Abstract
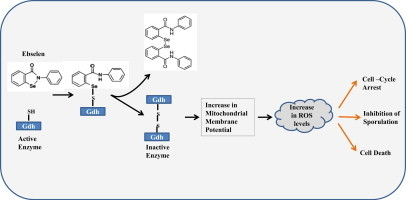
Ebselen is a synthetic, lipid-soluble seleno-organic compound. The high electrophilicity of ebselen enables it to react with multiple cysteine residues of various proteins. Despite extensive research on ebselen, its target molecules and mechanism of action remains less understood. We performed biochemical as well as in vivo experiments employing budding yeast as a model organism to understand the mode of action of ebselen. The growth curve analysis and FACS (florescence activated cell sorting) assays revealed that ebselen exerts growth inhibitory effects on yeast cells by causing a delay in cell cycle progression. We observed that ebselen exposure causes an increase in intracellular ROS levels and mitochondrial membrane potential, and that these effects were reversed by addition of antioxidants such as reduced glutathione (GSH) or N-acetyl-l-cysteine (NAC). Interestingly, a significant increase in ROS levels was noticed in gdh3-deleted cells compared to wild-type cells. Furthermore, we showed that ebselen inhibits GDH function by interacting with its cysteine residues, leading to the formation of inactive hexameric GDH. Two-dimensional gel electrophoresis revealed protein targets of ebselen including CPR1, the yeast homolog of Cyclophilin A. Additionally, ebselen treatment leads to the inhibition of yeast sporulation. These results indicate a novel direct connection between ebselen and redox homeostasis.
Keywords: Ebselen, ROS levels, Mitochondrial membrane potential, Glutamate dehydrogenase, Histone clipping, Yeast sporulation
Abbreviations: CypA, Cyclophilin A; DCFH-DA, 2,7-dichlorodihydrofluorescein diacetate; FACS, florescence activated cell sorting; GDH, glutamate dehydrogenase; GSH, glutathione; NAC, N-acetyl-l-cysteine; Ni-NTA, nickel-nitrilotriacetic acid; ROS, reactive oxygen species; SOD, superoxide dismutase
Graphical Abstract
Highlights
-
•
Ebselen treatment causes an increase in intracellular ROS levels and mitochondrial membrane potential.
-
•
Ebselen inactivates GDH3 and Cyclophilin A by interacting with their cysteine residues.
-
•
The ebselen-induced increase in ROS levels leads to inhibition of sporulation in yeast.
-
•
Yeast gdh3 deletion potentiates ROS generation upon ebselen treatment.
Introduction
A number of cellular defense mechanisms exist to quench free radicals and prevent intracellular damage by reducing the harmful effects of reactive oxygen species (ROS). These mechanisms or factors include low-molecular-weight antioxidants such as ascorbic acid and glutathione, and antioxidant enzymes such as thioredoxins, superoxide dismutase (SOD), catalase, and glutathione peroxidase [1–3]. These activities play a key role in minimizing the physiological levels of ROS. However, with continuous elevation of the levels of ROS, the defense systems can be exhausted, resulting in cellular damage. Normally functioning cells can sustain and tolerate background levels of damage, but if an imbalance occurs, cellular damage will be increased. ROS are reactive in nature and may cause damage to key cellular components including DNA, proteins, and lipids [4,5]. ROS, including hydrogen peroxide, the superoxide anion, and the hydroxyl radical are highly toxic oxidants which are inevitably produced in response to multiple stimuli [5–9]. Therefore, cells possess a complex system to neutralize the deleterious effects of ROS [10–13]. Because ROS are principal mediators of the cellular damage, compounds that regulate the fate of such species may be of great importance.
Ebselen is a synthetic, lipid-soluble seleno-organic compound having potent antioxidant capacity. It is also a novel anti-inflammatory agent having glutathione peroxidase-like activity [14–18]. It has therapeutic activity in neurological disorders, acute pancreatitis, noise-induced hearing loss, and cardiotoxicity. It also exhibits antiatherosclerotic, antithrombotic, and cytoprotective properties [15,19–21]. However, excessive amounts of ebselen are toxic to the cells. Ebselen is genotoxic above a concentration of 10 μM in V79 cells [22]. It induces apoptosis in HepG2 cells through a rapid depletion of intracellular thiols [23]. At high concentrations, it stimulates Ca2+ release from mitochondria via an NAD+ hydrolysis-dependent mechanism, and accelerates mitochondrial respiration and swelling, which are indicative of deterioration of the mitochondrial function [24]. A previous study from our laboratory documented the activation of DNA repair genes in yeast cells exposed to Ebselen [25].
Seleno-organic compounds exhibit strong electrophilic activity and are therefore capable of forming selenenyl-sulfide bonds with the cysteine residues in proteins [26–28]. The ability of ebselen to covalently react with proteins’ cysteine residues is thought to explain why the selenazal drugs modulate the activity of various inflammation-related enzymes, including lipoxygenase, nitric oxide synthase, and NADPH oxidase [29–31]. Various antioxidant enzymes have evolved to regulate the cellular levels of ROS. Glutathione (GSH), which is the most abundant peptide in cells, fulfills several functions, including directly scavenging of HO· and singlet oxygen [32]. Glutamate, which is required for the biosynthesis of GSH, is synthesized by GDH. The 2 isofunctional NADP-GDH of Saccharomyces cerevisiae (GDH1 and GDH3) [33–35] are involved in the synthesis of GSH, and the normal functioning of these enzymes is required for the regulation of ROS levels [34]. Multiple cysteine residues are seen in the primary structure of GDH3. Hence, selenazal drugs may modulate its activity, resulting in its defective functioning.
In this study, we report that ebselen potently inhibits chicken GDH by reacting with the enzyme's cysteine residues, leading to its inhibition. Ebselen exposure induces high intracellular ROS levels, and the deletion of yeast gdh3 potentiates ROS production, indicating that GDH3 is an in vivo target of this drug. Taken together, our results depicts GDH as a novel target of ebselen, and these observations can be used to design ebselen-based molecular therapeutics for the regulation of ROS levels under various conditions.
Materials and methods
Reagents and yeast strains
All reagents, unless otherwise stated, were purchased from Sigma–Aldrich (USA). Yeast strains were grown in SC (synthetic complete) medium. All experiments were performed on wild type strain W1588-4c (MATa ade2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1, ura3-1, RAD5+), BY4743 (MATa/α his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 LYS2/lys2Δ0 met15Δ0/MET15 ura3Δ0/ura3Δ0) gdh1, gdh2 and gdh3 KO generated in BY4743 background were purchased from Open Biosystem. For sporulation experiment USY61 (MATa/MATalpha ura3D0/ura3D0 his3D1/his3D1 CAN1/can1::Ste2::spHis5 flo8D0/flo8D0) yeast diploid strain was used, we got this strain as a kind gift from Ulrich Schlecht. Ebselen was dissolved in DMSO. Concentration of DMSO was kept below 0.1% in all experiments.
Growth sensitivity and methylene blue assays
To investigate the effect of ebselen on the growth of yeast mutants, wild type yeast strains were inoculated into YPD liquid medium and grown to saturation by incubating cultures at 30 °C and 200 rpm. Yeast saturated cultures were serially diluted (10−1, 10−2, 10−3, 10−4) in 1.0 ml of sterile double distilled water. 3 μl of cultures were spotted onto SC agar plates containing ebselen (2.5, 5.0, 7.5 and 10 μM) or DMSO. Plates were incubated at 30 °C and growth of the yeast strains were recorded at time intervals of 24, 48 and 72 h by scanning (HP scanjet G2410).
Wild type yeast cells were grown in YPD medium till log phase (OD600 equals to 0.6–0.8) and treated with ebselen at different concentrations (DMSO, 5, 10, 20, 30 and 50 μM) for 6 h. After treatment OD600 was recorded at regular intervals for growth curve analysis. Methylene blue assay was performed as described earlier [36,37] after 3 h of ebselen treatment, cells were stained with 100 μg/ml methylene blue to differentiate between live (unstained) and dead/metabolically inactive (dark blue colored) cells. Cells were observed under the bright field microscope by using LAS EZ-V1.7.0 software (LEICA DM500).
FACS analysis of yeast cells
Yeast cells in exponential phase were treated with alpha factor to synchronize cells in G1 phase. Cells were released in DMSO (control) or 25 μM ebselen containing media for 6 h. Samples were collected at regular intervals and harvested by centrifugation. Ethanol was added to cell pellets, with vigorous vortexing. Cells were collected by centrifugation and washed once with 50 mM sodium citrate buffer (pH 7.0). RNase A was added to the samples and incubated at 37 °C for 1 h. RNase A-treated samples were transferred to BD FACS flow containing 20 mg/ml propidium iodide (Sigma). Cellular DNA was detected by a BD FACS Aria III with BD FACS Diva software.
Detection of cellular ROS levels and assays for mitochondrial membrane potential (ΔΨ)
To measure ROS production we used 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) (Sigma, D6883). DCFH-DA is membrane-permeable and is trapped intracellularly following deacetylation. The resulting compound, DCFH, reacts with ROS (primarily H2O2 and hydroxyl radicals) to produce the oxidized fluorescent form 2,7-dichlorofluorescein (DCF). ROS analysis using DCFH-DA was performed as follows. Yeast cells were treated with 10 μM DCFH-DA in culture media for 1 h prior to harvesting. Cells were washed twice in ice-cold PBS (phosphate buffer saline), resuspended in same buffer and immediately observed under fluorescence microscope (AXIOVERT 4.0) using FITC filter. The membrane potential-dependent stain MitoTracker (Molecular Probes-Invitrogen) was used to assess the mitochondrial membrane potential of yeast cells. After treatment with drug approximately 1 × 107 yeast cells were harvested and washed with ice-cold PBS. Cells were resuspended in 100 μl of PBS followed by staining with MitoTracker. After staining cells were visualized under fluorescence microscope (AXIOVERT 4.0) using Rhodamine filter or by FACS. For analyzing the effect of reduced glutathione (GSH – 10 mM), or N-acetyl-l-cysteine (NAC – 20 mM) supplementation on ROS levels and mitochondrial membrane potential, they were added in exponential yeast culture 30 min prior to addition of ebselen. Cells were further grown for 3 h followed by staining with DCF-DA or MitoTracker and immediately analyzed by FACS.
Glutathione measurement assays
Glutathione levels were measured using the method described by Wu et al. [38]. Briefly, cells were grown to exponential phase and treated with DMSO (control) or ebselen for 3 h, washed with ice cold water, and resuspended in 250 μl of cold 1% 5-sulfosalicylic acid. Cells were broken by vigorous vortexing with glass beads and incubated at 4 °C for 15 min. The extract was centrifuged and supernatants were used to determine glutathione levels. Total glutathione was determined by adding 10 μl of lysate to 150 μl of assay mixture (0.1 M potassium phosphate, pH 7.0, 1 mM EDTA, 0.03 mg/ml 5,5′-dithiobis(2-nitrobenzoic acid), 0.12 unit of glutathione reductase). The samples were mixed and incubated for 5 min at room temperature followed by addition of 50 μl of NADPH (0.16 mg/ml). The formation of thiobis (2-nitrobenzoic acid) was measured spectrophotometrically at 420 nm over a 5-min period. Standard curves were generated for each experiment using 0–0.5 nmol of glutathione in 1% 5-sulfosalicylic acid. To measure GSSG alone, 100 μl lysate samples were derivatized by adding 2 μl of 97% 2-vinylpyridine, and the pH was adjusted by adding 2 μl of 25% triethanolamine followed by incubation at room temperature for 60 min. The samples were then assayed as described above for total GSSG. GSSG standards (0–0.1 nmol) were also treated with 2-vinylpyridine in an identical manner to the samples. Subtraction of the amount of GSSG in the lysate from the total glutathione concentration allowed a determination of GSH levels present in each sample.
Purification of human recombinant Cyclophilin A
Plasmid pET28a, encoding residues full length Cyclophilin A having N-ter hexahistidine tag, was transformed into Escherichia coli BL21 DE3 cells for expression. IPTG was added to induce the expression of Cyclophilin A, and the cultures were further incubated at 30 °C for 6 h. The cells producing Cyclophilin A were harvested, resuspended in 50 mM Tris–HCl buffer (pH 7.5) containing 2 mM beta-mercaptoethanol, 10% glycerol, and 0.5 M NaCl, and disrupted by sonication. The cell debris was removed by centrifugation (27,216g; 20 min), and the lysate was mixed gently with 1 ml (50% slurry) of nickel-nitrilotriacetic acid (Ni-NTA)–agarose resin (Qiagen) at 4 °C for 1 h. The Cyclophilin-bound Ni-NTA beads were then packed into an Econo-column (Bio-Rad) and washed with 100 ml of 50 mM Tris–HCl buffer (pH 7.5) containing 10% glycerol, 500 mM NaCl, and 5 mM imidazole . His6-tagged Cyclophilin A was eluted by a 3 ml elution buffer containing 500 mM imidazole. Eluted protein was concentrated and dialyzed to remove imidazole.
Analysis of glutamate dehydrogenase activity
To test the endopeptidase activity of GDH, an in vitro-assay system was developed as described earlier [39]. Briefly, for the in vitro proteolytic assay 0.5 μg of purified chicken GDH was mixed with 5.0 μg of chicken brain core histones in 20 μl reaction volume of buffer (25 mM Tris–Cl, pH 7.5, 150 mM NaCl, 10% glycerol, and 0.1 mM EDTA) and incubated at 37 °C for 1 h. 0.5 μM ebselen was used to check its effect on GDH activity. The reaction was stopped by boiling in SDS–PAGE sample loading buffer. Cleavage of H3 was monitored by resolving the reaction mixture on 15% SDS–PAGE.
Evaluation of effect of ebselen on purified GDH and Cyclophilin A
Purified chicken GDH was incubated with 0.5 μM ebselen in sodium phosphate buffer (pH 7.2) for 30 min at 37 °C. Similarly recombinant Cyclophilin A was incubated with increasing concentration of ebselen. The native and ebselen-modified GDH and Cyclophilin A were incubated with SDS sample buffer with or without a reducing agent for 5 min at 100 °C. The samples were separated by SDS–PAGE. The gel was trans-blotted onto a nitrocellulose membrane and western blotting was performed with anti-GDH (Sigma, SAB2100932-50UG) and anti-Cyclophilin A (Millipore, # 07-313) antibody.
Yeast sporulation assay
The following media were used for growth and sporulation of Saccharomyces cerevisiae: rich media YPD (1% yeast extract, 2% bactopeptone, 2% glucose), pre-sporulation media (0.5% ammonium sulfate, 0.17% yeast nitrogen base, 1% bactopeptone, 1% potassium acetate, 0.5% yeast extract, 1.02% potassium hydrogen phthalate) and sporulation media (2% potassium acetate). A fresh yeast colony was inoculated into 5 ml YPD and grown over night to saturation. 200 μl of this cell culture were inoculated into 30 ml of pre-sporulation media and grown until an optical density at 600 nm between 1.0 and 1.5 was reached. The cell suspension was centrifuged for 3 min at 3000 rpm and washed twice with 50 ml of pre-warmed water. Finally, the cell pellet was resuspended in 50 ml sporulation media. Cells were further grown for 24 h and sporulation was analyzed by visualizing cells under bright-field microscope (LEICA DM500) by using LAS EZ-V1.7.0 software.
For analysis of DNA content during sporulation, yeast strain USY61 were grown following sporulation protocol as described above. After transferring cells in sporulation media, 1 ml of cell suspension was taken out at regular intervals (0, 2, 4, 6 and 8 h) from DMSO, ammonium sulfate (2 mM) or ebselen (30 μM) treated samples. Cellular DNA was detected by a BD FACS Aria III with BD FACS Diva software.
Analysis of ROS levels Cda2 expression during sporulation
For analyzing ROS levels during sporulation yeast cells were treated with 10 μM DCFH-DA in culture media for 1 h prior to harvesting. Cells were washed twice in ice-cold PBS (phosphate buffer saline), resuspended in same buffer and immediately observed under fluorescence microscope (AXIOVERT 4.0) using FITC filter. To analyze the effect of ebselen on sporulation the expression of Cda2, a sporulation-specific chitin deacetylase involved in the biosynthesis of the spore wall component chitosan, was monitored. Experiment was conducted as described earlier [40]. Briefly, a colony from USY613 (USY61+ pCDA2-eGFP::HygB) was grown overnight till saturation in SC supplemented with Hygromycin B. 200 μl of this cell suspension was inoculated into 30 ml of pre-sporulation media (supplemented with Hygromycin B) and grown until an optical density between 1.2 and 1.6 was reached. The cell suspension was centrifuged and washed with 50 ml of pre-warmed water. Finally, the cell pellet was resuspended in 50 ml sporulation media containing DMSO (control), 2 mM ammonium sulfate or 30 μM ebselen and 10 ml cells harvested at regular intervals (0, 6, 12, 18, 24 h). Extracts were made from the cell pellet following TCA method. Cda2 expression was analyzed by western blotting using anti-GFP antibody.
Sample preparation for proteomic analysis
Cells were harvested and dispersed in lysis buffer (50 mM Tris–HCl, pH 7.5, 0.1% Triton X-100, 5% glycerol, 0.6 M NaCl, 5 mM EDTA, 5 mM DTT, 0.5 mM PMSF, protease inhibitor cocktail, 0.1 times the volume of solution containing 1 mg/ml DNase I, 0.25 mg/ml RNase A, 50 mM MgCl2). Samples were processed according to the 2D Clean-Up kit protocol (GE Healthcare). Protein concentration was determined by Bradford (Sigma) and 200 μg proteins were rehydrated on 7 cm Immobiline™ (pH 3–10) DryStrip (GE Healthcare) for 12 h in Immobiline DryStrip Reswelling Tray. First dimensional Isoelectric focusing (IEF) was performed in Ettan-IPGphor® Isoelectric focusing unit (GE Healthcare) followed by sequential equilibration in buffer containing DTT and IAA. Second dimensional SDS–PAGE was done. Selected differentially expressed protein spots were identified by mass spectrometry (National Centre for Biological Sciences, Bangalore, India).
Results
Increasing concentration of ebselen inhibits growth of yeast cells
To determine the effective dose of ebselen, we performed the spot test, growth curve analysis, and the methylene blue assay. For the spot test, we used increasing concentrations of ebselen (2.5, 5.0, 7.5, and 10.0 μM) incorporated in YPD (yeast extract powder 1%, peptone 2%, dextrose 2%) agar medium. We observed a dose-dependent decrease in the cell survival of wild type cells (Fig. 1A). For growth curve analysis, exponential phase-yeast cells were treated with ebselen (5.0, 10.0, 20.0, 30.0, and 50.0 μM) for 6 h. We found that increasing concentrations of ebselen were toxic to yeast cells, as shown by the reduction of growth (Fig. 1B). The result of growth curve analysis in liquid medium was consistent with that of methylene blue assay. For methylene blue assay, the cells were treated with ebselen at same concentrations mentioned above for 3 h. Live cells remained unstained while dead cells or metabolically inactive cells became dark blue. With increasing concentrations of ebselen (from 5 to 50 μM), the number of dead/metabolically inactive cells increased (Fig. 1C). Microscopic examination along with growth curve analysis indicated the toxicity of ebselen at higher doses.
Fig. 1.
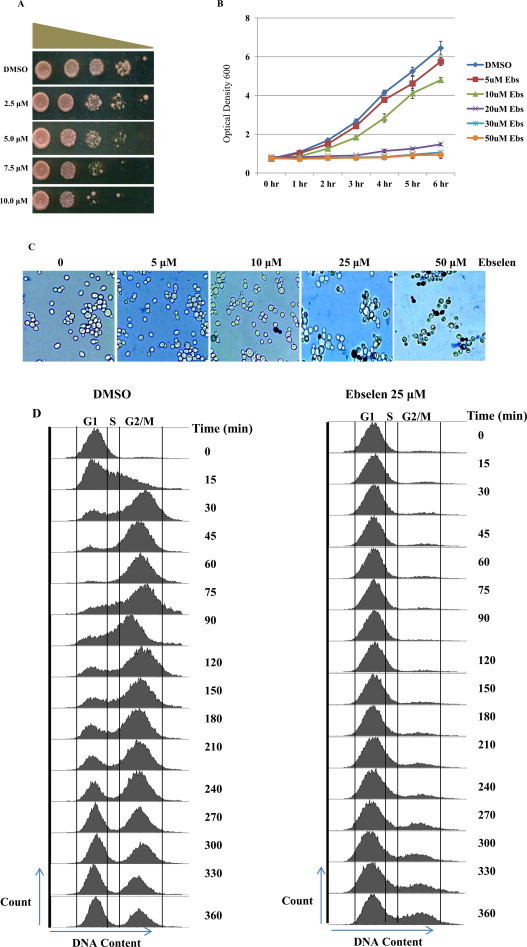
Increasing concentration of ebselen inhibits growth of yeast cells: (A) Spot test of wild type cells (WT1588-4C) in presence of DMSO (control), 2.5, 5.0, 7.5 and 10.0 μM ebselen. Yeast saturated cultures were serially diluted (10−1, 10−2, 10−3, 10−4) in 1.0 ml of sterile double distilled water. (B) Wild type yeast cells were grown in YPD medium until log phase reached at OD600 (0.6–0.8) then treated with ebselen at different concentrations (5, 10, 20, 30 and 50 μM) for 6 h. Growth was recorded by taking aliquots at regular interval. (C) Methylene blue assay was performed in treated and untreated cells and observed under microscope with magnification 400×. (D) FACS analysis showing the effect of the ebselen on yeast cell cycle progression. Wild-type cells were cultured in SC medium to exponential phase and treated with alpha factor to synchronize all cells in G1 phase. After synchronization cells were released in either DMSO (control) or 25 μM ebselen containing media. The culture was sampled at indicated time points and cellular DNA content was analyzed by FACS.
Ebselen treatment causes delay in cell cycle progression
Based on our growth curve experiments, we observed that the growth rate of yeast cells was significantly reduced upon ebselen treatment, indicating that the drug is possibly causing defects in normal cell cycle. To validate this hypothesis, we performed FACS analysis to observe the cell cycle progression in the presence or absence of ebselen. Exponentially growing yeast cells were synchronized by alpha factor treatment for 2 h. After synchronization G1 arrested cells were released in 25 μM ebselen or DMSO containing media. The DMSO treated cells quickly moved to G2 phase within 60 min of release from alpha factor arrest (Fig. 1D) while ebselen inhibited the movement of cells for prolonged time (Fig. 1D). Even after 360 min of release in ebselen containing media all cells were not able to progress to G2 phase suggesting that ebselen treatment causes delay in cell cycle progression.
Ebselen potentially inhibits the growth of wild type S. cerevisiae in a ROS-dependent manner by increasing the mitochondrial membrane potential (ΔΨ)
Next, we tried to define the intracellular molecular targets of ebselen in S. cerevisiae, aiming to understand the molecular insights of ROS generation upon ebselen treatment. Yeast in the exponential phase were treated with increasing concentrations of ebselen (5, 10, 20, and 30 μM ) or 1 mM hydrogen peroxide for 3 h. Hydrogen peroxide present in the medium served as a positive control for ROS. Notably, ebselen induced a dramatic increase in ROS generation, which was revealed by H2DHFA staining (Fig. 2A). We did not observe a ROS increase at low concentration of ebselen (5 and 10 μM) suggesting that high doses of ebselen are required to enhance ROS levels (Fig. 2A). Additionally our FACS analysis also exhibited similar results as observed in microscopic images. Ebselen treatment causes increase in ROS upon ebselen treatment in a dose dependent manner (Fig. 2C).
Fig. 2.
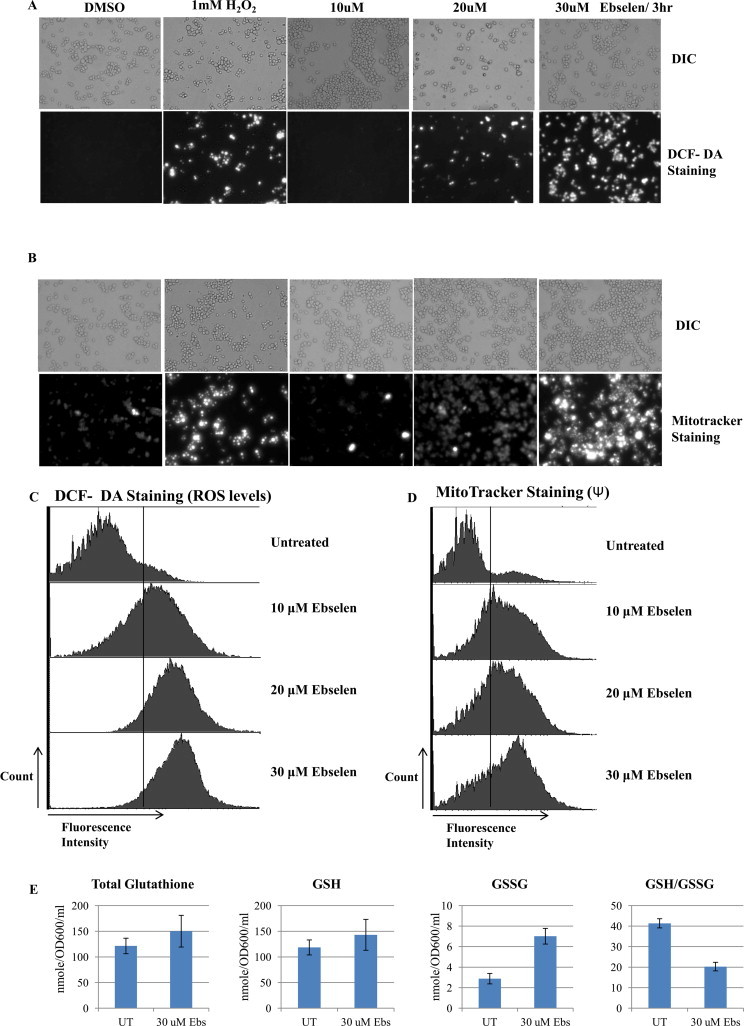
Ebselen treatment increases reactive oxygen species production and mitochondrial membrane potential in S. cerevisiae cells. ROS production detected by (A) DCF-DA and mitochondrial membrane potential by MitoTracker (B) in control cells and cells treated for 3 h. Cells were treated with 1 mM H2O2 for 3 h and it served as positive control. In A and B the upper panels show phase contrast microscopy; the lower panels show fluorescence microscopy of the same cells. (C and D) Yeast wild type strain was grown in SC media till exponential phase. Cells were treated with indicated concentration of ebselen for 3 h. The cells were then stained with DCF-DA or MitoTracker Red and examined by FACS as described in materials and methods. (E) Wild type cells were grown in DMSO or indicated concentration of ebselen for 3 h. GSH, GSSG, and the GSH:GSSG ratios were determined. Values are means S.D. of three independent cultures.
We used MitoTracker to monitor the mitochondrial membrane potential (ΔΨ). This probe irreversibly stains mitochondria in a ΔΨ -dependent fashion (the staining is more intense when ΔΨ is high). As shown in Fig. 2B, MitoTracker staining could be imaged without drug treatment only at enhanced contrast. However, with increasing concentrations of ebselen, especially at 20 or 30 μM, a strong MitoTracker staining was evident and ΔΨ increase was observed when 1 mM H2O2 was added (Fig. 2B). Similar increase in fluorescence was observed in FACS analysis (Fig. 2D). These results suggested that the abnormally elevated ΔΨ results from inhibition of ΔΨ-dependent processes in S. cerevisiae cells.
As an additional test to substantiate our observations we measured total glutathione, GSH and GSSG levels upon ebselen treatment. Oxidative stress is indicated by increased total glutathione, GSH and GSSG levels, and a decreased in GSH:GSSG ratio [38]. We calculated these parameters and compared wild type cells grown in DMSO (control) and 30 μM ebselen. Comparison of untreated versus the ebselen treated samples indicated that the treatment causes the elevation of oxidative stress parameters. Upon ebselen treatment total glutathione, GSH, and GSSG were elevated relative to untreated and the GSH: GSSG levels were reduced after treatment (Fig. 2E).
Supplementation of reduced glutathione (GSH) or N-acetyl cysteine (NAC) rescues ebselen induced increase in ROS/ mitochondrial membrane potential
To determine whether the ROS accumulation in ebselen treated cells is facilitated by increase in free radicals, we evaluated the effect of ebselen on ROS levels in presence of the antioxidants, such as N-acetyl-l-cysteine (NAC) and reduced glutathione (GSH). As shown in Fig. 3A, the addition of the 10 mM GSH or 30 mM NAC restored the growth of cells in the presence of ebselen (7.5 μM). NAC or GSH has been shown to exert antioxidant functions through scavenging ROS by the reaction with its thiol group. The ROS and mitochondrial membrane potential was measured upon ebselen treatment in presence or absence of NAC or GSH. As shown in Fig. 3B and C, the ROS level upon ebselen treatment was markedly suppressed by NAC or GSH. Taken together, these results suggest that ebselen causes generation of reactive oxygen species leading to growth inhibition/ cell death in yeast cells.
Fig. 3.
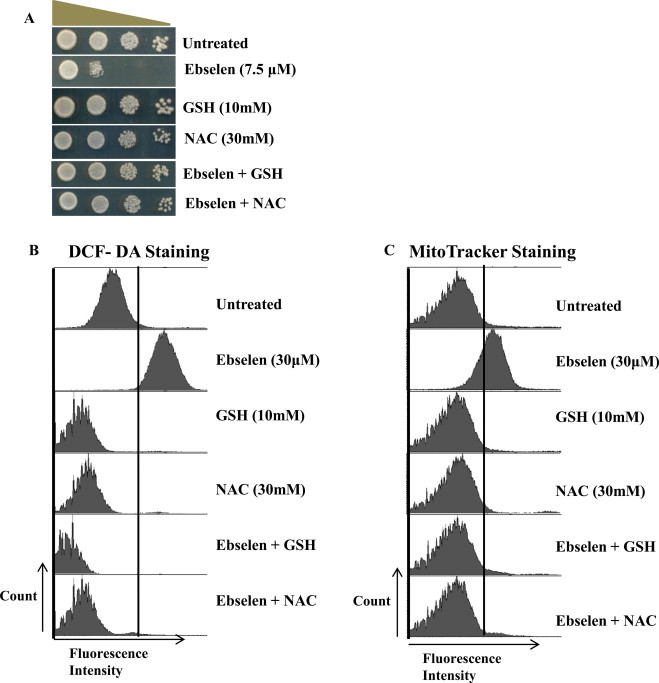
Effect of exogenous supply of GSH or NAC on ebselen induced ROS accumulation. (A) Spot test of wild type cells (WT1588-4C) supplemented with either NAC or GSH, and plates containing ebselen (7.5 μM) with either NAC or GSH. Yeast saturated cultures were serially diluted (10−1, 10−2, 10−3, 10−4) in 1.0 ml of sterile double distilled water and spotted onto the plates. Cells were cultured at 30 °C for 2–3 days. (D and E) Wild-type yeast strain grown in SC media supplemented with or without 10 mM GSH or 20 mM NAC for 1 h followed by exposure to 30 μM ebselen for 3 h. Yeast cells were processed for FACS analysis after staining with either DCF-DA (D) or MitoTracker Red (E).
gdh3-deletion in S. cerevisiae potentiates ROS generation by ebselen
Among the ROS-scavenging systems, the GSH system, which consists of GSH, GPx, and glutathione reductase, is the most important intracellular defense mechanism [41]. GPx catalyzes the reduction of H2O2 and oxidizes GSH to GSSG. GSSG is then reduced back to GSH by glutathione reductase [42]. Hence, the ability of the cells to reduce GSSG or synthesize GSH from glutamate is the key to effectively eliminate ROS-mediated cell damage [43]. The two isofunctional NADP-GDH of S. cerevisiae (GDH1 and GDH3) are involved in the synthesis of GSH and they differ in their allosteric properties and in the rates of α-ketoglutarate utilization [34]. Hence, we next investigated the functional role of glutamate dehydrogenases (GDH1, GDH2, and GDH3) in the ebselen-mediated ROS generation. To this end, we first tested whether gdh1, 2, or 3 deletions affected the levels of ROS under normal conditions. The basal levels of ROS were not changed significantly in DCF-DA stained gdh1/gdh2/gdh3-deleted cells compared to similarly stained wild type cells (Fig. 4A). When we investigated alterations in the ROS levels in the presence of ebselen, the ROS increase was prominent at 20 μM in gdh3Δ cells compared to that in gdh1Δ, gdh2Δ, and wild type cells (Fig. 4B).
Fig. 4.
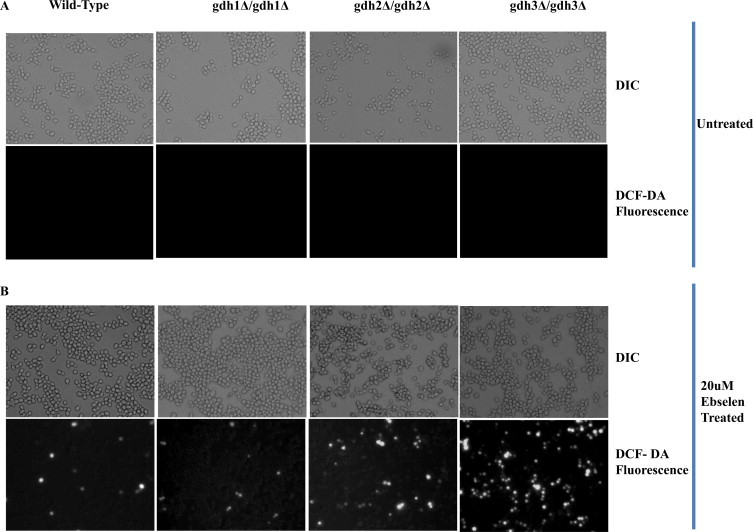
gdh3-dependent ROS generation by ebselen in S. cerevisiae. Wild-type and the gdh1, gdh2 and gdh3-deletion mutant were treated with 20 μM ebselen for 2 h. Then, they were stained with 10 μM H2DCFDA for 1 min, and the level of ROS was observed by fluorescence microscopy. (A) Background ROS levels in WT, gdh1, gdh2 and gdh3-deletion mutants. Upper panels show phase contrast microscopy; the lower panels show florescence microscopy of the same cells. (B) ROS level in mutant and wild-type cells.
Ebselen inhibits GDH by binding with its cysteine residue
Based on experiments performed on yeast cells, we found that GDH3 might a molecular target of ebselen. To further validate this hypothesis, we analyzed the effect of ebselen on purified chicken GDH by performing histone H3 digestion assay. We have identified GDH as a novel histone H3 protease [44]; hence, we performed H3 clipping by GDH in the presence or absence of ebselen. We observed the complete inhibition of H3 digestion/GDH activity in the presence of 0.5 μM ebselen (Fig. 5A, lane 5), suggesting that this drug interferes with the proteolytic activity of GDH. According to the literature, it is well established that ebselen acts as potent inhibitor of various proteins that have multiple cysteine residues [29–31]. In fact, ebselen makes covalent bonds with the proteins’ cysteine residues, making them inactive if that residue is critical for the function. Chicken GDH also has several cysteine residues (Fig. 5D); hence, we analyzed the GDH activity in the presence of β-mercaptoethanol and ebselen. Interestingly, GDH activity was restored when β-mercaptoethanol was added to the reaction mixture containing ebselen (Fig. 5A, lane 3). Ebselen did not react with the histones present in the reaction (Fig. 5B) because there is no difference in the pattern of histone bands in the presence or absence of ebselen when a non-reducing dye is used (Fig. 5B, compare lanes 2, 3 and 4).
Fig. 5.
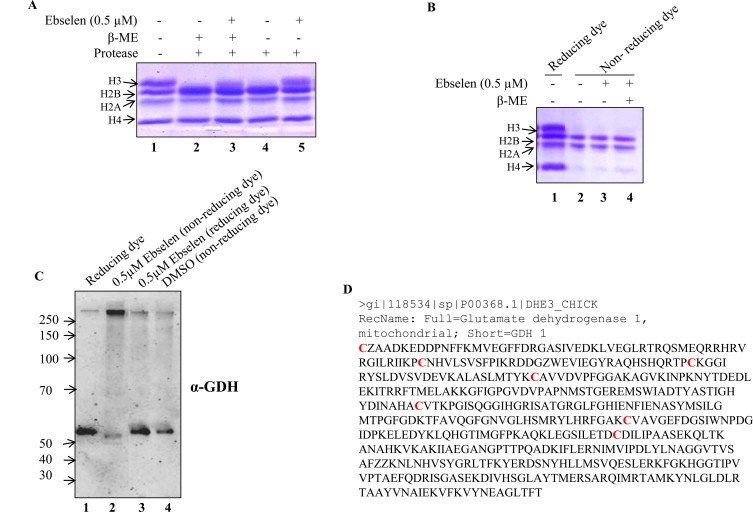
The effect of ebselen on GDH. (A) The effect of ebselen on GDH protease activity was analyzed by incubating purified core histones with chicken GDH in presence and/or absence of β-mercaptoethanol. (B and C) Effect of ebselen on core histones and on GDH protein profile was analyzed by resolving proteins on non-reducing and reducing SDS–PAGE, followed by coomassie brilliant blue R staining (B) and by western blotting with anti-GDH antibody (C) respectively. (D) The cysteine residues (labeled in red) of GDH were highlighted as the probable interacting site for ebselen. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
We next characterized the ebselen-treated GDH by non-reducing and reducing SDS–PAGE (Fig. 5C). When subjected to the non-reducing SDS–PAGE, the native (unoxidized/monomer) form of GDH migrated as a single protein band of about 50 kDa (Fig. 5C, lane 1). However, upon incubation with 0.5 μM ebselen, GDH was converted to a higher molecular weight protein species probably corresponding to the intermolecular cross-linking as observed in western blotting with GDH antibody (Fig. 5C, lane 2). Moreover, the formation of these modified proteins was not detected during the reducing SDS–PAGE (Fig. 5C, lane 3), and since the same concentration of DMSO was used in the control reaction showing no effect of solvent on GDH (Fig. 5D), we suggest that ebselen primarily formed selenenyl-sulfide (–Se–S–) and/or disulfide (–S–S–) linkages within GDH. Taken together, these results suggested that ebselen abrogates GDH activity by interacting with its cysteine residues and that this activity can be reversed in the presence of reducing agent.
Ebselen alters the proteome profile of yeast cells
To observe the effect of ebselen on the global protein profile, we performed proteome profiling using 2D gel electrophoresis (Fig. 6A), which revealed an alteration in the expression of a few proteins when comparing the control and 25 μM ebselen-treated samples. To identify those proteins, spots were excised from the two-dimensional gels, subjected to trypsin digestion, and then successfully analyzed by a mass spectrometry analysis. The details of identified protein spots are shown in Table 1. The identified proteins fall into several different functional classes, including protein synthesis (TMA19, RPL12A, EFB1, Eukaryotic translation initiation factor 5A-1) glycolytic enzymes (glyceraldehyde 3-phosphate dehydrogenase, Phosphoglycerate mutase), chaperone proteins (Hsp10, peptidyl-proryl cis–trans isomelase) and antioxidant protein Peroxiredoxin type-2.
Fig. 6.
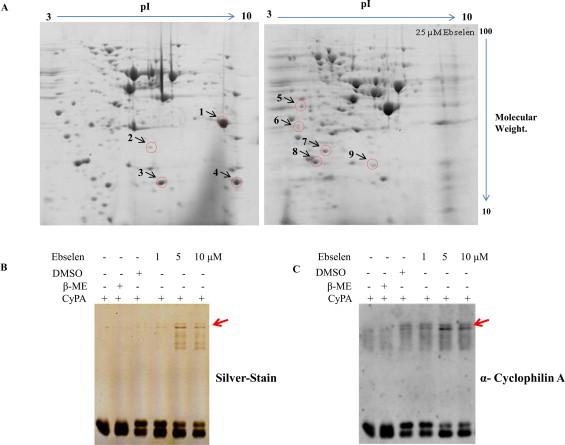
Ebselen alters the proteome profile of yeast cells: two dimensional gel electrophoresis gels are showing differentially expressed proteins in control (DMSO) and 25 μM ebselen treatment. (A) Proteins were separated in the first dimension on IEF gel (7 cm, pH 3–10) and then run on 12% SDS–PAGE. The red circles represent the protein spots which were excised from the gel for mass spectrometric analysis. The effect of ebselen on recombinant human Cyclophilin A. 5.0 μg CyPA incubated with increasing concentration of ebselen for 30 min, followed by running non-reducing SDS–PAGE (B) silver stained photograph, (C) corresponding cyclophilin A western signal image. Arrows indicate cyclophilin A complex. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Table 1.
List of spots identified by mass spectrometry.
| Spot No. | Protein description (OS = Saccharomyces cerevisiae) | Amino acid | Accession | MW [kDa] | pI | Score |
|---|---|---|---|---|---|---|
| 1 | Phosphoglycerate mutase 1 (PGM1) | 247 | P00950 | 27.6 | 8.84 | 2920.62 |
| 2 | Glyceraldehyde-3-phosphate dehydrogenase 3 (TDH3) | 332 | P00359 | 35.7 | 6.96 | 932.30 |
| 3 | 60S ribosomal protein L12-A (RPL12A) | 165 | P0CX53 | 17.8 | 9.41 | 4979.88 |
| 4 | 10 kDa heat shock protein, mitochondrial (HSP10) | 106 | P38910 | 11.4 | 9.00 | 4922.87 |
| 5 | Elongation factor 1-beta (EFB1) | 206 | P32471 | 22.6 | 4.45 | 10084.36 |
| 6 | Translationally-controlled tumor protein homolog (TMA19) | 167 | P35691 | 18.7 | 4.56 | 6741.90 |
| 7 | Eukaryotic translation initiation factor 5A-1 (HYP2) | 157 | P23301 | 17.1 | 4.96 | 8620.94 |
| 8 | Peroxiredoxin type-2 (AHP1) | 176 | P38013 | 19.1 | 5.16 | 5587.1 |
| 9 | Peptidyl-prolyl cis–trans isomerase (CPR1) | 162 | P14832 | 17.4 | 7.44 | 7673.50 |
One of the spot identified through mass spectrometry was CPR1. We performed non-reducing SDS–PAGE in the presence or absence of ebselen examining the protein Cyclophilin A (CypA, human homolog of yeast CPR1). CypA is conserved throughout the phylogenetic tree from yeast to human [45,46]. CypA possesses a peptidylprolyl cis–trans isomerase activity that converts the cis and trans configurations of the peptide bonds that precede the amino acid proline [47]. Interestingly, we observed the appearance of high molecular weight complexes with Cyclophilin A (Fig. 6B and C), suggesting that a phenomenon similar to that observed with GDH takes place with Cyclophilin A as well (Fig. 6C). Further studies should be carried out to determine the reasons of such alterations.
Ebselen inhibits the sporulation of yeast
The increase in ROS levels is known to adversely affect several biological pathways [6]. Based on our results, we found that ebselen targets yeast GDH3 to enhance cellular ROS, and GDH3 is known to be involved in the maintenance of ROS levels during the stationary phase [34]. With this background information, we propose that ebselen treatment might show effects on the sporulation of yeast cells. We first determined the sporulation of untreated cells (DMSO control) and observed that approximately all the cells sporulated within 12 h (Fig. 7A), while ammonium sulfate, which is a known inhibitor of sporulation, strongly reduced spore formation (Fig. 7A). In contrast, ebselen completely abolished the sporulation at a concentration of 20 and 30 μM (Fig. 7B). Even after 24 h, no spores were detected in the ebselen-treated culture. At lower concentrations (5 and 10 μM) of ebselen treatment, there was no significant difference in spore morphology compared to that of untreated cells, suggesting that, at higher doses of ebselen, sporulation was inhibited (Fig. 7B). Upon visual inspection of sporulating cultures by microscopy, marked morphological differences were observed. In the DMSO control, most cells formed an ascus with spores, whereas the ammonium sulfate-treated cells had round and inflated shapes (Fig. 7A). In contrast, cells that sporulated in the presence of ebselen (20 μM) accumulated small granular bodies of unknown nature (Fig. 7B, inset), but were devoid of spores, while 30 μM ebselen-treated cultures showed no morphological changes like ammonium sulfate-treated cells.
Fig. 7.
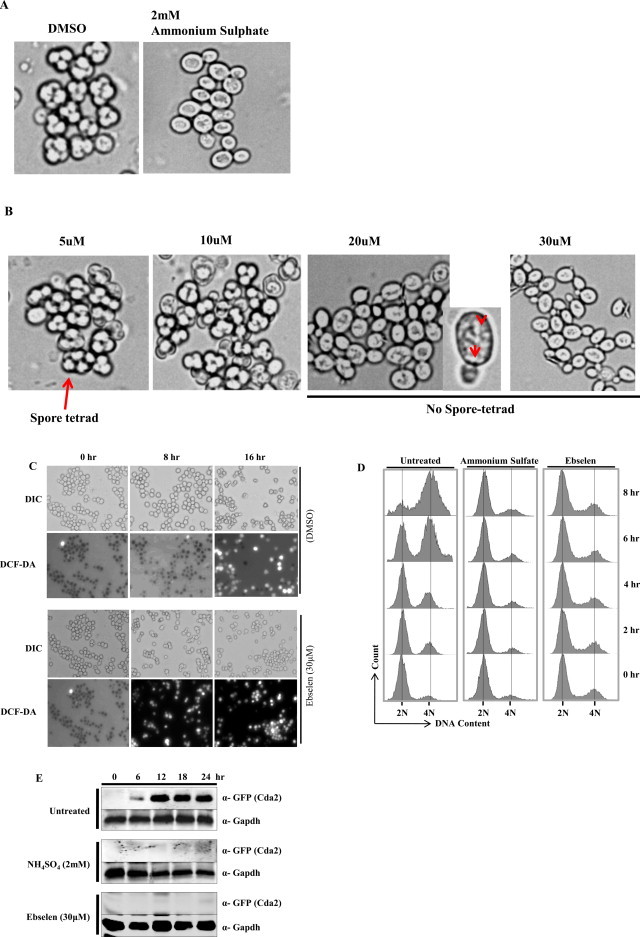
Ebselen strongly inhibits sporulation in yeast. (A) Microscopic images of cells sporulated for 24 h in the absence of drug (control), in the presence of 2 mM ammonium sulfate, (B) or increasing concentration of ebselen. Part of the 20 μM ebselen treated image was magnified; arrows indicate granular bodies of unknown origin. (C) Microscopic images of cells sporulated for indicated time (0, 8 and 16 h) in the absence of drug (DMSO), in the presence of 30 μM ebselen. The upper panels show phase contrast microscopy; the lower panels show fluorescence microscopy of the same cells after staining with DCF-DA. (D) Analysis of pre-meiotic DNA synthesis in a control (DMSO), and cells treated with ammonium sulfate (2 mM) or Ebselen (30 μM) through FACS. Samples were taken at regular interval as indicated in figure after induction of sporulation. Samples were subjected to FACS analysis and results were processed with BD FACS Diva software. (E) Yeast strain USY613 (USY61+ pCDA2-eGFP::HygB) was cultured as described in materials and methods and treated with 30 μM of ebselen or 2 mM ammonium sulfate for 24 h. 10 ml cells were harvested at regular intervals (0, 6, 12, 18, 24 h). Whole cell extracts were prepared by TCA extraction method and samples were subjected to western blot anlaysis using indicated antibodies. Tbp and Gapdh served as loading controls.
To substantiate our observations we have analyzed the ROS levels during the processes of sporulation. We observed ebselen treatment leads to increase in ROS levels at earlier time point (8 h) compared to DMSO treated (control) cells in which there was no increase in DCF-DA fluorescence (Fig. 7C). This result suggests that the early increase in ROS levels due to ebselen treatment causes inhibition of sporulation. Furthermore, we also analyzed the DNA content during the sporulation processes. Normally the total DNA content is duplicated (2C-4C) in the sporulation process. Interestingly, our result from FACS analysis revealed that the pre-meiotic DNA synthesis is inhibited in presence of ebselen (Fig. 7D). Ammonium sulfate (2 mM) treated cells served as positive control. Additionally, we also examined the expression of Cda2p, which is required for the formation of chitin wall of the spore tetrad. The relative increase in Cda2 levels upon ebselen treatment was significantly less compared to DMSO (control) treated samples as revealed by western blotting (Fig. 7E). Altogether, these results suggest that ebselen-dependent increase of ROS levels leads to inhibition of sporulation.
Discussions
Many mechanisms exist to regulate the cellular levels of ROS, otherwise their reactive nature may cause damage to key cellular components including DNA, proteins, and lipids [5]. When the cellular antioxidant capacity is exceeded, oxidative stress can result [48]. Deleterious pleiotropic effects of oxidative stress are observed in numerous disease states and are also implicated in a variety of drug-induced toxicities [49,50]. We examined the effect of ebselen on the cellular levels of ROS. Ebselen exhibits strong electrophilicity that underlies its ability to covalently react with protein cysteine residues to form selenyl-sulfide bonds [51]. It has been demonstrated that ebselen can react rapidly with free thiol groups including protein thiols and non-protein thiols, such as those of glutathione, N-acetyl-l-cysteine, dithioerythritol, and dihydrolipoate [21,23,52]. Our studies support the possibility that this electrophilicity allows ebselen to react with multiple cysteine residues of various proteins, representing the primary mechanism by which ebselen inhibits that particular protein/enzyme activity. According to the DHF staining results, we identified GDH as one of the targets of ebselen, subsequently leading to the increase of ROS levels. Non-reducing SDS–PAGE confirmed that ebselen is a chemically reactive species capable of converting GDH to the high-molecular weight (hexamer) protein species by a mechanism involving intermolecular cross-linking reactions, as already reported by our laboratory [44]. The disappearance of the modified form of GDH under the reducing SDS–PAGE indicates that the modified GDH protein is a disulfide-linked complex. It has been described in several studies that ebselen inhibits function of various thiol containing proteins for example Mycobacterium tuberculosis antigen 85 [53], NO synthases [54], H+/K(+)-ATPase [55], Lactate dehydrogenase [56], Heme enzyme indoleamine 2,3-dioxygenase (IDO) [57], Diguanylate cyclases [58], Alcohol dehydrogenase [59], JMJD2A [60] and many more. In all of these cases ebselen interferes with their SH groups leading to loss of their enzymatic activity. Furthermore the activity of these enzymes can be restored if reducing agents (DTT or beta-mercaptoethanol) are added along with ebselen; similarly GDH activity is also restored in presence of reducing agent (beta-mercaptoethanol) suggesting that like other known targets of ebselen ‘GDH’ is an another target. We have observed a similar phenomenon when GDH was treated with diamide [44]. Interestingly, yeast GDH3 was found to have a more significant role in the maintenance of ROS levels upon ebselen treatment than GDH1 and GDH2 (Fig. 4A and B). Our present study is in agreement with reported literature, which shows that GDH3 is required to maintain ROS levels during the stationary phase [34].
Cells are equipped with enzymatic and nonenzymatic antioxidant systems to eliminate ROS/RNS and maintain redox homeostasis [61–63]. A major class of enzymatic antioxidants, which catalyze the dismutation of O2− to H2O2 are the superoxide dismutases (SODs) [64]. Further conversion of H2O2 to H2O + O2 occurs through the action of catalase, a heme-based enzyme that is normally localized in the peroxisome [65]. H2O2 is converted to O2 through coupled reactions, with the conversion of reduced glutathione (GSH) to oxidized glutathione (GSSG), catalyzed by glutathione peroxidase (GPX) [66–68]. Our results demonstrated that ebselen treatment causes alteration in oxidative stress parameters, the oxidized glutathione (GSSG) was increased significantly while the total glutathione and GSH levels were also increased. These results suggest that ebselen treatment leads to oxidative stress.
The redox system is essential in maintaining cellular homeostasis [61,69]. Importantly, the increase in mitochondrial ΔΨ in yeast was shown to play a role in the cell death induced by acetic acid [70,71] or by expression of Bax [72]. In both of these cases, a mitochondrial ΔΨ increase was followed by strong elevation of the ROS levels. Based on the results from present study, we hypothesize that the generation of ROS and the increase in mitochondrial ΔΨ will lead to apoptotic cell death. It has been already shown that ebselen treatment causes the induction of apoptosis [23,73,74], which substantiates our hypothesis. Previously, it has been shown that ebselen treatment causes a dose- and time-dependent loss of mitochondrial membrane potential and release of cytochrome c [73]. Furthermore, ebselen also inhibits ATP hydrolysis [75] and causes deterioration of mitochondrial functioning [24]. Consistent with these studies we have observed alteration in mitochondrial membrane potential and increase in intracellular ROS levels indicating that mitochondrial physiology is severely affected following exposure to ebselen. An increase in ROS levels leads to an alteration in various cellular processes [76]. Accordingly, we found that yeast cells exhibited delayed cell cycle progression upon exposure to ebselen, suggesting that the cells takes more time to overcome the stress generated due to the treatment. Interestingly, at high dose ebselen treatment (50 μM), the extent of oxidative stress-induced cellular damage is irreversible because, as evidenced by methylene blue staining, it leads to cell death (Fig. 1C). Oxidative stress not only serves as a type of stimulus that can trigger stress-response signal-transduction pathways, but also can modulate cell death/survival through direct oxidative modifications of the target molecules, including DNA, lipids, and proteins. It is widely recognized that free radicals or ROS are involved in speeding the aging process or shortening the life span. At elevated concentrations, ROS exert various deleterious effects on normal cellular pathways; here, we showed the inhibitory effect of ROS on yeast sporulation (Fig. 7). Meiosis and sporulation in yeast and spermatogenesis in higher eukaryotes are analogous developmental pathways [77,78]. One of the key players in yeast sporulation is Cyclophilin A [79], which was identified by two-dimensional gel electrophoresis (Table 1) as one of the targets of ebselen. Our in vitro results clearly demonstrated that ebselen treatment results in the formation of high molecular weight complex of cyclophilin A (Fig 6B and C), but its enzymatic activity needs to be tested. Cyclophilin A is conserved from yeast to human and is encoded by the CPR1 gene [80]. Cyclophilin A is one of the members of a class of ubiquitous and highly conserved enzymes collectively known as peptidyl-prolyl cis–trans isomerases, or prolyl-isomerases, which catalyze the cis–trans isomerization of the peptide bonds preceding proline residues [47]. Its role in the process of yeast sporulation is being elucidated and was shown to be required for efficient sporulation [79]. Based on our present observations, we can hypothesize that ebselen treatment may negatively impact gametogenesis in humans by targeting Cyclophilin A, because the processes of yeast sporulation and spermatogenesis in human are conserved. Hence, future studies should be performed to validate these observations on human subjects.
Taken together, our present study sheds light on the mode of action of ebselen. We have identified the novel function of ebselen as an inducer of cellular ROS by inhibiting the function of GDH enzyme through a covalent crosslinking of its cysteine residues. Elevated levels of ROS lead to delayed cell cycle progression, inhibition of sporulation, and cell death. The remarkable reactivity of ebselen at micromolar concentrations with the components of the cellular redox system leads to various deleterious effects. Hence, further studies aiming to identify the cellular targets of ebselen are required to explore the mechanisms of action and the possible side effects of this promising clinically used agent. We hope that this study will aid in the enhanced understanding of the effects of ebselen on biological systems.
Authors contributions
Planned experiments; GKA VS PM RST
Performed experiments; GKA VS PM PS URG SB SC
Analyzed data; GKA VS PM RST
Contributed reagents; RST
Wrote the paper; GKA RST.
Acknowledgments
This work was supported by the Council of Scientific and Industrial Research (CSIR), Government of India to RST. GKA acknowledges CSIR (India) for providing fellowship. We are grateful to Sirano Dhe-Paganon for gifting us plasmid for expression of human recombinant Cyclophilin A. We acknowledge C-CAMP, NCBS Bangalore for mass spectrometry analysis. The members of chromatin biology lab are acknowledged for their helpful discussion through-out this study.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Aon M.A., Cortassa S., O’Rourke B. Redox-optimized ROS balance: a unifying hypothesis. Biochim. Biophys. Acta. 2010;1797:865–877. doi: 10.1016/j.bbabio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu J., Dong L., Outten C.E. The redox environment in the mitochondrial intermembrane space is maintained separately from the cytosol and matrix. J. Biol. Chem. 2008;283:29126–29134. doi: 10.1074/jbc.M803028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go Y.M., Jones D.P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecarini V., Gee J., Fioretti E., Amici M., Angeletti M., Eleuteri A.M., Keller J.N. Protein oxidation and cellular homeostasis: emphasis on metabolism. Biochim. Biophys. Acta. 2007;1773:93–104. doi: 10.1016/j.bbamcr.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 5.Avery S.V. Molecular targets of oxidative stress. Biochem. J. 2011;434:201–210. doi: 10.1042/BJ20101695. [DOI] [PubMed] [Google Scholar]
- 6.Bostek C.C. Oxygen toxicity: an introduction. AANA J. 1989;57:231–237. [PubMed] [Google Scholar]
- 7.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 8.Fridovich I. Superoxide radical: an endogenous toxicant. Annu. Rev. Pharmacol. Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- 9.Rhee S.G. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 10.Cerutti P.A. Prooxidant states and tumor promotion. Science. 1985;227:375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 11.Gobbel G.T., Bellinzona M., Vogt A.R., Gupta N., Fike J.R., Chan P.H. Response of postmitotic neurons to X-irradiation: implications for the role of DNA damage in neuronal apoptosis. J. Neurosci. 1998;18:147–155. doi: 10.1523/JNEUROSCI.18-01-00147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lushchak V.I., Gospodaryov D.V. Catalases protect cellular proteins from oxidative modification in Saccharomyces cerevisiae. Cell Biol. Int. 2005;29:187–192. doi: 10.1016/j.cellbi.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Lushchak V., Semchyshyn H., Mandryk S., Lushchak O. Possible role of superoxide dismutases in the yeast Saccharomyces cerevisiae under respiratory conditions. Arch. Biochem. Biophys. 2005;441:35–40. doi: 10.1016/j.abb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Sies H., Masumoto H. Ebselen as a glutathione peroxidase mimic and as a scavenger of peroxynitrite. Adv. Pharmacol. 1997;38:229–246. doi: 10.1016/s1054-3589(08)60986-2. [DOI] [PubMed] [Google Scholar]
- 15.Batna A., Fuchs C., Spiteller G. Lipid peroxidation in presence of ebselen. Chem. Phys. Lipids. 1997;87:149–158. doi: 10.1016/s0009-3084(97)00037-6. [DOI] [PubMed] [Google Scholar]
- 16.Muller A., Cadenas E., Graf P., Sies H. A novel biologically active seleno-organic compound – I. Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ 51 (Ebselen) Biochem. Pharmacol. 1984;33:3235–3239. doi: 10.1016/0006-2952(84)90083-2. [DOI] [PubMed] [Google Scholar]
- 17.Yang C.F., Shen H.M., Ong C.N. Protective effect of ebselen against hydrogen peroxide-induced cytotoxicity and DNA damage in HepG2 cells. Biochem. Pharmacol. 1999;57:273–279. doi: 10.1016/s0006-2952(98)00299-8. [DOI] [PubMed] [Google Scholar]
- 18.Sarma B.K., Mugesh G. Glutathione peroxidase (GPx)-like antioxidant activity of the organoselenium drug ebselen: unexpected complications with thiol exchange reactions. J. Am. Chem. Soc. 2005;127:11477–11485. doi: 10.1021/ja052794t. [DOI] [PubMed] [Google Scholar]
- 19.Chew P., Yuen D.Y., Stefanovic N., Pete J., Coughlan M.T., Jandeleit-Dahm K.A., Thomas M.C., Rosenfeldt F., Cooper M.E., de Haan J.B. Antiatherosclerotic and renoprotective effects of ebselen in the diabetic apolipoprotein E/GPx1-double knockout mouse. Diabetes. 2010;59:3198–3207. doi: 10.2337/db10-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindenblatt N., Schareck W., Belusa L., Nickels R.M., Menger M.D., Vollmar B. Anti-oxidant ebselen delays microvascular thrombus formation in the rat cremaster muscle by inhibiting platelet P-selectin expression. Thromb. Haemost. 2003;90:882–892. doi: 10.1160/TH02-09-0093. [DOI] [PubMed] [Google Scholar]
- 21.Schewe T. Molecular actions of ebselen – an antiinflammatory antioxidant. Gen. Pharmacol. 1995;26:1153–1169. doi: 10.1016/0306-3623(95)00003-j. [DOI] [PubMed] [Google Scholar]
- 22.Miorelli S.T., Rosa R.M., Moura D.J., Rocha J.C., Lobo L.A., Henriques J.A., Saffi J. Antioxidant and anti-mutagenic effects of ebselen in yeast and in cultured mammalian V79 cells. Mutagenesis. 2008;23:93–99. doi: 10.1093/mutage/gem048. [DOI] [PubMed] [Google Scholar]
- 23.Yang C.F., Shen H.M., Ong C.N. Ebselen induces apoptosis in HepG(2) cells through rapid depletion of intracellular thiols. Arch. Biochem. Biophys. 2000;374:142–152. doi: 10.1006/abbi.1999.1574. [DOI] [PubMed] [Google Scholar]
- 24.Gogvadze V., Klein S.D., Shigenaga M., Ames B.N., Richter C. Effect of ebselen on Ca2+ transport in mitochondria. Redox Rep. 2000;5:359–363. doi: 10.1179/135100000101535924. [DOI] [PubMed] [Google Scholar]
- 25.Azad G.K., Balkrishna S.J., Sathish N., Kumar S., Tomar R.S. Multifunctional ebselen drug functions through the activation of DNA damage response and alterations in nuclear proteins. Biochem. Pharmacol. 2012;83:296–303. doi: 10.1016/j.bcp.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Gopalakrishna R., Gundimeda U., Chen Z.H. Cancer-preventive selenocompounds induce a specific redox modification of cysteine-rich regions in Ca(2+)-dependent isoenzymes of protein kinase C. Arch. Biochem. Biophys. 1997;348:25–36. doi: 10.1006/abbi.1997.0334. [DOI] [PubMed] [Google Scholar]
- 27.Islam F., Watanabe Y., Morii H., Hayaishi O. Inhibition of rat brain prostaglandin D synthase by inorganic selenocompounds. Arch. Biochem. Biophys. 1991;289:161–166. doi: 10.1016/0003-9861(91)90456-s. [DOI] [PubMed] [Google Scholar]
- 28.Kim I.Y., Stadtman T.C. Inhibition of NF-kappaB DNA binding and nitric oxide induction in human T cells and lung adenocarcinoma cells by selenite treatment. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12904–12907. doi: 10.1073/pnas.94.24.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walther M., Holzhutter H.G., Kuban R.J., Wiesner R., Rathmann J., Kuhn H. The inhibition of mammalian 15-lipoxygenases by the anti-inflammatory drug ebselen: dual-type mechanism involving covalent linkage and alteration of the iron ligand sphere. Mol. Pharmacol. 1999;56:196–203. doi: 10.1124/mol.56.1.196. [DOI] [PubMed] [Google Scholar]
- 30.Zembowicz A., Hatchett R.J., Radziszewski W., Gryglewski R.J. Inhibition of endothelial nitric oxide synthase by ebselen. Prevention by thiols suggests the inactivation by ebselen of a critical thiol essential for the catalytic activity of nitric oxide synthase. J. Pharmacol. Exp. Ther. 1993;267:1112–1118. [PubMed] [Google Scholar]
- 31.Cotgreave I.A., Duddy S.K., Kass G.E., Thompson D., Moldeus P. Studies on the anti-inflammatory activity of ebselen. Ebselen interferes with granulocyte oxidative burst by dual inhibition of NADPH oxidase and protein kinase C? Biochem. Pharmacol. 1989;38:649–656. doi: 10.1016/0006-2952(89)90211-6. [DOI] [PubMed] [Google Scholar]
- 32.Deneke S.M., Fanburg B.L. Regulation of cellular glutathione. Am. J. Physiol. 1989;257:L163–L173. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- 33.Avendano A., Deluna A., Olivera H., Valenzuela L., Gonzalez A. GDH3 encodes a glutamate dehydrogenase isozyme, a previously unrecognized route for glutamate biosynthesis in Saccharomyces cerevisiae. J. Bacteriol. 1997;179:5594–5597. doi: 10.1128/jb.179.17.5594-5597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y.J., Kim K.J., Kang H.Y., Kim H.R., Maeng P.J. Involvement of GDH3-encoded NADP+-dependent glutamate dehydrogenase in yeast cell resistance to stress-induced apoptosis in stationary phase cells. J. Biol. Chem. 2012;287:44221–44233. doi: 10.1074/jbc.M112.375360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeLuna A., Avendano A., Riego L., Gonzalez A. NADP-glutamate dehydrogenase isoenzymes of Saccharomyces cerevisiae. Purification, kinetic properties, and physiological roles. J. Biol. Chem. 2001;276:43775–43783. doi: 10.1074/jbc.M107986200. [DOI] [PubMed] [Google Scholar]
- 36.Golla U., Singh V., Azad G.K., Singh P., Verma N., Mandal P., Chauhan S., Tomar R.S. Sen1p contributes to genomic integrity by regulating expression of ribonucleotide reductase 1 (RNR1) in Saccharomyces cerevisiae. PloS One. 2013;8:e64798. doi: 10.1371/journal.pone.0064798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azad G.K., Singh V., Golla U., Tomar R.S. Depletion of cellular iron by curcumin leads to alteration in histone acetylation and degradation of Sml1p in Saccharomyces cerevisiae. PloS One. 2013;8:e59003. doi: 10.1371/journal.pone.0059003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu C.Y., Bird A.J., Winge D.R., Eide D.J. Regulation of the yeast TSA1 peroxiredoxin by ZAP1 is an adaptive response to the oxidative stress of zinc deficiency. J. Biol. Chem. 2007;282:2184–2195. doi: 10.1074/jbc.M606639200. [DOI] [PubMed] [Google Scholar]
- 39.Mandal P., Azad G.K., Tomar R.S. Identification of a novel histone H3 specific protease activity in nuclei of chicken liver. Biochem. Biophys. Res. Commun. 2012;421:261–267. doi: 10.1016/j.bbrc.2012.03.149. [DOI] [PubMed] [Google Scholar]
- 40.Schlecht U., St Onge R.P., Walther T., Francois J.M., Davis R.W. Cationic amphiphilic drugs are potent inhibitors of yeast sporulation. PloS One. 2012;7:e42853. doi: 10.1371/journal.pone.0042853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy G., Sarma B.K., Phadnis P.P., Mugesh G. Selenium-containing enzymes in mammals: chemical perspectives. J. Chem. Sci. 2005;117:287–303. [Google Scholar]
- 42.Toppo S., Flohe L., Ursini F., Vanin S., Maiorino M. Catalytic mechanisms and specificities of glutathione peroxidases: variations of a basic scheme. BBA Gen. Subj. 2009;1790:1486–1500. doi: 10.1016/j.bbagen.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Wang X.F., Cynader M.S. Astrocytes provide cysteine to neurons by releasing glutathione. J. Neurochem. 2000;74:1434–1442. doi: 10.1046/j.1471-4159.2000.0741434.x. [DOI] [PubMed] [Google Scholar]
- 44.Mandal P., Verma N., Chauhan S., Tomar R.S. Unexpected histone H3 tail clipping activity of glutamate dehydrogenase. J. Biol. Chem. 2013 doi: 10.1074/jbc.M113.462531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang P., Heitman J. The cyclophilins. Genome Biol. 2005;6:226. doi: 10.1186/gb-2005-6-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obchoei S., Weakley S.M., Wongkham S., Wongkham C., Sawanyawisuth K., Yao Q., Chen C. Cyclophilin A enhances cell proliferation and tumor growth of liver fluke-associated cholangiocarcinoma. Mol. Cancer. 2011;10:102. doi: 10.1186/1476-4598-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F.X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989;337:476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- 48.Jones D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 49.Deavall D.G., Martin E.A., Horner J.M., Roberts R. Drug-induced oxidative stress and toxicity. J. Toxicol. 2012;2012:645460. doi: 10.1155/2012/645460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pereira C.V., Nadanaciva S., Oliveira P.J., Will Y. The contribution of oxidative stress to drug-induced organ toxicity and its detection in vitro and in vivo. Expert Opin. Drug Metab. Toxicol. 2012;8:219–237. doi: 10.1517/17425255.2012.645536. [DOI] [PubMed] [Google Scholar]
- 51.Sakurai T., Kanayama M., Shibata T., Itoh K., Kobayashi A., Yamamoto M., Uchida K. Ebselen, a seleno-organic antioxidant, as an electrophile. Chem. Res. Toxicol. 2006;19:1196–1204. doi: 10.1021/tx0601105. [DOI] [PubMed] [Google Scholar]
- 52.Haenen G.R.M.M., Derooij B.M., Vermeulen N.P.E., Bast A. Mechanism of the reaction of ebselen with endogenous thiols – dihydrolipoate is a better cofactor than glutathione in the peroxidase-activity of ebselen. Mol. Pharmacol. 1990;37:412–422. [PubMed] [Google Scholar]
- 53.Favrot L., Grzegorzewicz A.E., Lajiness D.H., Marvin R.K., Boucau J., Isailovic D., Jackson M., Ronning D.R. Mechanism of inhibition of Mycobacterium tuberculosis antigen 85 by ebselen. Nat. Commun. 2013;4:2748. doi: 10.1038/ncomms3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hattori R., Yui Y., Shinoda E., Inoue R., Aoyama T., Masayasu H., Kawai C., Sasayama S. Effect of ebselen on bovine and rat nitric oxide synthase activity is modified by thiols. Jpn. J. Pharmacol. 1996;72:191–193. doi: 10.1254/jjp.72.191. [DOI] [PubMed] [Google Scholar]
- 55.Tabuchi Y., Ogasawara T., Furuhama K. Mechanism of the inhibition of hog gastric H+,K(+)-ATPase by the seleno-organic compound ebselen. Arzneimittel-Forschung. 1994;44:51–54. [PubMed] [Google Scholar]
- 56.Lugokenski T.H., Muller L.G., Taube P.S., Rocha J.B., Pereira M.E. Inhibitory effect of ebselen on lactate dehydrogenase activity from mammals: a comparative study with diphenyl diselenide and diphenyl ditelluride. Drug Chem. Toxicol. 2011;34:66–76. doi: 10.3109/01480541003782294. [DOI] [PubMed] [Google Scholar]
- 57.Terentis A.C., Freewan M., Sempertegui Plaza T.S., Raftery M.J., Stocker R., Thomas S.R. The selenazal drug ebselen potently inhibits indoleamine 2,3-dioxygenase by targeting enzyme cysteine residues. Biochemistry. 2010;49:591–600. doi: 10.1021/bi901546e. [DOI] [PubMed] [Google Scholar]
- 58.Lieberman O.J., Orr M.W., Wang Y., Lee V.T. High-throughput screening using the differential radial capillary action of ligand assay identifies ebselen as an inhibitor of diguanylate cyclases. ACS Chem. Biol. 2013 doi: 10.1021/cb400485k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacob C., Maret W., Vallee B.L. Ebselen, a selenium-containing redox drug, releases zinc from metallothionein. Biochem. Biophys. Res. Commun. 1998;248:569–573. doi: 10.1006/bbrc.1998.9026. [DOI] [PubMed] [Google Scholar]
- 60.Sekirnik R., Rose N.R., Thalhammer A., Seden P.T., Mecinovic J., Schofield C.J. Inhibition of the histone lysine demethylase JMJD2A by ejection of structural Zn(II) Chem. Commun. (Camb.) 2009:6376–6378. doi: 10.1039/b916357c. [DOI] [PubMed] [Google Scholar]
- 61.Devasagayam T.P.A. Free radicals and antioxidants in human health foreword. Indian J. Biochem. Biol. 2009;46:5–9. [Google Scholar]
- 62.Devasagayam T.P., Tilak J.C., Boloor K.K., Sane K.S., Ghaskadbi S.S., Lele R.D. Free radicals and antioxidants in human health: current status and future prospects. J. Assoc. Phys. India. 2004;52:794–804. [PubMed] [Google Scholar]
- 63.Koharyova M., Kollarova M. Oxidative stress and thioredoxin system. Gen. Physiol. Biophys. 2008;27:71–84. [PubMed] [Google Scholar]
- 64.McCord J.M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 65.Chelikani P., Fita I., Loewen P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arthur J.R. The glutathione peroxidases. Cell. Mol. Life Sci. 2000;57:1825–1835. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tosatto S.C.E., Bosello V., Fogolari F., Mauri P., Roveri A., Toppo S., Flohe L., Ursini F., Maiorino M. The catalytic site of glutathione peroxidases. Antioxid. Redox signal. 2008;10:1515–1525. doi: 10.1089/ars.2008.2055. [DOI] [PubMed] [Google Scholar]
- 68.Imai H., Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic. Biol. Med. 2003;34:145–169. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 69.Herrero E., Ros J., Belli G., Cabiscol E. Redox control and oxidative stress in yeast cells. Biochim. Biophys. Acta. 2008;1780:1217–1235. doi: 10.1016/j.bbagen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 70.Ludovico P., Sousa M.J., Silva M.T., Leao C., Corte-Real M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology. 2001;147:2409–2415. doi: 10.1099/00221287-147-9-2409. [DOI] [PubMed] [Google Scholar]
- 71.Ludovico P., Rodrigues F., Almeida A., Silva M.T., Barrientos A., Corte-Real M. ytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Mol. Biol. Cell. 2002;13:2598–2606. doi: 10.1091/mbc.E01-12-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gross A., Pilcher K., Blachly-Dyson E., Basso E., Jockel J., Bassik M.C., Korsmeyer S.J., Forte M. Biochemical and genetic analysis of the mitochondrial response of yeast to BAX and BCL-X-L. Mol. Cell. Biol. 2000;20:3125–3136. doi: 10.1128/mcb.20.9.3125-3136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang C.F., Shen H.M., Ong C.N. Intracellular thiol depletion causes mitochondrial permeability transition in ebselen-induced apoptosis. Arch. Biochem. Biophys. 2000;380:319–330. doi: 10.1006/abbi.2000.1939. [DOI] [PubMed] [Google Scholar]
- 74.Sharma V., Tewari R., Sk U.H., Joseph C., Sen E. Ebselen sensitizes glioblastoma cells to tumor necrosis factor (TNFalpha)-induced apoptosis through two distinct pathways involving NF-kappaB downregulation and Fas-mediated formation of death inducing signaling complex. Int. J. Cancer. 2008;123:2204–2212. doi: 10.1002/ijc.23771. [DOI] [PubMed] [Google Scholar]
- 75.Furstenau C.R., Spier A.P., Rucker B., Luisa Berti S., Battastini A.M., Sarkis J.J. The effect of ebselen on adenine nucleotide hydrolysis by platelets from adult rats. Chem. Biol. Interact. 2004;148:93–99. doi: 10.1016/j.cbi.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 76.Jamieson D.J. Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast. 1998;14:1511–1527. doi: 10.1002/(SICI)1097-0061(199812)14:16<1511::AID-YEA356>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 77.Schlecht U., Primig M. Mining meiosis and gametogenesis with DNA microarrays. Reproduction. 2003;125:447–456. doi: 10.1530/rep.0.1250447. [DOI] [PubMed] [Google Scholar]
- 78.Schlecht U., Demougin P., Koch R., Hermida L., Wiederkehr C., Descombes P., Pineau C., Jegou B., Primig M. Expression profiling of mammalian male meiosis and gametogenesis identifies novel candidate genes for roles in the regulation of fertility. Mol. Biol. Cell. 2004;15:1031–1043. doi: 10.1091/mbc.E03-10-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arevalo-Rodriguez M., Heitman J. Cyclophilin A is localized to the nucleus and controls meiosis in Saccharomyces cerevisiae. Eukaryot. Cell. 2005;4:17–29. doi: 10.1128/EC.4.1.17-29.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haendler B., Keller R., Hiestand P.C., Kocher H.P., Wegmann G., Movva N.R. Yeast cyclophilin: isolation and characterization of the protein, cDNA and gene. Gene. 1989;83:39–46. doi: 10.1016/0378-1119(89)90401-0. [DOI] [PubMed] [Google Scholar]


