Abstract
Dehydroepiandrosterone (DHEA) and the dehydroepiandrosterone sulfate (DHEA-S) are steroids produced mainly by the adrenal cortex. There is evidence from both human and animal models suggesting beneficial effects of these steroids for obesity, diabetes mellitus, hypertension, and osteoporosis, conditions associated with the post-menopausal period. Accordingly, we hypothesized that DHEA supplementation in ovariectomized (OVX) female rats fed a high-fat diet would maintain glucose-induced insulin secretion (GSIS) and pancreatic islet function. OVX resulted in a 30% enlargement of the pancreatic islets area compared to the control rats, which was accompanied by a 50% reduction in the phosphorylation of AKT protein in the pancreatic islets. However, a short-term high-fat diet induced insulin resistance, accompanied by impaired GSIS in isolated pancreatic islets. These effects were reversed by DHEA treatment, with improved insulin sensitivity to levels similar to the control group, and with increased serine phosphorylation of the AKT protein. These data confirm the protective effect of DHEA on the endocrine pancreas in a situation of diet-induced overweight and low estrogen concentrations, a phenotype similar to that of the post-menopausal period.
Keywords: High fat diet, Menopause, Pancreatic islets, Insulin sensitivity, Insulin secretion, p-Akt/Akt
Abbreviations: DHEA, dehydroepiandrosterone; DHEA-S, dehydroepiandrosterone sulfate; HFD, high-fat diet; SHAM, sham-operated rats; SHL, sham rats fed a HFD; OVX, ovariectomized rats; OHL, ovariectomized rats fed HFD; OHLD, ovariectomized rats fed a HFD and treated with DHEA; Kitt, glucose disappearance rate; GTT, glucose tolerance test; GSIS, glucose-induced insulin secretion; SDS–PAGE, sodium dodecyl sulfate poly-acrylamide electrophoresis; PI, propidium iodide; PI3K, phosphatidylinositol-3-kinase; PI3K-PDK1-Akt, PI3K-3-phosphoinositide dependent kinase-Akt
Highlights
-
•
Dehydroepiandrosterone (DHEA) is a physiological precursor of androgens and estrogens.
-
•
Ovariectomized rats fed a high-fat diet showed insulin resistance and impaired glucose-induced insulin secretion.
-
•
These effects were reversed by DHEA treatment, with improved insulin secretion and sensitivity.
Introduction
Menopause coincides with an increase in body fatness and associated comorbidities such as insulin resistance, diabetes, and cardiovascular disease [1,2], which may be explained, at least in part, by reduced secretion of sex hormones, including estrogen [3]. Estrogen has been shown to play a pivotal role in regulating energy expenditure, body weight and fat distribution in women. Both human and murine models provide evidence for estrogen's protective role against obesity and type 2 diabetes (T2D) [4–6]. Experimental studies have shown that estrogen and its receptors protect pancreatic islets cells from lipotoxicity and apoptosis, as well as restores glucose-induced insulin secretion (GSIS) [6–12]. Furthermore, the functions of pancreatic β cells, including the regulation of insulin secretion, nutrient homeostasis, and even survival can be modulated by 17 β-estradiol [13].
It is suggested that there is a contribution of downstream elements in the insulin signaling pathway for β cell function and survival. For example, expression of multiple insulin signaling proteins is reduced in islets of patients with T2D [14,15]. Several lines of evidence indicate that activation of phosphoinositide 3-kinase (PI3K) signaling pathway plays an important role to regulate β cell mass and function. One of the major targets of PI3-kinase is the serine–threonine kinase Akt [16,17], which acts as a convergent target of several growth signals induced by growth factors and insulin. In fact, studies suggest that selective modulation of the Akt signaling could have positively impact for the design of pharmaceutical agents that induce β cell function and proliferation without adverse effect [18].
Dehydroepiandrosterone (DHEA), and its sulfated form DHEA-S, are synthesized by both adrenal and gonadal glands. These steroids are precursor of both androgens and estrogens. In humans, circulating DHEA and DHEA-S levels are markedly decreased with aging [19]. DHEA has been considered an alternative to estrogen replacement therapy since it has no effects on breast and endometrium cancer development [20]. Although no specific receptors for DHEA or DHEA-S have been identified, these steroids have been shown to have antioxidant and metabolic effects in different tissues [21,22]. Indeed, experimental evidence from animal models and studies on postmenopausal women has shown that DHEA supplementation improves insulin sensitivity and reduces fat mass gain, and obesity [23–26]. In addition, in streptozotocin-induced diabetic mice, DHEA was able to preserve pancreatic islet cell structure, while the administration of DHEA-S improved the insulin secretion [23,27].
We therefore hypothesized that DHEA supplementation in ovariectomized female rats fed a high-fat diet would maintain glucose-induced insulin secretion and pancreatic islet function.
Materials and methods
Experimental model
Female Wistar rats (8 weeks of age, weighing 150–180 g at the beginning of the experiments) were obtained from our breeding colony at the Institute of Biomedical Sciences. The animals were housed at constant room temperature, 12 h light and 12 h darkness cycle, 60% humidity, fed standard rat chow [3.8 kcal/g (63% carbohydrate, 26% proteins, and 11% fat), NUVILAB-CR, Colombo, PR, Brazil], and water made available ad libitum. The rats were anesthetized with thiopental (5 mg/100 g, i.p.; Cristália, São Paulo, SP, Brazil) and were submitted to sham surgery or bilateral ovariectomy (OVX). After the surgical procedure, the rats received standard chow or a high-fat diet (HFD) [5.4 kcal/g (26% carbohydrate, 15% proteins, and 59% fat), Prag Soluções Biociências Ltda, Jau, SP, Brazil] for the next 6 weeks. Half of the OVX rats fed a HFD (OHL group) was exposed to a second surgical procedure 3 weeks from the ovariectomy, which included the implantation of DHEA pellet (50 mg released by 21 days) into the subcutaneous back region (identified as the OHLD group). This study was approved by the ethical committee of the Institute of Biomedical Sciences, University of Sao Paulo (2708/CEEA). At the end of the protocol period the animals were killed with deep anesthesia, the pancreata were excised and the blood was used for the determination of DHEA using an ELISA kit. In addition, estradiol (E2), progesterone (P), luteinizing hormone (LH), follicle stimulating hormone (FSH), testosterone (T) and insulin concentrations were measured by radioimmunoassay (RIA).
Materials
Reagents for SDS–PAGE and immunoblotting were obtained from Bio-Rad (Richmond, CA). Human biosynthetic DHEA, Tris, aprotinin, dithiotreitol, Triton X-100, glycerol, Tween 20, bovine serum albumin (BSA, fraction V), propidium iodide (PI) were obtained from Sigma (St. Louis, MO). Human insulin from Lilly, American; nitrocellulose (0.45 lm) and enhanced chemiluminescence kit were purchased from Pharmacia (Uppsala, Sweden). Anti-phosphoserine473 Akt (pSer473Akt), anti-Akt and anti-α-Tubulin antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody against ERK1/2 was obtained from Millipore antibody (CA, USA), and anti-cleaved caspase 3 antibody was obtained from Cell Signaling Technology. Biotinylated goat anti-rabbit antibody was obtained from Vector Laboratories (Burlingame, CA). Rat insulin standards and anti-rat insulin antibodies were a gift from Dr. Leclercq-Meyer, Université Libre de Bruxelles, Belgium.
Insulin sensitivity and glucose tolerance
Under anesthesia (Thiopental 0.6 mg kg−1, i.p; Cristália, São Paulo, SP, Brazil), 12 h fasting animals underwent a 30-min insulin tolerance test (short ITT) and a glucose tolerance test (GTT). Briefly, blood glucose concentrations were measured using a glucometer (Acue check active Roche). The time points for the ITT were basal (0), 5, 10, 15, 20, 25 and 30 min after the intraperitoneal insulin injection (0.75 U/kg). The glucose disappearance rate (Kitt) was calculated as the slope of linear regression of glucose concentration from 5 to 30 min after insulin administration [28]. For GTT, the rats received 1 mg/g glucose through intraperitoneal injection and glucose concentrations was measured at the following time points: basal (0), 15, 30, 45, 60, 90, and 120 min after the infusion.
Morphometry of the endocrine pancreas
The animals were deeply anesthetized with a mixture of ketamine (5 mg/100 g) and xylazine (1 mg/100 g) intramuscularly, and were perfused through the heart with 0.9% PBS and 4% paraformaldehyde in 0.1 mol/l phosphate buffer. The pancreas were excised and dissected free from surrounding tissues and fixed by immersion in 4% formaldehyde–PBS solution for 4–6 h, followed by transfer to 30% sucrose PBS for cryoprotection. Frozen pancreas sections (12 μm) were cut on a cryostat and mounted on gelatin-coated slides. The pancreas sections were counter-stained with hematoxylin–eosin for morphometric analysis. The islet area was calculated by using NIH Image J program developed at the US National Institutes of Health and available on http://rsb.info.nih.gov/nih-image/.
Pancreatic islets isolation
The islets were isolated by collagenase digestion of the pancreata using the method described by Lacy and Kostianovsky (1967) [29]. Briefly, the pancreas was inflated with a Hanks solution containing 0.7 g/l type IV collagenase (Sigma–Aldrich Chemical, St. Louis, MO), excised and then maintained at 37 °C for about 25 min. The digested tissue was harvested and the islets were hand-picked. A Krebs–Henseleit buffer containing 115 mM NaCl, 5 mM KCl, 24 mM NaHCO3, 1 mM CaCl2, and 1 mM MgCl2 was used for isolation and pooling of the islets. Pancreatic islets were subjected to the following analysis: glucose-stimulated insulin secretion (GSIS), DNA fragmentation, and typical immunoblotting.
Glucose-stimulated insulin secretion (GSIS) assay
Pre-incubations of 5 islets were carried out at 37 °C for 30 min in 0.5 ml Krebs–Henseleit buffer and 0.2% albumin in the presence of 5.6 mM glucose. After 5.6 mM glucose pre-incubation, the following concentrations of glucose were used for incubation: 2.8 and 16.7 mM glucose for 60 min and equilibrated with a mixture of O2 (95%) and CO2 (5%). At the end of the incubation period, insulin concentrations were measured by RIA [30].
DNA fragmentation
DNA fragmentation was analyzed by flow cytometry after DNA staining with PI as described previously [31,32]. Briefly, 30 isolated pancreatic islets were suspended in 300 μl of hypotonic solution containing 50 mg/ml PI, 0.1% sodium citrate, and 0.1% Triton X-100, and incubated for 30 min at room temperature protected from light exposure. Fluorescence was measured in a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and the FL2 channel (orange/red fluorescence at 585/42 nm) and analyzed using the Cell Quest software (Becton Dickinson).
Immunoblotting
Batches of 300 islets from each group were homogenized by sonication (90 s) in 80 μl extraction buffer (100 mM Trizma, 1% SDS, 100 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM EDTA and 10 mM sodium vanadate), and boiled for 5 min. The extracts were then centrifuged at 12,000 rpm at 4 °C for 20 min to remove insoluble material. Protein determination of the supernatants was performed by the Bradford dye method (BioRad Laboratories, Hercules, CA). The proteins were treated with Laemmli sample buffer containing dithiotreitol and boiled for 5 min before loading onto 8% SDS–PAGE in a Bio-Rad miniature slab gel apparatus. Similar sized aliquots (30 μg) were subjected to SDS–PAGE. Electro-transfer of proteins from the gel to nitrocellulose was performed for 1 h at 120 V (constant) in a Bio-Rad miniature transfer apparatus. Non-specific protein binding to the nitrocellulose was reduced by preincubation for 1 h at 22 °C in blocking buffer containing 5% nonfat dry milk, 10 mM Tris, 150 mM NaCl, and 0.02% Tween 20. The nitrocellulose membranes were incubated overnight at 8 °C with specific antibodies diluted in blocking buffer added with 3% nonfat dry milk, and then washed for 30 min in blocking buffer without milk. The blots were subsequently incubated with peroxidase-conjugated secondary antibody for 1 h, and processed for enhanced chemiluminescence to visualize the immunoreactive bands. Band intensities were quantized by optical densitometry (Scion Image-Release Beta 3b, NIH, USA) of the developed autoradiography.
Statistical analysis
The results were expressed as means ± standard error (SEM). After Gaussian distribution analysis, differences in body weights were assessed by two-way repeated measures ANOVA (6 × 3) considering time and group as fixed factors and experimental units (female rats) as random factor (with a Tukey–Kramer test for multiple comparisons). Kitt, GSIS, and pancreatic islets area were assessed by one-way ANOVA. The alpha level of significance was set at P < 0.05.
Results
Characteristics of the animals
Circulating levels of sex hormones in the ovariectomized rats are presented in Table 1. Compared to sham-operated rats, ovariectomy was associated with a 0.5-fold decrease in estradiol, a 0.3-fold decrease in P and T concentrations, and a concomitant 6.5-fold increase in FSH and LH concentrations. In the ovariectomized rats, the DHEA pellet induced an approximately 2-fold increase in the blood DHEA levels, with a simultaneous increase in blood estradiol levels to levels similar to those measured in the sham-operated rats. In contrast, DHEA had no effect on blood LH, FSH, progesterone, and testosterone concentrations.
Table 1.
DHEA, E2 (estradiol), P (progesterne), LH (luteinizing hormone), FSH (follicle stimulating hormone) and T (testosterone) concentrations of the SHAM, OVX (ovariectomized) and OVX + DHEA rats after 6 weeks ovarian removal.
| SHAM | OVX | OVX + DHEA | P | |
|---|---|---|---|---|
| DHEA (pg/ml) | 22.7 ± 2.1a | 26.3 ± 3.8a | 51.5 ± 3.2b | <0.001 |
| E2 (pg/ml) | 52.8 ± 4.1a | 28.4 ± 3.7b | 57.5 ± 6.9a | <0.05 |
| P (ng/ml) | 26.0 ± 5.8a | 9.5 ± 1.6b | 9.7 ± 2.4b | <0.05 |
| LH (ng/ml) | 9.6 ± 0.5a | 63.2 ± 8.0b | 54.5 ± 5.7b | <0.01 |
| FSH (ng/ml) | 4.8 ± 0.2a | 31.0 ± 3.7b | 27.1 ± 2.8b | <0.05 |
| T (pg/ml) | 94.8 ± 16.6a | 29.3 ± 3.4b | 27.6 ± 4.5b | <0.05 |
Values are mean ± S.E.M. n = 8–10. Groups with distinct letters means significant difference (P < 0.05).
Table 2 describes the effects of HFD, ovariectomy and DHEA on body weight, insulin sensitivity, glucose tolerance, uterus weight, and pancreatic islet area of rats. The ovariectomy induced up to 1.9-fold increase in the body weight gain when compared to sham rats. Neither the HFD nor DHEA treatment had an effect on changes in body weight in the OVX and sham groups. OVX induced a 70% reduction in the uterus weight when compared to the sham group, but uterus weight was not altered by DHEA supplementation or HFD. The OVX group fed a HFD had greater insulin resistance and associated hyperinsulinemia compared to the sham groups, which was reversed by the treatment with DHEA. Pancreatic islet area was greater in the OVX groups than the sham groups, but was not altered by DHEA supplementation.
Table 2.
Changes in body weight and measures of insulin sensitivity in ovariectomized (OVX) or sham-operated (SHAM) rats treated with HFD and DHEA for 3 weeks.
| SHAM | SHL | OVX | OHL | OHLD | |
|---|---|---|---|---|---|
| Δ Body weight (g) | 49 ± 8.4a | 67 ± 7.5a | 95 ± 8.4b | 102 ± 7.4b | 100 ± 6.7b |
| Weight uterus (g) | 2.00 ± 0.30a | 1.70 ± 0.24a | 0.50 ± 0.03b | 0.50 ± 0.09b | 0.55 ± 0.05b |
| Insulin (ng/ml) | 0.38 ± 0.06ab | 0.72 ± 0.13ac | 0.26 ± 0.04b | 0.92 ± 0.09c | 0.37 ± 0.03b |
| Glucose (mg/dl) | 100 ± 3a | 119 ± 3ab | 103 ± 3a | 121 ± 4b | 108 ± 4a |
| AUC | 20.94 ± 7.51 | 21.12 ± 9.56 | 20.2 ± 8.91 | 23.12 ± 5.17 | 22.2 ± 8.76 |
| Kitt (%min−1) | 2.35 ± 0.20a | 2.28 ± 0.07a | 2.00 ± 0.14a | 1.00 ± 0.15b | 2.00 ± 0.14a |
| Islet area (μm2) | 10.470 ± 825a | 13.270 ± 1.006ab | 15.260 ± 1.225b | 17.230 ± 1.500b | 17.630 ± 1.154b |
Values are mean ± S.E.M. Similar letters indicate no significant difference, while different letters indicate significant difference (P < 0.05). Δ Body weight (BW final − initial); AUC: area under curve after 120 min of intraperitoneal glucose bolus; Kitt: glucose disappearance rate induced by 30 min Insulin Tolerance Test; SHL: sham rats fed a HFD; OVX: ovariectomized rats; OHL: ovariectomized rats fed HFD; OHLD: ovariectomized rats fed a HFD and treated with DHEA. n = 4–25.
The area under curve (AUC) of glucose tolerance test was not different between the groups (P > 0.05). However analyses of the GTT curve identified 2 time points with significant differences, so that 15 min after glucose load the SHAM group showed lower glucose levels when compared to SHL group (P < 0.05). Furthermore, 60 min after glucose load the OHL group showed higher glucose levels than OVX group (P < 0.05) (Fig. 1).
Fig. 1.
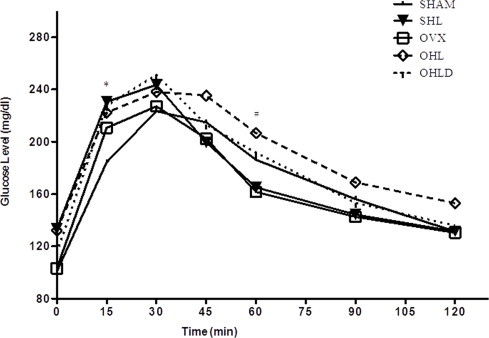
Glucose tolerance test during 120 min after intraperitoneal glucose bolus. *Indicates that SHAM is different from SHL (P < 0.05). #Indicates that OVX is different from OHL (P < 0.05).
Static insulin secretion
As expected, insulin secretion from the isolated pancreatic islets of the SHAM group was 3-fold higher after incubation with 16.7 mM glucose compared to 2.8 mM glucose (Fig. 2A and B). Ovariectomy or exposure to the HFD alone had no effect on GSIS. In contrast, ovariectomy combined with HFD impaired insulin secretion (Fig. 2B), which was reversed with DHEA treatment.
Fig. 2.
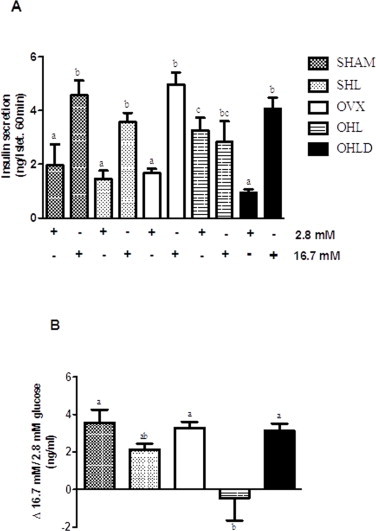
GSIS of isolated pancreatic islets in SHAM, SHL, OVX, OHL and OHLD groups. Panel A is the 60-min insulin secretion at 2.8 mM glucose (basal) and 16.7 mM glucose (stimulus). Panel B is the difference between the stimulus and the basal insulin secretion. Values are mean ± S.E.M., and distinct letters indicates significant differences (P < 0.05).
DNA fragmentation
The proportion of cells with DNA fragmentation was similar between the OVX groups with values around 10% (data not shown).
Immunoblotting analysis
Fig. 3A illustrates the protein expression of phosphoserine Akt (p-Akt), and the protein expression of Akt, ERK1/2, caspase-3, using α-tubulin as a control. Both HFD and OVX resulted in a 50% reduction in the phosphorylation state of Akt, with no additive effect of these two conditions, which was reversed by DHEA treatment (Fig. 3B). There were no effects of HFD or OVX on protein expression of ERK1/2 and caspase-3 in the studied conditions (Fig. 3C and D).
Fig. 3.
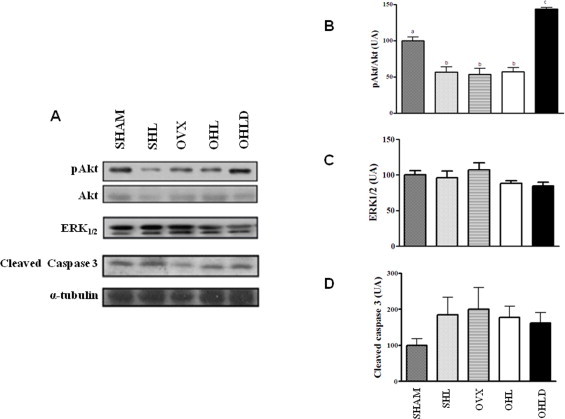
Protein levels of Akt, ERK1/2, and cleaved caspase 3 of isolated pancreatic islets in SHAM, SHL, OVX, OHL and OHLD groups. Panel A is the immunobloting of pAkt, Akt, ERK1/2, cleaved caspase 3 and α-tubulin. Panel B is the stoichiometric relationship between the serine phosphorylation status of Akt (p-Akt) and the protein expression of Akt (pAkt/Akt). Panel C is the protein level of ERK1/2. Panel D is the protein level of cleaved caspase-3. The values are expressed as the mean ± S.E.M. and distinct letters indicate significance (P < 0.05).
Discussion
The levels of the sex steroid hormone DHEA decrease between the ages of 25 and 75 years, and has been related to body weight gain, obesity and insulin resistance [33]. Conversely, studies have demonstrated that concentrations of DHEA and other sex steroid hormones are reduced with obesity and type 2 diabetes [34]. In addition, numerous studies using animal models have demonstrated that DHEA treatment increases insulin sensitivity [35] and GSIS [27,36].
In our study, despite the mild increase in body weight in OVX animals, OVX in combination with a HFD (OHL rats) had no impact on body weight over the study period. However, the combination of OVX and HFD resulted in insulin resistance and impaired GSIS of the isolated pancreatic islets, which were reversed with DHEA supplementation. These findings reinforce the known effect of ovarian hormones on reducing the susceptibility of females to HFD-induced metabolic disturbance [37].
We suggest that DHEA treatment is involved in some aspects of pathway-selective insulin resistance, which are independent of its effects on body weight. This is supported by a study that showed that a single DHEA injection in rats with streptozotocin-induced diabetes improves glucose metabolism-related signaling pathway via protein kinase B (Akt) and reversed impaired GLUT-4-related signaling in muscle [38]. In our study OHL rats were the only group that presented with insulin resistance and functional beta cell damage, the latter probably caused by a defect in insulin signaling in β cells. Consistent with these reports, we showed that DHEA supplementation, which increased estradiol levels, also increased the p-Akt/Akt ratio in pancreatic islets from OHLD animals, suggesting a possible role for Akt in normal glucose metabolism and β beta cell function in OHLD animals. Indeed, there is evidence showing that protein kinase Akt is important for the peripheral actions of insulin, such as glucose homeostasis, β cell growth and survival [39].
Studies also have suggested that Akt plays a important role in the regulation of distal components of the secretory pathway in the β cell [40]. Transgenic mice with diminished Akt activity in their β cells present with glucose intolerance due to a defect in insulin secretion at the level of exocytosis process, which appears to be independent of the function of voltage-gated Ca2+ channels [40]. Furthermore, acute inhibition of PI3K-PDK1-Akt pathway increased GSIS by upregulation of specific intracellular fusion events from newcomer granules [41]. On the other hand, inhibition of class IA phosphatidylinositol 3-kinase (PI3K) also resulted in glucose intolerance and reduced GSIS [42].
In the present study, extracellular signal-regulated protein kinases 1 and 2, ERK1/2, which is involved on cellular proliferation, growth, and differentiation, were not altered by HFD, ovariectomy or DHEA treatment. Similarly, pathways related to cell death were not involved, since no changes in DNA fragmentation and cleaved caspase 3 expressions were observed in any of the treatment groups.
In summary, our study showed that OVX in combination with a HFD resulted in a significant increase in body weight, as well as a reduction in insulin sensitivity and secretion. Independent of changes in body weight, the changes in insulin sensitivity and secretion were normalized after 3 weeks of the DHEA pellet insertion. This effect was mediated by an increase in the p-Akt/Akt ratio. These findings, in addition to the higher prevalence of insulin resistance and type 2 diabetes in women after menopause, provide support for a potential therapeutic role of DHEA in post-menopausal women that have some features of the metabolic syndrome.
Acknowledgments
The authors are grateful to Luciene M. Ribeiro for the excellent technical assistance. We also gratefully acknowledge the helpful statistical discussions with professor Flávio O. Pires. This work was supported by grants from FAPESP, CNPq and CAPES (Brazil).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Katherine Veras, Email: katherine.veras@gmail.com.
Carla Roberta de Oliveira Carvalho, Email: carlacarvalho.usp@gmail.com.
References
- 1.Tchernof A., Calles-Escandon J., Sites C.K., Poehlman E.T. Menopause, central body fatness, and insulin resistance: effects of hormone-replacement therapy. Coron. Artery Dis. 1998;9:503–511. doi: 10.1097/00019501-199809080-00006. [DOI] [PubMed] [Google Scholar]
- 2.Polotsky H.N., Polotsky A.J. Metabolic implications of menopause. Semin. Reprod. Med. 2010;28:426–434. doi: 10.1055/s-0030-1262902. [DOI] [PubMed] [Google Scholar]
- 3.Brown L.M., Gent L., Davis K., Clegg D.J. Metabolic impact of sex hormones on obesity. Brain Res. 2010;1350:77–85. doi: 10.1016/j.brainres.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musatov S., Chen W., Pfaff D.W., Mobbs C.V., Yang X.J., Clegg D.J., Kaplitt M.G., Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl. Acad. Sci. U.S.A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingstone C., Collison M. Sex steroids and insulin resistance. Clin. Sci. (Lond) 2002;102:151–166. doi: 10.1042/cs1020151. [DOI] [PubMed] [Google Scholar]
- 6.Liu S., Mauvais-Jarvis F. Minireview: Estrogenic protection of beta-cell failure in metabolic diseases. Endocrinology. 2010;151:859–864. doi: 10.1210/en.2009-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Seifi S., Green I.C., Perrin D. Insulin release and steroid-hormone binding in isolated islets of langerhans in the rat: effects of ovariectomy. J. Endocrinol. 1981;90:59–67. doi: 10.1677/joe.0.0900059. [DOI] [PubMed] [Google Scholar]
- 8.Choi S.B., Jang J.S., Park S. Estrogen and exercise may enhance beta-cell function and mass via insulin receptor substrate 2 induction in ovariectomized diabetic rats. Endocrinology. 2005;146:4786–4794. doi: 10.1210/en.2004-1653. [DOI] [PubMed] [Google Scholar]
- 9.Le May C., Chu K., Hu M., Ortega C.S., Simpson E.R., Korach K.S., Tsai M.J., Mauvais-Jarvis F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiano J.P., Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat. Rev. Endocrinol. 2012;8:342–351. doi: 10.1038/nrendo.2011.242. [DOI] [PubMed] [Google Scholar]
- 11.Nadal A., Alonso-Magdalena P., Soriano S., Ripoll C., Fuentes E., Quesada I., Ropero A.B. Role of estrogen receptors alpha, beta and GPER1/GPR30 in pancreatic beta-cells. Front. Biosci. 2011;16:251–260. doi: 10.2741/3686. [DOI] [PubMed] [Google Scholar]
- 12.Ropero A.B., Pang Y., Alonso-Magdalena P., Thomas P., Nadal A. Role of ERβ and GPR30 in the endocrine pancreas: a matter of estrogen dose. Steroids. 2012;77:951–958. doi: 10.1016/j.steroids.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Mauvais-Jarvis F., Clegg D.J., Hevener A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhodes C.J., White M.F., Leahy J.L., Kahn S.E. Direct autocrine action of insulin on β-cells: does it make physiological sense? Diabetes. 2013;62:2157–2163. doi: 10.2337/db13-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldfine A.B., Kulkarni R.N. Modulation of β-cell function: a translational journey from the bench to the bedside. Diabetes Obes. Metab. 2012;14(Suppl. 3):152–160. doi: 10.1111/j.1463-1326.2012.01647.x. [DOI] [PubMed] [Google Scholar]
- 16.Xuan S. Defective insulin secretion in pancreatic beta cells lacking type 1 IGF receptor. J. Clin. Invest. 2002;110:1011–1019. doi: 10.1172/JCI15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulkarni R.N., Brüning J.C., Winnay J.N., Postic C., Magnuson M.A., Kahn C.R. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 18.Elghazi L., Balcazar N., Bernal-Mizrachi E. Emerging role of protein kinase B/Akt signaling in pancreatic beta-cell mass and function. Int. J. Biochem. Cell Biol. 2006;38:157–163. doi: 10.1016/j.biocel.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Vermeulen A. Dehydroepiandrosterone sulfate and aging. Ann. N.Y. Acad. Sci. 1995;774:121–127. doi: 10.1111/j.1749-6632.1995.tb17376.x. [DOI] [PubMed] [Google Scholar]
- 20.Labrie F. Extragonadal synthesis of sex steroids: intracrinology. Ann. Endocrinol. (Paris) 2003;64:95–107. [PubMed] [Google Scholar]
- 21.Cameron D.R., Braunstein G.D. The use of dehydroepiandrosterone therapy in clinical practice. Treat. Endocrinol. 2005;4:95–114. doi: 10.2165/00024677-200504020-00004. [DOI] [PubMed] [Google Scholar]
- 22.Camporez J.P., Akamine E.H., Davel A.P., Franci C.R., Rossoni L.V., Carvalho C.R. Dehydroepiandrosterone protects against oxidative stress-induced endothelial dysfunction in ovariectomized rats. J. Physiol. 2011;589:2585–2596. doi: 10.1113/jphysiol.2011.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman D.L., Leiter E.H., Schwizer R.W. Therapeutic effects of dehydroepiandrosterone (DHEA) in diabetic mice. Diabetes. 1982;31:830–833. doi: 10.2337/diab.31.9.830. [DOI] [PubMed] [Google Scholar]
- 24.Perzyło K., Kulik-Rechberger B., Gałczyński K., Rechberger T. Intracrinology and dehydroepiandrosterone – a new perspective for the use of androgens in hormone replacement therapy in postmenopausal women. Ginekol. Pol. 2011;82:690–695. [PubMed] [Google Scholar]
- 25.Gómez-Santos C., Larqué E., Granero E., Hernández-Morante J.J., Garaulet M. Dehydroepiandrosterone-sulphate replacement improves the human plasma fatty acid profile in plasma of obese women. Steroids. 2011;76:1425–1432. doi: 10.1016/j.steroids.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Yen T.T., Allan J.A., Pearson D.V., Acton J.M., Greenberg M.M. Prevention of obesity in Avy/a mice by dehydroepiandrosterone. Lipids. 1977;12:409–413. doi: 10.1007/BF02533624. [DOI] [PubMed] [Google Scholar]
- 27.Medina M.C. Dehydroepiandrosterone increases beta-cell mass and improves the glucose-induced insulin secretion by pancreatic islets from aged rats. FEBS Lett. 2006;580:285–290. doi: 10.1016/j.febslet.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Bonora E., Targher G., Alberiche M., Bonadonna R.C., Saggiani F., Zenere M.B., Monauni T., Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 29.Lacy P.E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 30.Scott A.M., Atwater I., Rojas E. A method for the simultaneous measurement of insulin release and B cell membrane potential in single mouse islets of Langerhans. Diabetologia. 1981;21:470–475. doi: 10.1007/BF00257788. [DOI] [PubMed] [Google Scholar]
- 31.Nicoletti I., Migliorati G., Pagliacci M.C., Grignani F., Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 32.Azevedo-Martins A.K., Monteiro A.P., Lima C.L., Lenzen S., Curi R. Fatty acid-induced toxicity and neutral lipid accumulation in insulin-producing RINm5F cells. Toxicol. In Vitro. 2006;20:1106–1113. doi: 10.1016/j.tiv.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Barrett-Connor E., Khaw K.T., Yen S.S. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N. Engl. J. Med. 1986;315:1519–1524. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- 34.Lea-Currie Y.R., Wen P., McIntosh M.K. Dehydroepiandrosterone-sulfate (DHEAS) reduces adipocyte hyperplasia associated with feeding rats a high-fat diet. Int. J. Obes. Relat. Metab. Disord. 1997;21:1058–1064. doi: 10.1038/sj.ijo.0800516. [DOI] [PubMed] [Google Scholar]
- 35.Campbell C.S., Caperuto L.C., Hirata A.E., Araujo E.P., Velloso L.A., Saad M.J., Carvalho C.R. The phosphatidylinositol/AKT/atypical PKC pathway is involved in the improved insulin sensitivity by DHEA in muscle and liver of rats in vivo. Life Sci. 2004;76:57–70. doi: 10.1016/j.lfs.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Dillon J.S. Dehydroepiandrosterone sulfate and beta-cell function: enhanced glucose-induced insulin secretion and altered gene expression in rodent pancreatic beta-cells. Diabetes. 2000;49:2012–2020. doi: 10.2337/diabetes.49.12.2012. [DOI] [PubMed] [Google Scholar]
- 37.Hong J., Stubbins R.E., Smith R.R., Harvey A.E., Núñez N.P. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr. J. 2009;8:11. doi: 10.1186/1475-2891-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato K., Iemitsu M., Aizawa K., Ajisaka R. DHEA improves impaired activation of Akt and PKC zeta/lambda-GLUT4 pathway in skeletal muscle and improves hyperglycaemia in streptozotocin-induced diabetes rats. Acta Physiol. (Oxf.) 2009;197:217–225. doi: 10.1111/j.1748-1716.2009.02011.x. [DOI] [PubMed] [Google Scholar]
- 39.Dickson L.M., Rhodes C.J. Pancreatic beta-cell growth and survival in the onset of type 2 diabetes: a role for protein kinase B in the Akt? Am. J. Physiol. Endocrinol. Metab. 2004;287:E192–E198. doi: 10.1152/ajpendo.00031.2004. [DOI] [PubMed] [Google Scholar]
- 40.Bernal-Mizrachi E., Fatrai S., Johnson J.D., Ohsugi M., Otani K., Han Z., Polonsky K.S., Permutt M.A. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J. Clin. Invest. 2004;114:928–936. doi: 10.1172/JCI20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aoyagi K., Ohara-Imaizumi M., Nishiwaki C., Nakamichi Y., Ueki K., Kadowaki T., Nagamatsu S. Acute inhibition of PI3K-PDK1-Akt pathway potentiates insulin secretion through upregulation of newcomer granule fusions in pancreatic β-cells. PLoS One. 2012;7:e47381. doi: 10.1371/journal.pone.0047381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaneko K. Class IA phosphatidylinositol 3-kinase in pancreatic β cells controls insulin secretion by multiple mechanisms. Cell Metab. 2010;12:619–632. doi: 10.1016/j.cmet.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


