fig. 4.
Decrease of neural crest cells in hair follicles from Pygo null newborns. a, a’ Two different fields of vision of hair follicles from back skin of Wnt1-cre::Pygo(−/−) newborn mice. b, b’ Hair follicles from Wnt1-cre::Pygo(+/+) newborn mice. There was about a ∼10 % significant decrease in hair follicle sections that contained detectable neural crest cells in the forming hair shaft. Bar, 50 μm
Zig-zag hair development is dependent on Eda-A1/Edar signalling [38] and Eda-A1 is a Wnt target gene [7, 21]. As zig-zag hair numbers and morphology were affected by the mutation, we were interested in determining whether neural crest cells express Eda-A1. Neural tube explants from day 9.5 mouse embryos yielded large numbers of rapidly migrating and proliferating neural crest cells (Fig. 5a). Immunocytochemistry showed that neural crest cells were immuno-positive for Eda-A1 (Fig. 5b). All cells were Eda-A1 immunoreactive, but there was a range of intensities of fluorescence. There were groups of cells showing intense immunofluorescence (Fig. 5b middle) intermingled with cells that showed low intensity of Eda-A1 fluorescence (e.g., Fig. 5b, upper left)
Fig. 5.
Embryonic mouse neural crest cells express Eda-A1. a Neural tube explant from E9.5 C57Bl/6J mouse embryos. Eighteen hours post-explantation, neural tubes were surrounded by a halo of thousands of emigrating and proliferating neural crest cells. b Cultures were fixed at 18 h post-explantation and processed for immunocytochemistry with antibodies against Eda-A1. All neural crest cells showed Eda-A1 immunoreactivity; subsets of cells were intensely immunoreactive. Asterisk indicates neural crest cells; nt neural tube. Number of explants stained and observed: 5. Bars, 50 μm
Discussion
Wnt signalling in the epidermis at the pre-placode stage, i.e. prior to hair stage 0, is an absolute requirement for hair follicle induction [10, 15, 28, 38] — its origin is, however, unknown. Since canonical Wnt signalling is a characteristic of neural crest cells, because neural crest cells express Pygo2 [17] and invade the epidermis [4, 17, 34, 40], and since pre-placode epidermal areas express Pygo2 [22], we asked in this study whether neural crest cells could participate in regulating hair follicle formation. We found that deleting the Pygo2 gene specifically in neural crest cells during early embryonic development perturbed hair formation in diverse ways. Hair follicles were sparser and thinner in Wnt1-cre(+/−)::Pygo(−/−) newborns. There was a decrease in the number of zig-zag hair in surviving mutant animals and zig-zag hair morphology was altered. There was a delay in hair follicle development and a diminished number of neural crest cells present in hair follicles of Wnt1-cre(+/−)::Pygo(−/−) mice. Furthermore, we showed that mouse neural crest cells express Eda-A1. Li et al. [23] observed an approximate 30 % decrease in hair density in global Pygo2 knockout animals. We observed a 19 % decrease in hair density, which shows that the decrease in hair density in the absence of Pygo2 is to a large part neural crest-related.
Ectodermal Wnt/β-catenin signalling as well as ectodermal Eda-1A signalling through its cognate receptor, Edar, and activation of the down-stream target genes, NFκB and Wnt10a/b, are essential for zig-zag hair induction and morphology, respectively (Andl 2002) [1, 16, 24, 37, 38]. Eda-A1 is a Wnt target gene [7, 21] and is expressed in neural crest cells (this study), while the cognate receptor, Edar is induced by mesenchyme derived activin [21] but is expressed exclusively in the epidermis and becomes limited to the hair placodes [1, 9, 16, 25, 48, 50]. The Eda-A1/Edar signalling network is thought to be involved in regulating hair thickness, as in humans enhanced Edar signalling leads to coarser hair [27]. Taken together, the reduction in hair thickness and perturbed zig-zag hair development in Wnt1-cre(+/−)::Pygo2(−/−) mice can be explained by diminished Eda-A1 production as a consequence of attenuated Wnt1 signalling in neural crest cells.
Other indications of perturbed hair formation was a delay in hair development in Wnt1-cre(+/−)::Pygo2(−/−) newborns and significantly fewer neural crest cells in hair follicles. Canonical Wnt signalling in neural crest cells is involved in neural crest initiation, migration, proliferation and differentiation [12, 14, 29]. Attenuated Wnt signalling as a consequence of deleting Pygo2 in neural crest cells can thus explain the reduced number of neural crest cells in hair follicles, which may compound the observed deficits in hair development. Neural crest cells are, however, known for their capacity to compensate for cell loss, as after partial ablation of the neural crest, neighbouring neural crest can change their pathways and fill in the gaps [20].
In addition to its role in canonical Wnt signalling, Pygo2 can also bind to the promoter of several histone genes in a cell context-specific way; in particular Pygo2 enhances acetylation of histone H3 [13, 26]. Whether altered histone modification plays a role in hair follicle induction remains to be determined.
Inductive fields where hair follicle induction takes place are proposed to be formed by interacting, self-organizing gradients of stimulatory and inhibitory signals (reaction-diffusion mechanism) that involve Wnt signalling and the Wnt inhibitor, DKK [35]. In view of the existing literature and our data in the present study we suggest a working model for the involvement of neural crest cells in hair follicle formation (Fig. 6). Neural crest cells express Wnt proteins, in particular Wnt1 [5] and Wnt3a [44], which activate the canonical Wnt pathway. Neural crest cells also express the cognate receptors frizzled (Fzd) and LRP [43, 46, 47, 49], enabling autocrine Wnt signalling in neural crest cells (Fig. 6a). As a result neural crest cells are able to produce the Wnt target gene Eda-A1 ([32]; this study). Eda-A1 is a membrane bound protein that gets cleaved and released as a soluble protein and could thus occupy Edar receptors on neighbouring epidermal cells (Fig. 6b). DKK4 is an Eda-A1/Edar target gene [9] and is transiently expressed in the epidermis in pre-placodal locations ([2]; Fig. 6c). DKK expression affects placode size and hair spacing [2, 9, 35]. Due to reduced Wnt signalling and consequent reduced Eda expression, placode size and hair spacing would thus be expected to be perturbed in Wnt1-cre(+/−)::Pygo(−/−) mice, which is in agreement with our observations. Additional work, which is beyond the scope of this study, will be required to corroborate the role of neural crest cells in hair development in the mouse.
Fig. 6.
Hypothetical model for mechanisms underlying pre-placode stage neural crest-derived epidermal Wnt signalling. a Neural crest cells that have invaded the ectoderm [34] are in close apposition with epidermal stem cells at pre-placode stages. Neural crest cells express Wnt proteins [8, 44] and their cognate receptors [43, 46, 47, 49]. Therefore neural crest cells can undergo autocrine Wnt signalling. Epidermal stem cells within the future placode express the Eda-A1 receptor, Edar [3, 32]. No activated beta-catenin indicative of Wnt signalling in the dermis is detected at that early stage of hair development [3]. b As a consequence of cell-autonomous Wnt signalling, neural crest cells produce Wnt target genes, including Eda-A1 ([21]; this study). c Eda-A1/Edar signalling in epidermal cells leads to DKK4 expression. In Wnt1-cre(+/−)::Pygo(−/−) mice, it is expected that the DKK:Wnt ratio is altered and Eda-A1 protein levels are reduced. Together these changes can explain the here observed disturbances in hair follicle thickness, hair density, as well as zig-zag hair formation and morphology
Acknowledgments
This work was supported by Medical Research Council grant 22358, and Newcastle University. MSB thanks Profs Steven Potter (Pygo2), Philippe Soriano (R26R-LacZ) and Andrew McMahon (Wnt1-cre) for providing the respective mouse lines, and Prof Heather Cordell for advice on statistical analysis.
Conflict of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Developmental Cell. 2002;2(5):643–653. doi: 10.1016/S1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- 2.Bazzi H, Fantauzzo KA, Richardson GD, et al. The Wnt inhibitor, Dickkopf 4, is induced by canonical Wnt signalling during ectodermal appendage morphogenesis. Developmental Biology. 2007;305(2):498–507. doi: 10.1016/j.ydbio.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 3.Chen, D., Jarrell, A., Guo, C., et al. (2012). Dermal β-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development, 139(8), 1522–1533. [DOI] [PMC free article] [PubMed]
- 4.Clewes O, Narytnyk A, Gillinder KR, et al. Human epidermal neural crest stem cells (hEPI-NCSC)--characterization and directed differentiation into osteocytes and melanocytes. Stem Cell Reviews. 2011;7(4):799–814. doi: 10.1007/s12015-011-9255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danielian PS, Muccino D, Rowitch DH, et al. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Current Biology. 1998;8(24):1323–1326. doi: 10.1016/S0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 6.Dunn KJ, Brady M, Ochsenbauer-Jambor C, et al. WNT1 and WNT3a promote expansion of melanocytes through distinct modes of action. Pigment Cell Research. 2005;18(3):167–180. doi: 10.1111/j.1600-0749.2005.00226.x. [DOI] [PubMed] [Google Scholar]
- 7.Durmowicz MC, Cui CY, Schlessinger D. The EDA gene is a target of, but does not regulate Wnt signaling. Gene. 2002;285(1-2):203–211. doi: 10.1016/S0378-1119(02)00407-9. [DOI] [PubMed] [Google Scholar]
- 8.Echelard Y, Vassileva G, McMahon AP. Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development. 1994;120(8):2213–2224. doi: 10.1242/dev.120.8.2213. [DOI] [PubMed] [Google Scholar]
- 9.Fliniaux I, Mikkola ML, Lefebvre S, Thesleff I. Identification of dkk4 as a target of Eda-A1/Edar pathway reveals an unexpected role of ectodysplasin as inhibitor of Wnt signalling in ectodermal placodes. Developmental Biology. 2008;320(1):60–71. doi: 10.1016/j.ydbio.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Fu, J., & Hsu, W. (2012) Epidermal Wnt controls hair follicle induction by orchestrating dynamic signaling crosstalk between the epidermis and dermis. Journal of Investigative Dermatology. Nov 29. doi:10.1038/jid.2012.407. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 11.Glotzer DJ, Zelzer E, Olsen BR. Impaired skin and hair follicle development in Runx2 deficient mice. Developmental Biology. 2008;315(2):459–473. doi: 10.1016/j.ydbio.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes & Development. 2008;22(17):2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu B, Watanabe K, Dai X. Pygo2 regulates histone gene expression and H3 K56 acetylation in human mammary epithelial cells. Cell Cycle. 2012;11(1):79–87. doi: 10.4161/cc.11.1.18402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hari L, Miescher I, Shakhova O, et al. Temporal control of neural crest lineage generation by Wnt/β-catenin signaling. Development. 2012;139(12):2107–2117. doi: 10.1242/dev.073064. [DOI] [PubMed] [Google Scholar]
- 15.Huelsken J, Vogel R, Erdmann B, et al. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105(4):533–545. doi: 10.1016/S0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 16.Headon DJ, Overbeek PA. Involvement of a novel Tnf receptor homologue in hair follicle induction. Nature Genetics. 1999;22(4):370–374. doi: 10.1038/11943. [DOI] [PubMed] [Google Scholar]
- 17.Hu YF, Zhang ZJ, Sieber-Blum M. An epidermal neural crest stem cell (EPI-NCSC) molecular signature. Stem Cells. 2006;24(12):2692–2702. doi: 10.1634/stemcells.2006-0233. [DOI] [PubMed] [Google Scholar]
- 18.Ito K, Morita T, Sieber-Blum M. In vitro clonal analysis of mouse neural crest development. Developmental Biology. 1993;157(2):517–525. doi: 10.1006/dbio.1993.1154. [DOI] [PubMed] [Google Scholar]
- 19.Jessen S, Gu B, Dai X. Pygopus and the Wnt signaling pathway: a diverse set of connections. Bioessays. 2008;30(5):448–456. doi: 10.1002/bies.20757. [DOI] [PubMed] [Google Scholar]
- 20.Kulesa P, Bronner-Fraser M, Fraser S. In ovo time-lapse analysis after dorsal neural tube ablation shows rerouting of chick hindbrain neural crest. Development. 2000;127:2843–2852. doi: 10.1242/dev.127.13.2843. [DOI] [PubMed] [Google Scholar]
- 21.Laurikkala J, Pispa J, Jung HS, et al. Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Development. 2002;129(10):2541–2553. doi: 10.1242/dev.129.10.2541. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Mackay DR, Ma J, Dai X. Cloning and developmental expression of mouse pygopus 2, a putative Wnt signaling component. Genomics. 2004;84(2):398–405. doi: 10.1016/j.ygeno.2004.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Rhéaume C, Teng A, et al. Developmental phenotypes and reduced Wnt signaling in mice deficient for pygopus 2. Genesis. 2007;45(5):318–325. doi: 10.1002/dvg.20299. [DOI] [PubMed] [Google Scholar]
- 24.Mikkola ML, Pispa J, Pekkanen M, et al. Ectodysplasin, a protein required for epithelial morphogenesis, is a novel TNF homologue and promotes cell-matrix adhesion. Mechanisms of Development. 1999;88(2):133–146. doi: 10.1016/S0925-4773(99)00180-X. [DOI] [PubMed] [Google Scholar]
- 25.Mikkola ML, Thesleff I. Ectodysplasin signaling in development. Cytokine & Growth Factor Reviews. 2003;14(3-4):211–224. doi: 10.1016/S1359-6101(03)00020-0. [DOI] [PubMed] [Google Scholar]
- 26.Miller TC, Rutherford TJ, Johnson CM, et al. Allosteric remodelling of the histone H3 binding pocket in the Pygo2 PHD finger triggered by its binding to the B9L/BCL9 co-factor. Journal of Molecular Biology. 2010;401(5):969–984. doi: 10.1016/j.jmb.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mou C, Thomason HA, Willan PM, et al. Enhanced ectodysplasin-A receptor (EDAR) signaling alters multiple fiber characteristics to produce the East Asian hair form. Human Mutation. 2008;29(12):1405–1411. doi: 10.1002/humu.20795. [DOI] [PubMed] [Google Scholar]
- 28.Närhi K, Järvinen E, Birchmeier W, et al. Sustained epithelial beta-catenin activity induces precocious hair development but disrupts hair follicle down-growth and hair shaft formation. Development. 2008;135(6):1019–1028. doi: 10.1242/dev.016550. [DOI] [PubMed] [Google Scholar]
- 29.Patapoutian A, Reichardt LF. Roles of Wnt proteins in neural development and maintenance. Current Opinion in Neurobiology. 2000;10(3):392–399. doi: 10.1016/S0959-4388(00)00100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pennisi DJ, Wilkinson L, Kolle G, et al. Crim1KST264/KST264 mice display a disruption of the Crim1 gene resulting in perinatal lethality with defects in multiple organ systems. Developmental Dynamics. 2007;236(2):502–511. doi: 10.1002/dvdy.21015. [DOI] [PubMed] [Google Scholar]
- 31.Paus R, Müller-Röver S, Van Der Veen C, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. Journal of Investigative Dermatology. 1999;113(4):523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- 32.Pummila M, Fliniaux I, Jaatinen R, et al. Ectodysplasin has a dual role in ectodermal organogenesis: inhibition of Bmp activity and induction of Shh expression. Development. 2007;134(1):117–125. doi: 10.1242/dev.02708. [DOI] [PubMed] [Google Scholar]
- 33.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3(11):e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson MK, Sieber-Blum M. Pluripotent neural crest cells in the developing skin of the quail embryo. Developmental Biology. 1993;157(2):348–358. doi: 10.1006/dbio.1993.1140. [DOI] [PubMed] [Google Scholar]
- 35.Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science. 2006;314(5804):1447–1450. doi: 10.1126/science.1130088. [DOI] [PubMed] [Google Scholar]
- 36.Schlake T. Determination of hair structure and shape. Seminars in Cell & Developmental Biology. 2007;18:267–273. doi: 10.1016/j.semcdb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt-Ullrich R, Aebischer T, Hülsken J, et al. Requirement of NF-kappaB/Rel for the development of hair follicles and other epidermal appendices. Development. 2001;128(19):3843–3853. doi: 10.1242/dev.128.19.3843. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27(3):247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- 39.Schwab KR, Patterson LT, Hartman HA, et al. Pygo1 and Pygo2 roles in Wnt signaling in mammalian kidney development. BMC Biology. 2007;5:15. doi: 10.1186/1741-7007-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sieber-Blum M, Grim M, Hu YF, Szeder V. Pluripotent neural crest stem cells in the adult hair follicle. Developmental Dynamics. 2004;231(2):258–269. doi: 10.1002/dvdy.20129. [DOI] [PubMed] [Google Scholar]
- 41.Sieber-Blum M, Hu Y. Epidermal neural crest stem cells (EPI-NCSC) and pluripotency. Stem Cell Reviews. 2008;4(4):256–260. doi: 10.1007/s12015-008-9042-0. [DOI] [PubMed] [Google Scholar]
- 42.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genetics. 1999;21(1):70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 43.Sisson BE, Topczewski J. Expression of five frizzleds during zebrafish craniofacial development. Gene Expression Patterns. 2009;9(7):520–527. doi: 10.1016/j.gep.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun X, Zhang R, Lin X, Xu X. Wnt3a regulates the development of cardiac neural crest cells by modulating expression of cysteine-rich intestinal protein 2 in rhombomere 6. Circulation Research. 2008;102(7):831–839. doi: 10.1161/CIRCRESAHA.107.166488. [DOI] [PubMed] [Google Scholar]
- 45.Szeder V, Grim M, Halata Z, Sieber-Blum M. Neural crest origin of mammalian Merkel cells. Developmental Biology. 2003;253(2):258–263. doi: 10.1016/S0012-1606(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 46.van Gijn ME, Blankesteijn WM, Smits JF, et al. Frizzled 2 is transiently expressed in neural crest-containing areas during development of the heart and great arteries in the mouse. Anat Embryol (Berl). 2001;203(3):185–192. doi: 10.1007/s004290000152. [DOI] [PubMed] [Google Scholar]
- 47.Wu J, Saint-Jeannet JP, Klein PS. Wnt-frizzled signaling in neural crest formation. Trends in Neurosciences. 2003;26(1):40–45. doi: 10.1016/S0166-2236(02)00011-5. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Tomann P, Andl T, et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Developmental Cell. 2009;17(1):49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Y, Snead ML. Derivation of cranial neural crest-like cells from human embryonic stem cells. Biochemical and Biophysical Research Communications. 2008;376(3):542–547. doi: 10.1016/j.bbrc.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou P, Byrne C, Jacobs J, Fuchs E. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes & Development. 1995;9(6):700–713. doi: 10.1101/gad.9.6.700. [DOI] [PubMed] [Google Scholar]