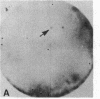
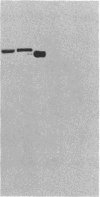
Abstract
Despite many biochemical and structural similarities between muscle and nonmuscle myosins, their genes appear to have completely diverged, since muscle myosin molecular clones will not hybridize to RNA from nonmuscle sources. Here we report the isolation and characterization of a partial myosin heavy chain (MHC) cDNA clone from the slime mold Dictyostelium discoideum. We have isolated this clone from a lambda gt11 expression cDNA library by antibody screening. In contrast to the highly conserved sarcomeric muscle MHC multigene families in other organisms, there appears to be only one gene encoding MHC in the Dictyostelium genome. The cloned portion of this gene does not hybridize to the genomic DNAs of other eukaryotic organisms. Analysis of the predicted amino acid sequence of the partial Dictyostelium MHC clone shows that while there is no sequence homology to known striated muscle MHCs, the structure- and coiled-coil-forming capacities have been conserved.
Full text
PDF
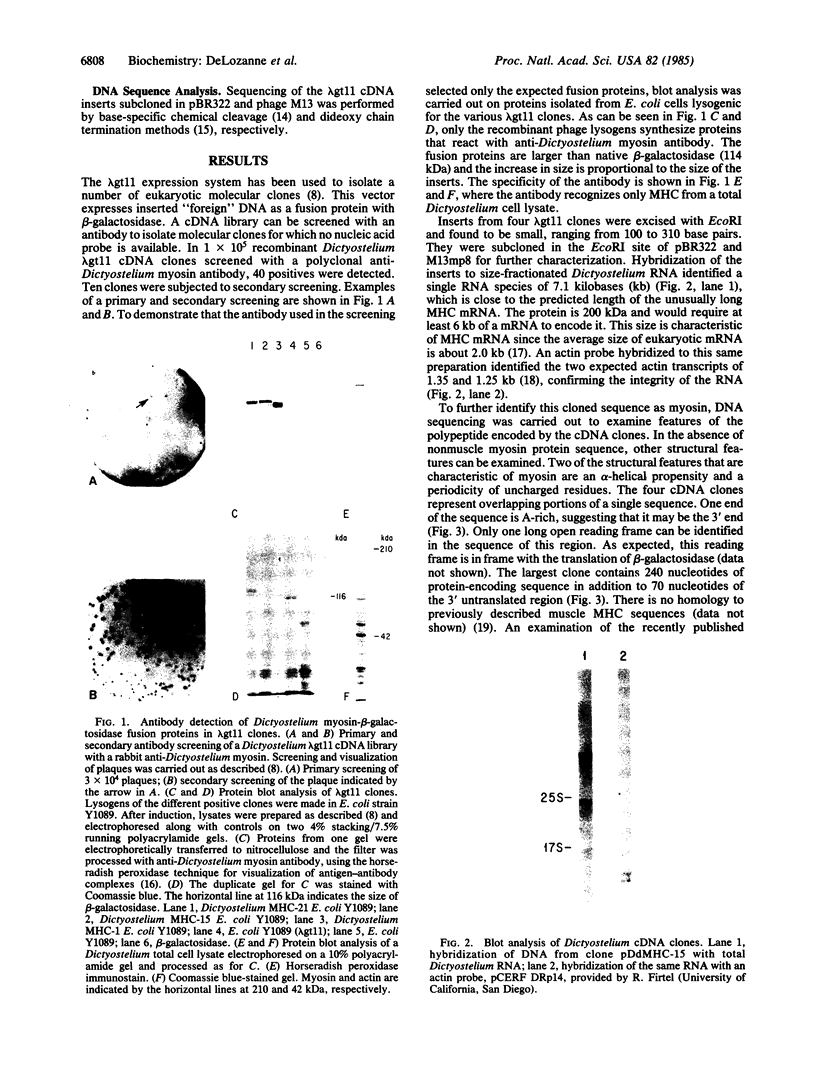


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Côté G. P., Robinson E. A., Appella E., Korn E. D. Amino acid sequence of a segment of the Acanthamoeba myosin II heavy chain containing all three regulatory phosphorylation sites. J Biol Chem. 1984 Oct 25;259(20):12781–12787. [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Firtel R. A. Multigene families encoding actin and tubulin. Cell. 1981 Apr;24(1):6–7. doi: 10.1016/0092-8674(81)90494-3. [DOI] [PubMed] [Google Scholar]
- Kavinsky C. J., Umeda P. K., Sinha A. M., Elzinga M., Tong S. W., Zak R., Jakovcic S., Rabinowitz M. Cloned mRNA sequences for two types of embryonic myosin heavy chains from chick skeletal muscle. I. DNA and derived amino acid sequence of light meromyosin. J Biol Chem. 1983 Apr 25;258(8):5196–5205. [PubMed] [Google Scholar]
- Kindle K. L., Firtel R. A. Identification and analysis of Dictyostelium actin genes, a family of moderately repeated genes. Cell. 1978 Nov;15(3):763–778. doi: 10.1016/0092-8674(78)90262-3. [DOI] [PubMed] [Google Scholar]
- Kuczmarski E. R., Spudich J. A. Regulation of myosin self-assembly: phosphorylation of Dictyostelium heavy chain inhibits formation of thick filaments. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7292–7296. doi: 10.1073/pnas.77.12.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWEY S., COHEN C. Studies on the structure of myosin. J Mol Biol. 1962 Apr;4:293–308. doi: 10.1016/s0022-2836(62)80007-2. [DOI] [PubMed] [Google Scholar]
- Leinwand L. A., Saez L., McNally E., Nadal-Ginard B. Isolation and characterization of human myosin heavy chain genes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3716–3720. doi: 10.1073/pnas.80.12.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: sequence components of heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Feb;4(2):77–93. doi: 10.1016/0092-8674(75)90113-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A. D., Karn J. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature. 1982 Sep 16;299(5880):226–231. doi: 10.1038/299226a0. [DOI] [PubMed] [Google Scholar]
- Medford R. M., Wydro R. M., Nguyen H. T., Nadal-Ginard B. Cytoplasmic processing of myosin heavy chain messenger RNA: evidence provided by using a recombinant DNA plasmid. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5749–5753. doi: 10.1073/pnas.77.10.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H. T., Gubits R. M., Wydro R. M., Nadal-Ginard B. Sarcomeric myosin heavy chain is coded by a highly conserved multigene family. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5230–5234. doi: 10.1073/pnas.79.17.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rozek C. E., Davidson N. Drosophila has one myosin heavy-chain gene with three developmentally regulated transcripts. Cell. 1983 Jan;32(1):23–34. doi: 10.1016/0092-8674(83)90493-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A. M., Umeda P. K., Kavinsky C. J., Rajamanickam C., Hsu H. J., Jakovcic S., Rabinowitz M. Molecular cloning of mRNA sequences for cardiac alpha- and beta-form myosin heavy chains: expression in ventricles of normal, hypothyroid, and thyrotoxic rabbits. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5847–5851. doi: 10.1073/pnas.79.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen R. G., Schwartz K., Bouveret P., Sell S. M., Gros F. Contractile protein isozymes in muscle development: identification of an embryonic form of myosin heavy chain. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5197–5201. doi: 10.1073/pnas.76.10.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydro R. M., Nguyen H. T., Gubits R. M., Nadal-Ginard B. Characterization of sarcomeric myosin heavy chain genes. J Biol Chem. 1983 Jan 10;258(1):670–678. [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]