Key Points
Polyphosphate suppresses complement via the terminal pathway by destabilizing C5b,6, thereby reducing the lytic capacity of the membrane attack complex.
Polyphosphate, a novel negative regulator of complement, bridges coagulation and complement and is a potential therapeutic target.
Abstract
Polyphosphate, synthesized by all cells, is a linear polymer of inorganic phosphate. When released into the circulation, it exerts prothrombotic and proinflammatory activities by modulating steps in the coagulation cascade. We examined the role of polyphosphate in regulating the evolutionarily related proteolytic cascade complement. In erythrocyte lysis assays, polyphosphate comprising more than 1000 phosphate units suppressed total hemolytic activity with a concentration to reduce maximal lysis to 50% that was 10-fold lower than with monophosphate. In the ion- and enzyme-independent terminal pathway complement assay, polyphosphate suppressed complement in a concentration- and size-dependent manner. Phosphatase-treated polyphosphate lost its ability to suppress complement, confirming that polymer integrity is required. Sequential addition of polyphosphate to the terminal pathway assay showed that polyphosphate interferes with complement only when added before formation of the C5b-7 complex. Physicochemical analyses using native gels, gel filtration, and differential scanning fluorimetry revealed that polyphosphate binds to and destabilizes C5b,6, thereby reducing the capacity of the membrane attack complex to bind to and lyse the target cell. In summary, we have added another function to polyphosphate in blood, demonstrating that it dampens the innate immune response by suppressing complement. These findings further establish the complex relationship between coagulation and innate immunity.
Introduction
Inorganic polyphosphate (polyP) is a linear polymer of orthophosphate, linked by phosphoanhydride bonds.1-3 It is found in all mammalian cells and lower organisms, localized in lysosomes, dense granules, mitochondria, and nuclei. The polymer varies in length from cell to cell and in different organisms, ranging from 60 to 100 units in human platelets to up to thousands of phosphate units in some bacteria.4-6 In platelets, polyP is localized in dense granules7 and released on activation, whereupon it is found in platelet-rich thrombi at concentrations of 1 to 3 μM.8 At physiologic pH, each internal unit has a monovalent negative charge, and thus the polymers are highly anionic. This property led to the finding that polyP is a physiologic surface on which factor XII, prekallikrein, and high molecular weight kininogen assemble for contact activation of coagulation.9 Subsequent studies confirmed that polyP is prothrombotic and proinflammatory in in vivo mouse models8,10 and that there are multiple steps in the coagulation cascade at which polyP acts to achieve this end.8,11-17 The effects of polyP on coagulation are concentration- and size-dependent.14 Thus, platelet-sized polyP (P60-100) primarily accelerates thrombin-mediated activation of factor XI and factor V, whereas larger-size polyP triggers coagulation via contact activation of factor XII and enhances fibrin polymerization.
The observation that polyP modulates coagulation raised the question as to whether polyP also regulates the evolutionarily related blood-borne proteolytic cascade complement. The complement system comprises more than 30 soluble and membrane-bound proteins, contributing to innate and adaptive immunity and aiding in disposal of danger-associated molecular patterns (for reviews, see Morgan18 and Ricklin and Lambris19). Complement activation is achieved via 3 pathways: the lectin pathway, classical pathway, and alternative pathway. These pathways converge with C3 convertase-mediated transformation of C3 into C3a and C3b. The C3a anaphylatoxin recruits leukocytes and activates platelets.20 C3b deposition on bacteria promotes phagocytosis by leukocytes and is required for formation of the C5 convertase that cleaves C5 into C5a and C5b. C5b rapidly binds to C6, forming a tight C5b,6 complex, which then binds to C7, yielding the C5b-7 complex. This attaches to the outer leaflet of a target membrane. The subsequent addition of the heterotrimeric C8αβγ stabilizes and anchors the now C5b-8 complex to the cell by inducing a conformational change in C8 and burying a hydrophobic tail through the lipid bilayer. Multiple C9 subunits finally join for assembly of the C5b-9 pore-like, lytic membrane attack complex (MAC).21
Coagulation and complement are coordinately regulated to limit blood loss, eliminate pathogens and damaged cells, and promote healing. Although often viewed as distinct, they are highly integrated, with several recently identified cross-talk pathways.22-30 For example, thrombin activates C5 and enhances formation of the MAC.31 Conversely, C5a promotes expression of tissue factor and plays a role in the antiphospholipid antibody-associated fetal loss syndome.32 Several other biochemical connections have been documented.29,33,34
In spite of evidence of links between coagulation and complement, the possibility that polyP regulates complement in mammalian systems is unexplored. In a mutant Neisseria meningitidis, lack of the polyphosphatase that normally degrades polyP renders the bacteria resistant to complement-mediated death, indicating that polyP facilitates evasion from complement-mediated killing.35 We tested the hypothesis that polyP provides a bridge between complement and coagulation in the human system.36 We show that polyP significantly suppresses complement via the terminal pathway (TP) and that this occurs in a concentration-dependent, size-dependent, and ion chelation-independent manner. PolyP binds to and destabilizes C5b,6, interfering with optimal assembly and membrane binding of the MAC. The findings reveal a novel mechanism by which complement is regulated and underline the complex relationship between coagulation and complement.
Materials and methods
Reagents
Chemicals were purchased from Sigma Aldrich (St. Louis, MO) unless otherwise stated. PolyP preparations were synthesized, size-fractionated, and quantified by the malachite green assay,14 with concentrations expressed in phosphate monomers (monomer formula: NaPO3). The number of phosphate units making up polyP is indicated in the subscript. Gelatin veronal buffer (pH 7.4), normal human serum (NHS), and human plasma-derived C5b,6, C7, C8, and C9 were from Complement Technology Inc. (Tyler, TX). Rabbit erythrocytes (rRBCs) were freshly obtained from citrated venous blood after venipuncture performed by Animal Care Services personnel at the University of British Columbia (Vancouver, BC, Canada), with approval by the University of British Columbia Animal Ethics Committee. Chicken erythrocytes (cRBCs) were from Colorado Serum Company (Denver, CO). Hemolytic assays were performed in 96-well nontreated microplates from Corning (Amsterdam, The Netherlands). Sypro Orange was from Invitrogen (Burlington, ON, Canada).
Hemolytic assays
Erythrocyte lysis assays18,37 were used to measure complement-mediated hemolytic activity (supplemental Data, available on the Blood Web site). Lysis was first determined relative to the control of 100% lysis with H2O. For total hemolytic and TP assays, concentrations of NHS and C5b,6 to initiate complement activation were established from pilot studies to obtain 70% to 80% erythrocyte lysis at 30 minutes (supplemental Figures 1-3). Results were normalized to this amount of lysis and shown in figures as “relative RBC lysis.” Results are representative of experiments performed a minimum of 3 times each.
Native polyacrylamide gel electrophoresis, analytic gel filtration, and differential scanning fluorimetry
Native polyacrylamide gel electrophoresis (PAGE), analytic gel filtration, and differential scanning fluorimetry (DSF; also referred to as thermal shift assay)38 were used to assess polyP-protein interactions. Methods for the first 2 are described in supplemental Data. For DSF, 25 µL reaction mixes consisting of 0.4 mg/mL protein (0.14 mg/mL for C5b,6), varying concentrations of polyP diluted in Hepes buffered saline (20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid and 150 mM NaCl at pH 7.4), and 5× Sypro Orange were loaded into MicroAmp Fast Optical 96-well Reaction Plates (Applied Biosystems, Carlsbad, CA). Samples were heat denatured in an Applied Biosystems StepOnePlus Real-Time PCR System, using a ramp configuration starting at 25°C and increasing at 1°C min−1 to 95°C. Fluorescence (in relative fluorescence units) was measured every 30 seconds.38 Data for each curve were normalized to the maximum and the minimum of the curve.
Statistical analyses
Analyses were performed with GraphPad Prism version 5.0 (San Diego, CA). Where indicated, 1-way analysis of variance with Bonferroni’s multiple comparison tests were performed. Results shown are means ± standard error of the mean. Statistical significance refers to P < .05.
Results
PolyP suppresses total hemolytic activity in a concentration-dependent manner
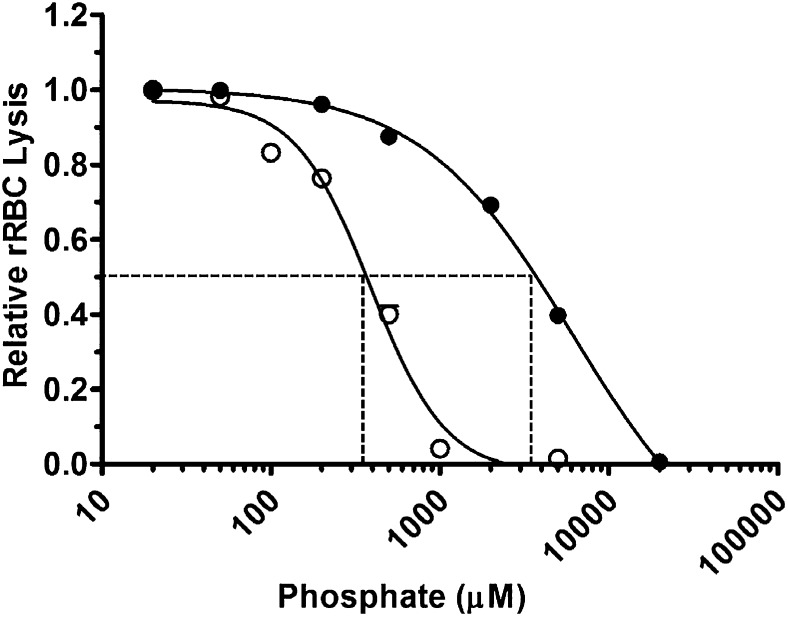
We first assessed the effects of polyP on total complement-mediated hemolytic activity. NHS diluted to yield 70% to 80% lysis was preincubated with varying concentrations of polyP that include more than 1000 orthophosphate units (NaPO3) per chain (polyP>1000) and that promote coagulation.14 Orthophosphate (P1) was used as a control. Reactions were initiated by the addition of rRBCs, and hemolysis was measured after 30 minutes (Figure 1). P1 and polyP>1000 suppressed hemolysis in a concentration-dependent manner. However, at equivalent molar concentrations of the monomeric form, polyP>1000 was strikingly more effective at suppressing hemolysis. The concentration required to achieve half maximal inhibition of lysis (IC50) was ∼3.5 mM for P1 and ∼350 µM for polyP>1000 (Figure 1). Because complement activation via the alternative and classical pathways is dependent in part on the presence of ionic calcium and magnesium, it is likely that the observed suppression of total hemolytic activity by monophosphate concentrations exceeding ∼5 mM was induced primarily by chelation of those ions. However, chelation would not account for the enhanced activity of polyP compared with P1, as the ionic strengths of the polyP and P1 at equivalent monomeric concentrations are similar. Thus, polyP suppresses total hemolytic activity.
Figure 1.
PolyP suppresses total hemolytic activity. rRBCs were incubated with 4.5% serum in the presence of increasing concentrations of P1 (●) or polyP>1000 (○). Values were normalized to baseline lysis in the absence of phosphate. Curves were fitted to a nonlinear regression inhibitory dose–response model to determine IC50 for P1 and polyP, shown by dotted lines. Results are representative of 3 experiments, each performed in triplicate.
PolyP, but not monophosphate, suppresses complement-mediated lysis via the TP in serum
We examined the effect of polyP on the TP of complement. The TP is initiated by rapid binding of C5b to C6 and the subsequent and sequential binding of C7, C8, and several C9 molecules to form the C5b-9 MAC. This pathway is ion-independent, and thus any effects of polyP would also be independent of its capacity to chelate cations.
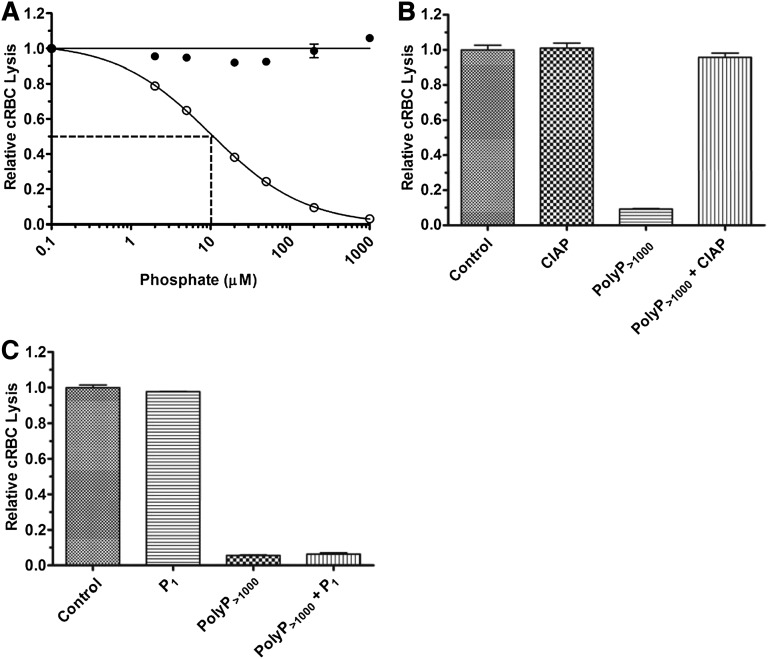
We first studied the effects of polyP on the TP of complement in NHS. TP activity was initiated by adding a limiting amount of exogenous purified C5b,6 to achieve 70% to 80% lysis after 30 minutes. A molar excess of EDTA was used to prevent upstream activation of complement and generation of endogenous C5b,6. In the absence of C5b,6, there was no detectable complement-mediated cRBC lysis (data not shown). P1 at concentrations ranging from 0.1 to 1000 µM had no effect on complement-mediated hemolysis in NHS via the TP (Figure 2A). In contrast, polyP>1000 inhibited the TP in NHS in a concentration-dependent manner, with an IC50 of ∼10 µM polyP>1000 (Figure 2A). To verify that the effects observed were dependent on the integrity of the polymers, we pretreated polyP with CIAP, which cleaves polyP into monomeric units.39 CIAP alone had no effect on the TP, whereas CIAP treatment of the polyP>1000 completely abrogated its ability to dampen hemolysis via the TP (Figure 2B). In addition, coincubation of up to 2 mM P1 in combination with polyP>1000 at a concentration of 200 µM had no effect on the suppressive properties of polyP>1000 (Figure 2C).
Figure 2.
PolyP suppresses TP hemolytic activity in serum. (A) P1 (●) or polyP>1000 (○) were titrated into the TP assay in the presence of 2% serum and 250 pM C5b,6. The IC50 for polyP is shown by dotted lines and was determined as in Figure 1. Results are representative of more than 5 experiments, each performed in triplicate. (B) 600 µM polyP>1000 was incubated overnight with 400 U/mL calf intestinal alkaline phosphatase (CIAP) and then added to the TP assay at a final polyP>1000 concentration of 100 µM. The control represents lysis in the absence of CIAP and polyP, but with an equivalent concentration of CIAP digestion buffer. “CIAP” represents lysis in the presence of CIAP but absence of polyP. The findings indicate that suppression of the TP by polyP requires the integrity of the polymer. Values were normalized to baseline lysis from the control condition. Each column represents quadruplicate data points. (C) 2 mM P1 and 200 µM polyP>1000 were added singly or in combination in the TP assay. Values were normalized to baseline lysis from the control condition in which no phosphate was added. Excess monomer could not overcome suppressive properties of polyP. n = 3 independent experiments, each performed in triplicate.
Suppression of TP hemolytic activity is dependent on polyP chain length
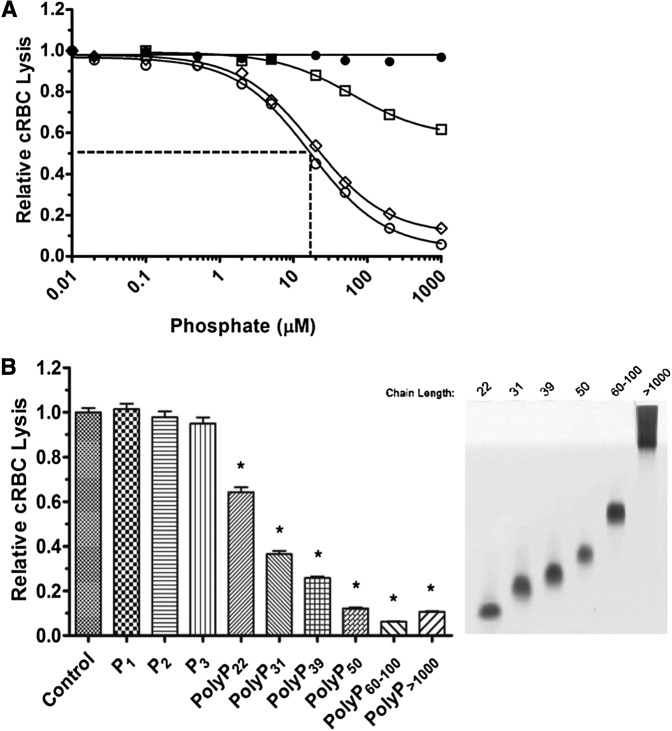
The effects of polyP on coagulation are dependent on chain length. We therefore tested the effect of different chain lengths of polyP on TP hemolytic activity. Medium chain-length polyP (polyP40-160) had a similar concentration-dependent suppressive effect as polyP>1000 on TP-mediated hemolysis (Figure 3A). However, shorter-length polyP (polyP<30) was less potent, and an IC50 could not be achieved even with 5 mM polyP<30. A wider range of different polyP chain lengths was examined (Figure 3B). At equivalent 100 µM concentrations, P1, diphosphate (Na4P2O7) (P2), and triphosphate (Na5P3O10) (P3) had no effect on TP hemolytic activity. However, polyP with a mean length of 22 orthophosphate units (polyP22) significantly dampened TP hemolytic activity, and the extent of suppression by longer-chain polyP increased in a size-dependent manner. PolyP released from thrombin-stimulated platelets also suppressed TP complement activity (not shown).
Figure 3.
PolyP suppresses the TP in a size-dependent manner. (A) P1 (●), polyP<30 (□), polyP40-160 (▪), and polyP>1000 (○) were titrated into the TP assay in the presence of 2% serum and 250 pM C5b,6. The IC50 is shown only for polyP>1000. The TP was suppressed in a size-dependent manner. Results are representative of 3 experiments, each performed in triplicate. (B) On the left, 100 µM of different size polyPs were added to the TP assay as above. On the right, the different-size polyPs used in the TP assay were separated on a Tris-Borate-EDTA-urea gel and stained with toluidine blue. *Comparisons to control without phosphate (P < .001; n = 4).
PolyP depends on early TP components to suppress lytic activity
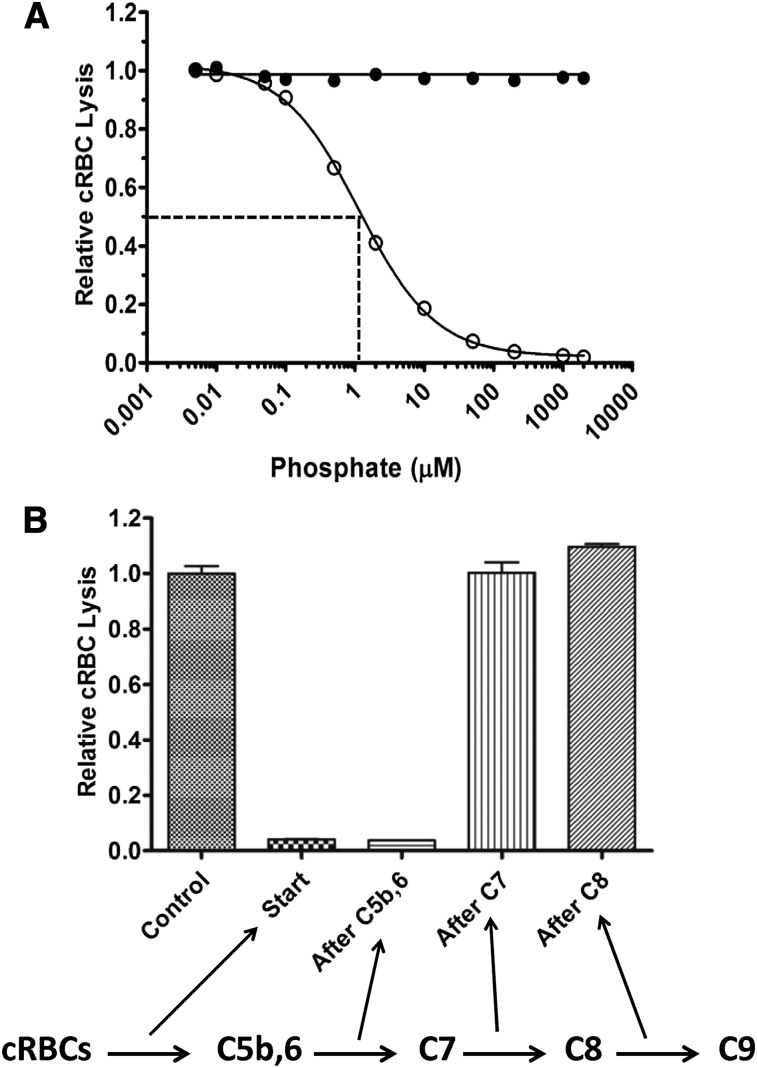
We next examined whether polyP suppresses TP hemolytic activity through direct interactions with TP components (C5b,6, C7, C8, or C9) or whether other serum factors are required. Purified TP components C7, C8, and C9 were therefore used as the source of complement, rather than NHS. TP hemolytic activity was measured by sequentially adding cRBCs, varying concentrations of polyP, and then C5b,6, C7, C8, and C9. Similar to findings with NHS, polyP dose-dependently suppressed lysis, whereas P1 had no effect (Figure 4A). The IC50 of polyP>1000 in this system was ∼1 µM. The effect of polyP was not specific to the species of the target erythrocytes, as TP hemolytic activity was also suppressed by polyP when human erythrocytes were used (data not shown). Overall, the findings indicate that polyP interferes with TP complement-mediated lysis of RBCs by binding directly to 1 or more of the TP complement components or to the target red cell membrane.
Figure 4.
PolyP directly affects the function of TP components. (A) In a purified system, P1 (●) or polyP>1000 (○) were titrated into the TP assay in the presence of 20 pM C5b,6 and excess C7, C8, and C9. PolyP suppresses the TP in a similar manner as in serum (Figure 2A). Results are representative of 3 experiments, each performed in triplicate. (B) The TP assay was performed by sequentially adding cRBCs, C5b,6, C7, C8, and C9, with 200 µM polyP>1000 added at different steps, as indicated by arrows below the figure. Values were normalized to baseline lysis in the control condition without the addition of polyP. Each column represents quadruplicate data points. Relative lysis between control, after C7, and after C8 conditions were not statistically significant (P > .05).
PolyP interferes with TP hemolytic activity at early steps in assembly of the MAC
We delineated the steps in the TP at which polyP interferes with the function of the MAC to lyse erythrocytes. PolyP was added at different steps of the TP assay (ie, before C5b,6, after C5b,6, after C7, after C8, or with C9; Figure 4B). PolyP>1000 at a concentration of 200 µM suppressed lysis to less than 5% of maximal lysis (ie, without polyP) when added before or immediately after C5b,6. In contrast, polyP had no effect on cRBC lysis in the TP assay when added after C7, after C8, or with C9. Equivalent results were obtained with polyP60-100 (data not shown). Thus, polyP interferes with optimal MAC function/assembly by destabilizing C5b,6, limiting normal C5b,6 interaction with C7, or causing further downstream TP complexes (C5b-7, C5b-8, or C5b-9) to be unstable or incapable of attaching or inserting into the RBC membrane. Once C5b-7 forms, however, polyP can no longer modulate MAC function.
To determine whether polyP interferes with incorporation of the terminal complex onto or into the membrane of cRBCs, we added varying concentrations of polyP to the cells, followed by a 5-minute incubation with equimolar concentrations of purified C5b,6, C7, and C8. Under these conditions, the C5b-8 complex integrates into the membrane, causing minimal lysis. Cells were pelleted, and the amount of unbound C5b, reflecting unbound C5b-8, was assessed by Western immunoblot under reducing conditions (supplemental Figure 4A). More C5b was recovered in the supernatant when polyP was included, and this effect was dose-dependent. In a similar manner, by incubating C5b,6 and C7 (without C8 or C9), the presence of polyP caused reduced binding of the C5b-7 complex to the erythrocyte membrane (supplemental Figure 4B). The findings indicate that polyP interferes with binding/integration of the C5b-7 and C5b-8 complexes to/into the cell membrane.
PolyP alters the stability of C5b,6 and C6, but not C5 or C7
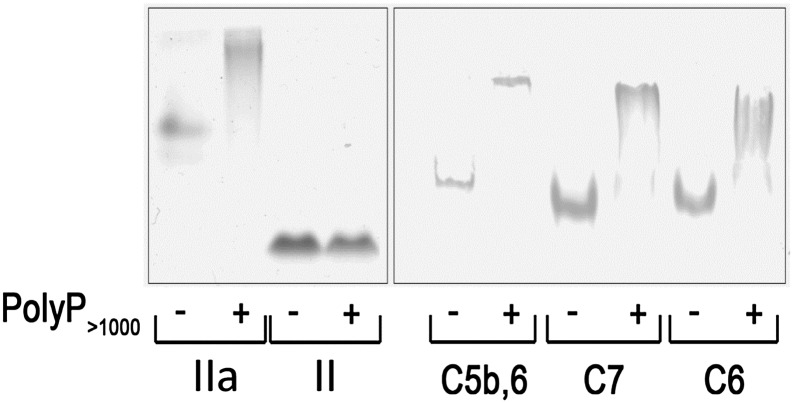
We investigated whether polyP interacts directly with different components of the TP and whether it alters the stability and, thus, the function of these components. In the first approach, native PAGE was used to assess polyP–protein binding. Platelet-derived polyP binds to thrombin (distribution coefficient, ∼5 nM), but not to prothrombin13; findings that are evident on native gels (Figure 5). Similar to thrombin’s interaction with polyP, we showed that C5b,6, C6, and C7 all exhibited band shifts in the presence of polyP consistent with a physical interaction under these experimental conditions.
Figure 5.
PolyP reduces the mobility of TP proteins in native gels. Two micrograms of protein were incubated with or without 6 µg polyP>1000 and resolved by native PAGE. The gel was stained with Coomassie blue for detection. PolyP binds to and causes a shift in the migration of thrombin (IIa) but does not bind to or affect migration of prothrombin (II). PolyP causes a gel shift in C5b,6, C7, and C8. Results are representative of 4 independent experiments.
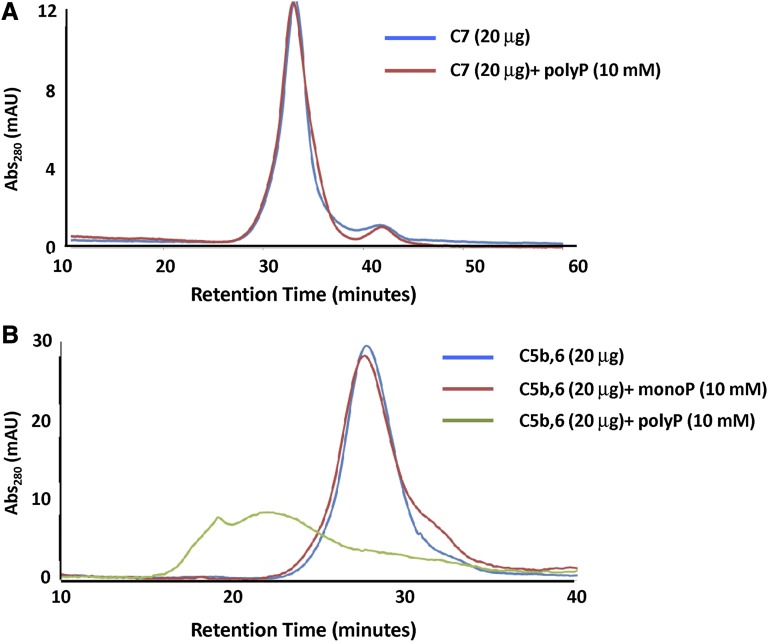
In a second approach, we performed gel filtration studies with either C5b,6 or C7 in the presence or absence of polyP>1000. PolyP had no effect on the chromatogram for C7 (Figure 6A). This was somewhat surprising, given the gel shift data, but is likely attributable to different buffer conditions. Notably, coincubation of polyP caused a shift to a higher oligomerized state in the C5b,6 elution profile (Figure 6B), indicating a direct interaction of C5b,6 with polyP. The broader C5b,6-polyP peak is consistent with polyP destabilizing C5b,6. In the absence of C6, C5b is highly unstable and forms aggregates,40 and thus C5b was not independently examined.
Figure 6.
Gel filtration to assess interactions of C5b,6 and C7 with polyP. The effect of polyP on the gel filtration elution profiles of C5b,6 and C7 was evaluated. (A) The presence of polyP>1000 (10 mM) does not affect the retention time or elution profile of C7. (B) P1 (monoP) (10 mM) does not affect the retention time or elution profile of C5b,6. However, the presence of polyP (10 mM) binds to and shifts C5b,6 to a more highly oligomerized or aggregated state.
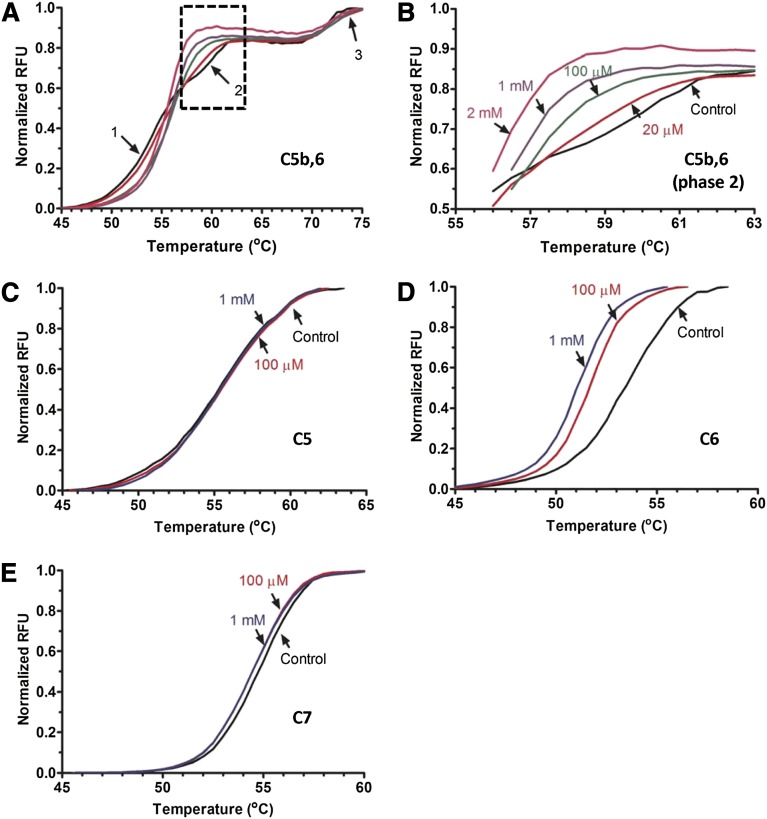
In the third approach, DSF was used to determine the thermal stability of the complement proteins in the presence and absence of polyP. This technique involves heat-denaturing proteins and exposing internal hydrophobic regions to the aqueous environment, detected by the fluorescent dye Sypro Orange.38 Interactions between a protein and a binding partner often change the thermal stability of the protein, observed as a shift in the melting (denaturation) curve. In the absence of polyP, the melting curve of C5b,6 (Figure 7A) exhibits 3 transition phases (arrows), likely as a result of the differential thermal stabilities between C5b,6 domains. When C5b,6 was incubated with polyP>1000, a dose-dependent leftward shift in the second transition phase at 59.5°C was observed (expanded in Figure 7B), evidence that polyP binds to a distinct domain on C5b,6. PolyP had no effect on the melting curve of C5 but caused a dose-dependent destabilization shift in the C6 melting curve (Figure 7C-D, respectively), suggesting that polyP interacts with C5b,6 via C6. In spite of the gel shift showing an interaction between polyP and C7, only a minimal polyP-induced shift in the DSF C7 curve was observed, and only in the presence of a high concentration (1 mM) of polyP (Figure 7E). In a similar manner, although thrombin binds to polyP,13 we also did not detect a polyP-induced change in thrombin’s thermal stability measured by DSF (supplemental Figure 5). PolyP60-100 had the same effect as polyP>1000 for all proteins tested.
Figure 7.
PolyP alters the thermal stability of C5b,6. C5b,6 (A-B), C5 (C), C6 (D), and C7 (E) were individually incubated with a range of concentrations of polyP>1000, and relative fluorescence was measured as the proteins were thermally denatured. An increase in relative fluorescence indicates protein denaturation. Controls are protein without phosphate. For C5b,6 (A), arrows with numbers indicate the 3 transition phases exhibited during denaturation in the absence of polyP. (B) Focus on the second transition phase of C5b,6 (boxed region in A), where the lines from A are labeled with the different concentrations of polyP (from 0 [control] to 2 mM) that were added. PolyP induces concentration-dependent denaturation of C5b,6 and C6, but not C5 or C7. Results were similar with polyP60-100 (not shown). Results are representative of 3 independent experiments.
Discussion
We have shown that polyP dampens complement via the TP, that this occurs in a concentration- and polymer size-dependent manner, and that the mechanisms involve, at least in part, destabilization of C5b,6, thereby interfering with optimal lytic function of the C5b-9 MAC. This is the first account of a complement-inhibitory function for polyP in a eukaryotic system. Previously, Zhang et al35 used mutant forms of N meningitidis, which lack the exopolyphosphatase that cleaves polyP, and showed that these bacteria are resistant to complement-mediated killing. Moreover, exogenous polyP protected wild-type bacteria from complement-mediated death. Thus, in these bacteria, polyP is a weapon of survival, used to overcome host innate immunity.41 Our findings are in line with theirs, supporting the notion that polyP in humans may dampen innate immune responses via suppression of complement.
Previous studies established that polyP initiates activation of coagulation and inflammation via the contact pathway, accelerates generation of factor Va by factor Xa and thrombin, and enhances the stability of the fibrin clot.9,16 The biochemical studies were validated in vivo in mice, where injections of polyP caused a thrombotic and proinflammatory response, the latter mediated by increased generation of factor XIIa, kallikrein, and bradykinin.8,10 That polyP can inhibit complement and limit the innate immune response appears at odds with its well-characterized procoagulant and proinflammatory properties. How can this be explained?
It is not unprecedented in the coagulation system for a molecule to have dual and functionally opposing activities that are delicately balanced to maintain homeostasis. For example, thrombin, through dynamic allosteric changes and interactions with cofactors, receptors, and substrates, elicits procoagulant and anticoagulant properties that are orchestrated to ensure rapid and localized responses to injury and bleeding while preventing unrestrained thrombosis (reviewed in DiCera42 and Lechtenberg et al.43 Thrombin is also promiscuous in its role in regulating inflammation and innate immunity. It induces proinflammatory cytokine release,44-46 is chemotactic, and activates complement C5.31,47 Conversely, thrombin enhances vascular endothelial barrier protection48 and generates activated thrombin activatable fibrinolysis inhibitor, which, in turn, inactivates proinflammatory mediators bradykinin, osteopontin, C3a, and C5a.49 It therefore is not surprising that polyP has diverse functions. PolyP in the plasma50 or released from cells may provide local protection to host cells (eg, platelets, endothelial cells, leukocytes) while inducing proinflammatory/procoagulant effects at other sites. These properties may be differentially exhibited on the basis of the pathophysiologic situation, the cellular source of the polyP, the presence of ions in the target pathways, the extracellular concentration of the polyP, and/or the size of the polymer at the site of action.
Polymer size is indeed important in the function of polyP in the coagulation system, where it dictates the specific steps in the cascade at which it optimally performs.14 More extensive study of the role of polyP in the multiple complement pathways may similarly reveal size-dependent differential regulation. Our studies suggest that polymer size may distinguish the effects of polyP in coagulation and complement. PolyP preparations with lengths less than 30 orthophosphate units had no effect on plasma clotting time,14 whereas polyP>1000 shortens the clotting time at concentrations of 5 to 20 μM (supplemental Figure 6). In vivo studies that revealed the prothrombotic and proinflammatory properties of polyP were performed, using polymers with chain lengths that exceeded 45 orthophosphate units,8,10 and thus, it is not known whether shorter-length polymers would have the same effect. The shortest-length polymers used in our in vitro studies were polyP22 and polyP31, sizes that would be expected to have minimal effects on coagulation. Notably, these could still suppress the TP. Because we have not yet evaluated the role of polyP in vivo in animal models of human disease associated with excess complement activation, we can only speculate that using shorter forms of polyP might selectively reduce activation of complement through the TP. It is also possible that lower concentrations than what have been used in vivo8,10 might spare the prothrombotic and proinflammatory effects of polyP and favor complement inhibition and dampening of inflammation. These studies, using a range of sizes and concentrations of polyP in different disease models, will be important to perform, as therapies targeting polyP might be tailored to independently modulate coagulation and complement to meet patients' needs.
Platelets play a central role in maintaining hemostasis and promoting thrombus growth and are also critical in immune surveillance (reviewed in Rondina et al51). In addition to being a source of polyP, they express several complement receptors, store complement regulatory and activating factors, and provide a surface for generation of C3a, C5a, and MAC assembly.20,52,53 Although platelets participate in the innate immune response by promoting complement activation, this is tightly regulated by platelet-derived negative regulators of complement, such as factor H, clusterin, CD55, and CD59. Indeed, the platelet must protect itself from excess activation and complement-mediated destruction. This is accomplished in part by the release of the major fluid phase-negative regulator, factor H.54 PolyP, released from activated platelets, is believed to participate in the initiation and activation of coagulation but might also modulate the local inflammatory response by suppressing complement activation. In humans, low platelet levels of polyP are found in patients with dense granule storage pool diseases,55,56 such as Hermansky Pudlak Syndrome57 and Chediak Higashi Disease.58 Patients with these disorders exhibit a bleeding diathesis but, interestingly, often suffer from largely unexplained recurrent, life-threatening infections. These may be a result of leukocyte defects. Alternatively, the immune defects may be partly caused by alterations in complement that could be partially attributed to reduced polyP release. It will be interesting to evaluate complement activation in these patients.
We explored the mechanisms by which polyP suppresses complement activation. As an anionic polymer, polyP chelates divalent metal ions.59 Initiation and activation of complement until formation of the C5 convertases is dependent on Mg2+ and Ca2+. In our studies using the total hemolytic activity assay, increasing concentrations of monophosphate (P1) suppressed cell lysis, but only at molar concentrations equivalent to or greater than the concentrations of ionic calcium and magnesium in the assay system and required for complement activation. PolyP>1000 at equivalent molar concentrations was much more potent in suppressing complement-mediated cell lysis, suggesting that chelation of calcium and magnesium ions was not the only mechanism. Although we subsequently focused on examining the effect of polyP on the TP, which is ion-independent, preliminary findings from our laboratory indicate that polyP binds to C1-esterase inhibitor, factor B, and factor H, but not to factor D, factor I, or C3b (supplemental Figure 7). Further studies will uncover the relevance of these findings with respect to regulating complement via 1 or more of the complement activation pathways.
The TP of complement is, in effect, a point of no return that, without intervention by negative regulators, results in formation of the MAC. Three major negative regulators of the TP that prevent cell lysis have been described, all of which variably bind to TP components to prevent formation and integration of the MAC. CD59 is a glycosylphosphatidylinositol-linked protein that binds to C8 and C9 and prevents C9 from polymerizing.60 CD59 deficiencies are associated with paroxysmal nocturnal hemoglobinuria.61 Clusterin binds to C7, C8, and C9, inducing a conformational change that reduces the capacity of the C5b-9 complex to integrate into the membrane.62,63 Vitronectin binds to C5b-7 in the fluid phase (but not to C5b,6), allowing formation of a soluble MAC that cannot bind to the membrane.64 PolyP is a fourth negative regulator of the TP that also binds to TP components to reduce cell lysis.
Several lines of physicochemical evidence led to the conclusion that polyP binds to and destabilizes the earliest component of the TP, such that formation of a functional MAC is reduced. Electromobility shift assays in native gels showed that polyP directly interacts with several complement components. These findings, which do not necessarily indicate a functional relationship, prompted us to use other approaches to establish the mechanisms. In gel filtration studies, polyP binds directly to C5b,6, whereas DSF clearly shows that polyP destabilizes C5b,6 in a concentration-dependent manner. By sequentially adding the TP components to the cRBCs in the TP hemolytic assay, we determined that polyP significantly suppresses lysis when added before or after C5b,6, but not after C7, and that polyP interferes with C5b-7 and C5b-8 anchoring to the membrane. Further structure function studies will be necessary to identify the molecular structures involved and whether there are additional mechanisms.
In summary, we have uncovered a novel mechanism by which complement is negatively regulated. Future studies will focus on delineating the in vivo relevance, using mouse models of human disease and exploring how polyP might regulate other steps in the complement system. The interplay among polyP, complement, and coagulation is important to understand, as alterations in their cross-talk may manifest as disease and diagnostic and therapeutic insights may be gained.
Supplementary Material
Acknowledgments
J.M.W. is supported in part by a scholarship from the Canadian Institutes of Health Research. J.H.F. is supported by a Banting Fellowship. E.M.C. is supported by operating grants from the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, and the Canada Foundations for Innovation. He holds a CSL Behring Research Chair and a Tier 1 Canada Research Chair in Endothelial Cell Biology, is an adjunct scientist with the Canadian Blood Services, and is a member of the University of British Columbia Life Sciences Institute. N.C.S. is supported by operating grants from the Canadian Institutes of Health Research and holds a Tier 1 Canada Research Chair in Antibiotic Discovery and a Senior International Research Scholar award from the Howard Hughes Medical Institute. This work was supported in part by National Institutes of Health Grant R01 HL47014 (to J.H.M.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.M.C. directed all research and wrote the paper; J.M.W. designed and performed the experiments and helped write the paper; J.H.F. and M.J.K. helped design the studies, established conditions for hemolytic assays, and helped write the paper; V.L. and L.M.O. performed hemolytic assays and gel filtration; G.A.W., E.L., and N.C.S. helped perform and analyze DSF and gel filtration; and S.A.S. and J.H.M. prepared polyP and helped in designing experiments and preparing the paper.
Conflict-of-interest disclosure: J.H.M. and S.A.S. are coinvestigators on patents pertaining to medical uses of polyphosphate. E.M.C., J.M.W., M.J.K., J.H.F., and L.M.O. are coinvestigators on a patent pertaining to medical uses of polyphosphate. The remaining authors declare no competing financial interests.
Correspondence: Edward M. Conway, Centre for Blood Research, 4306-2350 Health Sciences Mall, University of British Columbia, Vancouver, BC V6T 1Z3, Canada; e-mail: ed.conway@ubc.ca.
References
- 1.Harold FM. Inorganic polyphosphates in biology: structure, metabolism, and function. Bacteriol Rev. 1966;30(4):772–794. doi: 10.1128/br.30.4.772-794.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caen J, Wu Q. Hageman factor, platelets and polyphosphates: early history and recent connection. J Thromb Haemost. 2010;8(8):1670–1674. doi: 10.1111/j.1538-7836.2010.03893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao NN, Kornberg A. Inorganic polyphosphate regulates responses of Escherichia coli to nutritional stringencies, environmental stresses and survival in the stationary phase. Prog Mol Subcell Biol. 1999;23:183–195. doi: 10.1007/978-3-642-58444-2_9. [DOI] [PubMed] [Google Scholar]
- 4.Kornberg A, Rao NN, Ault-Riché D. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem. 1999;68:89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- 5.Brown MR, Kornberg A. Inorganic polyphosphate in the origin and survival of species. Proc Natl Acad Sci USA. 2004;101(46):16085–16087. doi: 10.1073/pnas.0406909101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown MR, Kornberg A. The long and short of it - polyphosphate, PPK and bacterial survival. Trends Biochem Sci. 2008;33(6):284–290. doi: 10.1016/j.tibs.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279(43):44250–44257. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- 8.Müller F, Mutch NJ, Schenk WA, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139(6):1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci USA. 2006;103(4):903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae JS, Lee W, Rezaie AR. Polyphosphate elicits pro-inflammatory responses that are counteracted by activated protein C in both cellular and animal models. J Thromb Haemost. 2012;10(6):1145–1151. doi: 10.1111/j.1538-7836.2012.04671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller F, Renné T. Platelet polyphosphates: the nexus of primary and secondary hemostasis. Scand J Clin Lab Invest. 2011;71(2):82–86. doi: 10.3109/00365513.2010.550312. [DOI] [PubMed] [Google Scholar]
- 12.Mutch NJ, Engel R, Uitte de Willige S, Philippou H, Ariëns RA. Polyphosphate modifies the fibrin network and down-regulates fibrinolysis by attenuating binding of tPA and plasminogen to fibrin. Blood. 2010;115(19):3980–3988. doi: 10.1182/blood-2009-11-254029. [DOI] [PubMed] [Google Scholar]
- 13.Mutch NJ, Myles T, Leung LL, Morrissey JH. Polyphosphate binds with high affinity to exosite II of thrombin. J Thromb Haemost. 2010;8(3):548–555. doi: 10.1111/j.1538-7836.2009.03723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith SA, Choi SH, Davis-Harrison R, et al. Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood. 2010;116(20):4353–4359. doi: 10.1182/blood-2010-01-266791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SA, Morrissey JH. Polyphosphate as a general procoagulant agent. J Thromb Haemost. 2008;6(10):1750–1756. doi: 10.1111/j.1538-7836.2008.03104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith SA, Morrissey JH. Polyphosphate enhances fibrin clot structure. Blood. 2008;112(7):2810–2816. doi: 10.1182/blood-2008-03-145755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Meijden PE, Heemskerk JW. Polyphosphates: a link between platelet activation, intrinsic coagulation and inflammation? Expert Rev Hematol. 2010;3(3):269–272. doi: 10.1586/ehm.10.26. [DOI] [PubMed] [Google Scholar]
- 18.Morgan BP. The complement system: an overview. Methods Mol Biol. 2000;150:1–13. doi: 10.1385/1-59259-056-X:1. [DOI] [PubMed] [Google Scholar]
- 19.Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat Biotechnol. 2007;25(11):1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Conde I, Crúz MA, Zhang H, López JA, Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J Exp Med. 2005;201(6):871–879. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadders MA, Bubeck D, Roversi P, et al. Assembly and regulation of the membrane attack complex based on structures of C5b6 and sC5b9. Cell Rep. 2012;1(3):200–207. doi: 10.1016/j.celrep.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delvaeye M, Conway EM. Coagulation and innate immune responses: can we view them separately? Blood. 2009;114(12):2367–2374. doi: 10.1182/blood-2009-05-199208. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie. 2010;30(1):5–6, 8-9. [PubMed] [Google Scholar]
- 24.Weiler H. Regulation of inflammation by the protein C system. Crit Care Med. 2010;38(2 Suppl):S18–S25. doi: 10.1097/CCM.0b013e3181c9cbb5. [DOI] [PubMed] [Google Scholar]
- 25.Rezaie AR. Regulation of the protein C anticoagulant and antiinflammatory pathways. Curr Med Chem. 2010;17(19):2059–2069. doi: 10.2174/092986710791233706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oehmcke S, Herwald H. Contact system activation in severe infectious diseases. J Mol Med (Berl) 2010;88(2):121–126. doi: 10.1007/s00109-009-0564-y. [DOI] [PubMed] [Google Scholar]
- 27.Luyendyk JP, Sullivan BP, Guo GL, Wang R. Tissue factor-deficiency and protease activated receptor-1-deficiency reduce inflammation elicited by diet-induced steatohepatitis in mice. Am J Pathol. 2010;176(1):177–186. doi: 10.2353/ajpath.2010.090672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.La Bonte LR, Pavlov VI, Tan YS, et al. Mannose-binding lectin-associated serine protease-1 is a significant contributor to coagulation in a murine model of occlusive thrombosis. J Immunol. 2012;188(2):885–891. doi: 10.4049/jimmunol.1102916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi K, Chang WC, Takahashi M, et al. Mannose-binding lectin and its associated proteases (MASPs) mediate coagulation and its deficiency is a risk factor in developing complications from infection, including disseminated intravascular coagulation. Immunobiology. 2011;216(1-2):96–102. doi: 10.1016/j.imbio.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeerleder S. C1-inhibitor: more than a serine protease inhibitor. Semin Thromb Hemost. 2011;37(4):362–374. doi: 10.1055/s-0031-1276585. [DOI] [PubMed] [Google Scholar]
- 31.Krisinger MJ, Goebeler V, Lu Z, et al. Thrombin generates previously unidentified C5 products that support the terminal complement activation pathway. Blood. 2012;120(8):1717–1725. doi: 10.1182/blood-2012-02-412080. [DOI] [PubMed] [Google Scholar]
- 32.Girardi G, Mackman N. Tissue factor in antiphospholipid antibody-induced pregnancy loss: a pro-inflammatory molecule. Lupus. 2008;17(10):931–936. doi: 10.1177/0961203308094994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krarup A, Wallis R, Presanis JS, Gál P, Sim RB. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS ONE. 2007;2(7):e623. doi: 10.1371/journal.pone.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin W, Ghebrehiwet B, Peerschke EI. Expression of complement components and inhibitors on platelet microparticles. Platelets. 2008;19(3):225–233. doi: 10.1080/09537100701777311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q, Li Y, Tang CM. The role of the exopolyphosphatase PPX in avoidance by Neisseria meningitidis of complement-mediated killing. J Biol Chem. 2010;285(44):34259–34268. doi: 10.1074/jbc.M110.154393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimura A, Ikeo K, Nonaka M. Evolutionary origin of the vertebrate blood complement and coagulation systems inferred from liver EST analysis of lamprey. Dev Comp Immunol. 2009;33(1):77–87. doi: 10.1016/j.dci.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Rawal N, Pangburn MK. C5 convertase of the alternative pathway of complement. Kinetic analysis of the free and surface-bound forms of the enzyme. J Biol Chem. 1998;273(27):16828–16835. doi: 10.1074/jbc.273.27.16828. [DOI] [PubMed] [Google Scholar]
- 38.Niesen FH, Berglund H, Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc. 2007;2(9):2212–2221. doi: 10.1038/nprot.2007.321. [DOI] [PubMed] [Google Scholar]
- 39.Lorenz B, Schröder HC. Mammalian intestinal alkaline phosphatase acts as highly active exopolyphosphatase. Biochim Biophys Acta. 2001;1547(2):254–261. doi: 10.1016/s0167-4838(01)00193-5. [DOI] [PubMed] [Google Scholar]
- 40.Lachmann PJ, Thompson RA. Reactive lysis: the complement-mediated lysis of unsensitized cells. II. The characterization of activated reactor as C56 and the participation of C8 and C9. J Exp Med. 1970;131(4):643–657. doi: 10.1084/jem.131.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tinsley CR, Gotschlich EC. Cloning and characterization of the meningococcal polyphosphate kinase gene: production of polyphosphate synthesis mutants. Infect Immun. 1995;63(5):1624–1630. doi: 10.1128/iai.63.5.1624-1630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Cera E. Thrombin as procoagulant and anticoagulant. J Thromb Haemost. 2007;5(Suppl 1):196–202. doi: 10.1111/j.1538-7836.2007.02485.x. [DOI] [PubMed] [Google Scholar]
- 43.Lechtenberg BC, Freund SM, Huntington JA. An ensemble view of thrombin allostery. Biol Chem. 2012;393(9):889–898. doi: 10.1515/hsz-2012-0178. [DOI] [PubMed] [Google Scholar]
- 44.Drake WT, Lopes NN, Fenton JW, II, Issekutz AC. Thrombin enhancement of interleukin-1 and tumor necrosis factor-alpha induced polymorphonuclear leukocyte migration. Lab Invest. 1992;67(5):617–627. [PubMed] [Google Scholar]
- 45.Fujita T, Yamabe H, Shimada M, et al. Thrombin enhances the production of monocyte chemoattractant protein-1 and macrophage inflammatory protein-2 in cultured rat glomerular epithelial cells. Nephrol Dial Transplant. 2008;23(11):3412–3417. doi: 10.1093/ndt/gfn352. [DOI] [PubMed] [Google Scholar]
- 46.Wadgaonkar R, Somnay K, Garcia JG. Thrombin induced secretion of macrophage migration inhibitory factor (MIF) and its effect on nuclear signaling in endothelium. J Cell Biochem. 2008;105(5):1279–1288. doi: 10.1002/jcb.21928. [DOI] [PubMed] [Google Scholar]
- 47.Huber-Lang M, Sarma JV, Zetoune FS, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12(6):682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 48.Bae JS, Kim YU, Park MK, Rezaie AR. Concentration dependent dual effect of thrombin in endothelial cells via Par-1 and Pi3 Kinase. J Cell Physiol. 2009;219(3):744–751. doi: 10.1002/jcp.21718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morser J. Thrombomodulin links coagulation to inflammation and immunity. Curr Drug Targets. 2012;13(3):421–431. doi: 10.2174/138945012799424606. [DOI] [PubMed] [Google Scholar]
- 50.Lorenz B, Leuck J, Köhl D, Muller WE, Schröder HC. Anti-HIV-1 activity of inorganic polyphosphates. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14(2):110–118. doi: 10.1097/00042560-199702010-00003. [DOI] [PubMed] [Google Scholar]
- 51.Rondina MT, Weyrich AS, Zimmerman GA. Platelets as cellular effectors of inflammation in vascular diseases. Circ Res. 2013;112(11):1506–1519. doi: 10.1161/CIRCRESAHA.113.300512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmaier AH, Amenta S, Xiong T, Heda GD, Gewirtz AM. Expression of platelet C1 inhibitor. Blood. 1993;82(2):465–474. [PubMed] [Google Scholar]
- 53.Wiedmer T, Esmon CT, Sims PJ. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood. 1986;68(4):875–880. [PubMed] [Google Scholar]
- 54.Licht C, Pluthero FG, Li L, et al. Platelet-associated complement factor H in healthy persons and patients with atypical HUS. Blood. 2009;114(20):4538–4545. doi: 10.1182/blood-2009-03-205096. [DOI] [PubMed] [Google Scholar]
- 55.Gunay-Aygun M, Huizing M, Gahl WA. Molecular defects that affect platelet dense granules. Semin Thromb Hemost. 2004;30(5):537–547. doi: 10.1055/s-2004-835674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nurden A, Nurden P. Advances in our understanding of the molecular basis of disorders of platelet function. J Thromb Haemost. 2011;9(Suppl 1):76–91. doi: 10.1111/j.1538-7836.2011.04274.x. [DOI] [PubMed] [Google Scholar]
- 57.Hurford MT, Sebastiano C. Hermansky-pudlak syndrome: report of a case and review of the literature. Int J Clin Exp Pathol. 2008;1(6):550–554. [PMC free article] [PubMed] [Google Scholar]
- 58.Kaplan J, De Domenico I, Ward DM. Chediak-Higashi syndrome. Curr Opin Hematol. 2008;15(1):22–29. doi: 10.1097/MOH.0b013e3282f2bcce. [DOI] [PubMed] [Google Scholar]
- 59.Docampo R, Moreno SN. Acidocalcisomes. Cell Calcium. 2011;50(2):113–119. doi: 10.1016/j.ceca.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ninomiya H, Sims PJ. The human complement regulatory protein CD59 binds to the alpha-chain of C8 and to the “b” domain of C9. J Biol Chem. 1992;267(19):13675–13680. [PubMed] [Google Scholar]
- 61.Risitano AM. Paroxysmal nocturnal hemoglobinuria and the complement system: recent insights and novel anticomplement strategies. Adv Exp Med Biol. 2013;735:155–172. doi: 10.1007/978-1-4614-4118-2_10. [DOI] [PubMed] [Google Scholar]
- 62.Tschopp J, Chonn A, Hertig S, French LE. Clusterin, the human apolipoprotein and complement inhibitor, binds to complement C7, C8 beta, and the b domain of C9. J Immunol. 1993;151(4):2159–2165. [PubMed] [Google Scholar]
- 63.Falgarone G, Chiocchia G. Chapter 8: Clusterin: A multifacet protein at the crossroad of inflammation and autoimmunity. Adv Cancer Res. 2009;104:139–170. doi: 10.1016/S0065-230X(09)04008-1. [DOI] [PubMed] [Google Scholar]
- 64.Podack ER, Kolb WP, Müller-Eberhard HJ. The SC5b-7 complex: formation, isolation, properties, and subunit composition. J Immunol. 1977;119(6):2024–2029. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.