Abstract
Background
After the first year following kidney transplantation, 3–5% of grafts fail each year but detailed studies of how grafts progress to failure are lacking. This study aimed to analyze the functional stability of kidney transplants between 1 and 5-years post-transplant and to identify initially well-functioning grafts with progressive decline in allograft function.
Methods
The study included 788 adult conventional kidney transplants performed at Mayo Clinic Rochester between 1/2000 and 12/2005 with a minimum graft survival and follow-up of 2.6 years. The MDRD equation for estimating glomerular filtration rate (eGFRMDRD) was used to calculate the slope of renal function over time using all available serum creatinine values between 1 and 5 years post-transplant.
Results
The majority of transplants had good function (eGFRMDRD ≥ 40 ml/min) at 1 year with positive eGFRMDRD slope between 1 and 5 years post-transplant. However, a subset of grafts with 1 year eGFRMDRD ≥ 40 ml/min exhibited strongly negative eGFRMDRD slope between 1 and 5-years suggestive of progressive loss of graft function. Forty-one percent of this subset reached graft failure during follow-up, accounting for 69% of allograft failures occurring after 2.5 years post-transplant. This pattern of progressive decline in eGFR despite good early function was associated with, but not fully attributable to, factors suggestive of enhanced anti-donor immunity.
Conclusions
Longitudinal analysis of serial eGFR measurements identifies initially well-functioning kidney transplants at high risk for subsequent graft loss. For this subset, further studies are needed to identify modifiable causes of functional decline.
Keywords: kidney transplantation, glomerular filtration rate, graft survival, chronic allograft nephropathy, proteinuria
INTRODUCTION
Kidney transplantation is a successful treatment for end-stage renal disease (ESRD) but remains associated with a graft failure rate of 3–5% per year following the first post-transplant year [1]. Early identification of grafts at highest risk for failure is a clear prerequisite for developing strategies to improve long-term outcomes [2–8].
Donor specific antibody, subclinical intragraft inflammation, recurrent disease and polyomavirus infection have been associated with shortened graft survival [2, 7]. Regardless of the underlying cause, the path to graft failure is typically preceded by a period of functional decline. Several studies have reported associations between graft function and subsequent loss but the majority of these have focused on correlating rates of graft failure with a single functional measurement within the first post-transplant year [3, 5, 9–11]. Although these studies show that low allograft function in the early post-transplant period (≤1-year) is associated with increased risk of subsequent graft failure, it is unlikely that the ongoing annual loss of 3–5% of transplants during long-term follow-up remains closely linked with low initial graft function. For example, Magott-Proceleweska et al recently showed that while eGFRMDRD <40 ml/min at 6-months is associated with increased risk of graft loss, 33% of those grafts had eGFR improvement by 2-years with 94% 5-year graft survival [9].
We hypothesized that low renal function at 1-year post-transplant would identify recipients at high risk for early graft failure but that risk prediction for graft failures occurring during longer term follow-up would require a more longitudinal analysis of function. We pursued a two-stage approach to analyzing the association between eGFR and graft failure in a large cohort of kidney transplant recipients followed for ≥5 years. A single eGFRMDRD value at 1-year post-transplant was used to determine a level of early graft function below which subsequent survival was significantly reduced. For recipients with eGFRMDRD above this cut-off value, we used all available eGFRMDRD measurements between 1 and 5-years post-transplant to determine the graft functional stability (slope of eGFR). The results indicate that: (a) Low 1-year eGFR is primarily predictive of graft failure occurring within a short time-frame post-transplantation; (b) A substantial subset of allografts with high 1-year eGFR undergo progressive decline in eGFR after the first post-transplant year and this accounts for the majority of graft failures occurring during extended follow-up. (c) Analysis of eGFR trends by MDRD (and other formula-based approaches) using large numbers of serum creatinine measurements per patient, provides important prognostic information despite known discrepancies between estimated and true GFR measurements in kidney transplant recipients [7, 12].
RESULTS
Correlating kidney transplant survival with 1-year eGFRMDRD
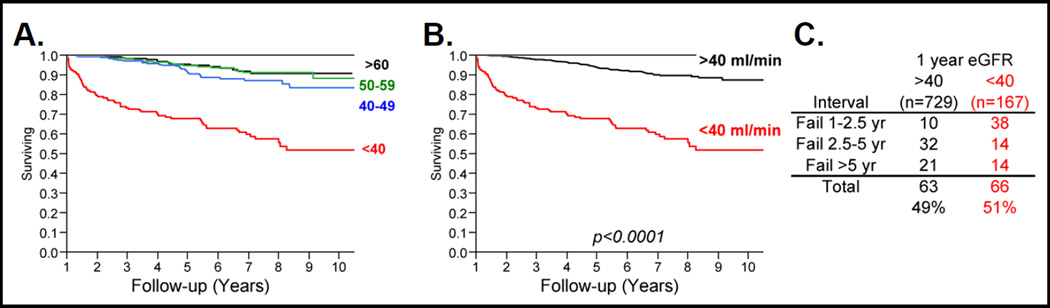
All adult conventional renal transplants between 2000 and 2005 that remained functional for ≥1 year were identified. From a total of 925 transplants, 896 (89%) had eGFRMDRD recorded 1-year post-transplant (Figure 1). Subsequent graft survival rates were determined for different ranges of 1-year eGFRMDRD (<20, 20–29, 30–39, 40–49, 50–59 ml/min) and were compared to the survival rate for transplants with eGFRMDRD ≥60 ml/min (data not shown). This analysis indicated that all 1-year eGFRMDRD ranges below 40 ml/min had significantly lower subsequent graft survival while those with eGFRMDRD between 40 and 59 ml/min had similar graft survival rates to the ≥60 ml/min group (Figure 2A). For subsequent analyses, therefore, transplants with 1-year eGFRMDRD ≥40 ml/min and <40 ml/min were designated as “High GFR" and “Low GFR" respectively (Figure 2B).
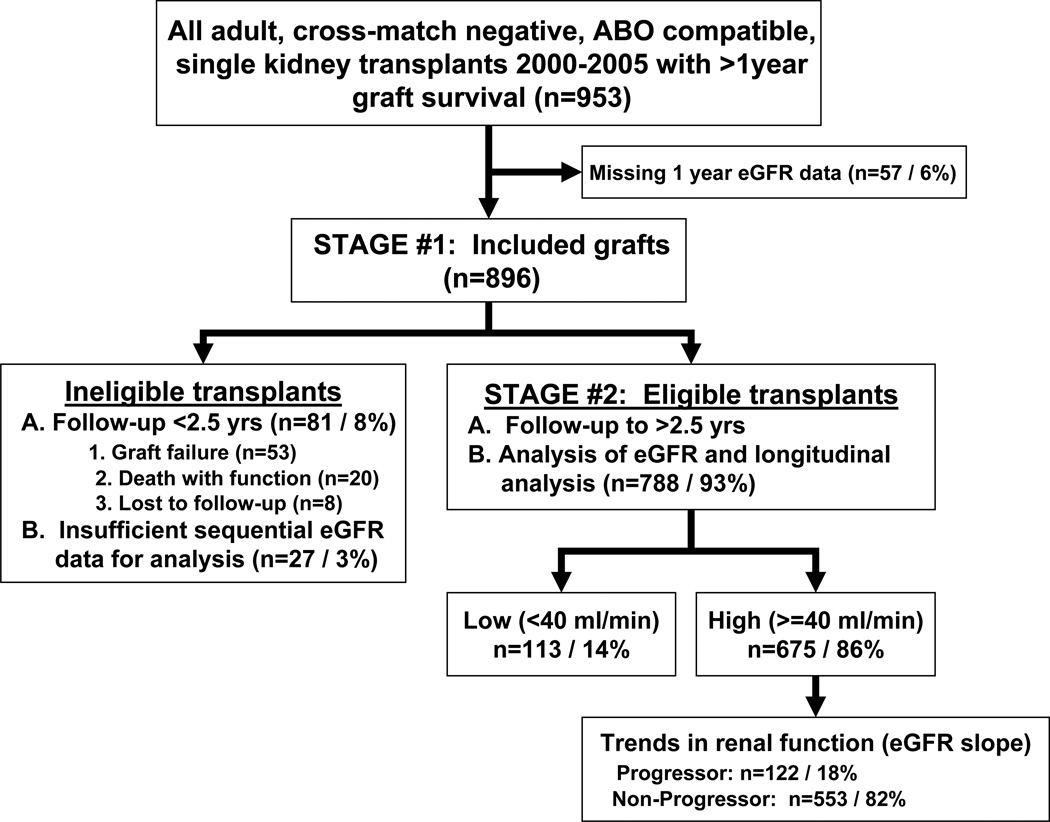
Figure 1.
Flow diagram of the overall study design.
Figure 2.
A. Graft survival trends from 1 year post-transplant for groups of kidney transplants with 1-year eGFRMDRD ≥ 60, 50–59, 40–49 and < 40 ml/min. B. Graft survival trends from 1 year post-transplant for kidney transplants with 1-year eGFRMDRD < 40 and ≥ 40 ml/min. C. Numbers of graft failures among kidney transplant groups with 1-year eGFRMDRD < 40 and ≥ 40 ml/min that occurred from 1 to 2.5 years, 2.5 to 5 years and >5 years post-transplant.
In total, 129/896 transplants (14.4%) failed during follow-up of 62.3 ± 26.9 months following 1-year eGFRMDRD measurement. Between 1 and 2.5-years post-transplant the majority of graft failures (38/48; 79%) occurred within the Low GFR group. In contrast, graft failures later than 2.5-years post-transplant occurred predominantly within the High GFR group (53/81; 65%). Thus, 49% of all graft failures during this follow-up period would have been incorrectly categorized as having a good prognosis based on eGFRMDRD at 1-year post-transplant (Figure 2C).
Combining the 1-year eGFRMDRD and slope of renal function between 1–5 years to identify grafts at high risk for graft loss
In the next stage of the study, longitudinal trends in renal function during the first 2.5-years were analyzed with a view to identification of well-functioning transplants at increased risk for later graft failure. For this analysis, allografts which failed or were lost to follow-up prior to 2.5-years post-transplant (81/896, 8%) or which had insufficient eGFR measurements (27/896, 3%) were omitted, leaving 788 transplants eligible for analysis – 113 categorized as Low GFR and 675 as High GFR (Figure 1). Characteristics of the total group are summarized in Table 1A and those of the Low GFR and High GFR subsets in Table 1B. Of note, while 1-year eGFRMDRD was lower among the 70 allografts from this cohort that failed during follow-up compared to all other outcomes, there was considerable overlap of individual 1-year eGFRMDRD values for all outcomes (Supplemental Digital Content, Figure S1A).
Table 1.
Characteristics of kidney transplants eligible for longitudinal analysis of renal function by slope of eGFRMDRD
| A. | B. | C. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| High eGFR - quintiles | ||||||||||
| Eligible | Low | High | P-Value | 0–20% | 21–40% | 41–60% | 61–80% | 81–100% | ||
| # Patients | 788 | 113 | 675 | 135 | 135 | 135 | 135 | 135 | ||
| 1 year eGFRMDRD | 53.2 ± 13 | 33.7 ± 4.3 | 56.5 ± 10.9 | <0.0001 | 57.1 ± 12.1 | 57.2 ± 11.2 | 57.2 ± 11.4 | 55.4 ± 10.5 | 55.6 ± 9.4 | |
| # Serum Creatinine / Patient | 39.9 ± 30.6 | 52 ± 37.5 | 37.8 ± 28.8 | 0.0002 | 47.7 ± 31.1 | 39.8 ± 27 | 35.3 ± 26.5 | 33.6 ± 24.9 | 32.5 ± 31.7 | |
| Slope of line (%) | −1.4 ± 9% | 0.6 ± 8.5% | −1.7 ± 9% | 0.0083 | −15.2 ± 10% | −3.4 ± 1.3% | 0 ± 0.7% | 2.5 ± 0.8% | 7.4 ± 4% | |
| Median slope (%) | 0.20% | 0.01% | 1.38% | −12.1% | −3.39% | 0.01% | 2.43% | 6.09% | ||
| Slope of line (ml/min/yr) | −0.9 ± 5 | 0.1 ± 2.8 | −1 ± 5.3 | −8.7 ± 6.2 | −2 ± 0.9 | 0 ± 0.4 | 1.4 ± 0.6 | 4.1 ± 2.3 | ||
| Median slope (ml/min/yr) | 0.11 | 0.51 | 0.00 | −6.57 | −1.84 | 0.00 | 1.33 | 3.49 | ||
| Graft loss (death censored) | 9% (n70) | 19% (n22) | 7% (n48) | <0.0001 | 31% (n42) | 2% (n3) | 1% (n2) | 1% (n1) | 0% (n0) | |
| Graft failure <5 years | 53 | 16 | 37 | 36 | 0 | 1 | 0 | 0 | ||
| Graft failure >5 years | 17 | 6 | 11 | 6 | 3 | 1 | 1 | 0 | ||
| Mean follow-up (days) | 2436 ± 663 | 2401 ± 737 | 2442 ± 650 | 0.5801 | 2110 ± 634 | 2527 ± 640 | 2574 ± 627 | 2537 ± 617 | 2441 ± 652 | |
| Range of follow-up (days) | [968 – 3897] | [1006 – 3882] | [968 – 3897] | [968 – 3897] | [1448 – 3868] | [1284 – 3879] | [1075 – 3876] | [1087 – 3786] | ||
| Demographics | ||||||||||
| Recipient age at transplant | 51.9 ± 13.8 | 54.8 ± 12.5 | 51.4 ± 14 | 0.0142 | 49.3 ± 14.1 | 47.7 ± 13.7 | 52.9 ± 14.2 | 52.7 ± 13.3 | 54.2 ± 13.7 | |
| Recipient race (%caucasian) | 94% (n738) | 97% (n110) | 94% (n628) | 0.1508 | 97% (n130) | 95% (n127) | 92% (n124) | 96% (n128) | 90% (n119) | |
| Recipient gender (% female) | 41% (n327) | 55% (n62) | 39% (n265) | 0.0018 | 47% (n64) | 48% (n65) | 33% (n44) | 30% (n41) | 38% (n51) | |
| Donor type (% deceased) | 21% (n164) | 19% (n22) | 21% (n142) | 0.7039 | 21% (n29) | 15% (n20) | 24% (n32) | 25% (n34) | 20% (n27) | |
| Donor age at transplant | 41.4 ± 12.8 | 47.7 ± 12.1 | 40.3 ± 12.7 | <0.0001 | 41.4 ± 13.3 | 41.6 ± 12.5 | 40.2 ± 13.2 | 39.6 ± 12.4 | 38.6 ± 11.8 | |
| Induction (% Thymoglobulin) | 82% (n647) | 80% (n90) | 83% (n557) | 0.4609 | 76% (n102) | 79% (n107) | 80% (n108) | 86% (n116) | 92% (n124) | |
| Primary Immunosup (%CI) | 77% (n606) | 73% (n83) | 77% (n523) | 0.3247 | 77% (n104) | 79% (n106) | 77% (n104) | 79% (n106) | 76% (n103) | |
| Pre-transplant DSA+ | 14% (n64/474) | 17% (n12/72) | 13% (n52/402) | 0.3935 | 18% (n13/72) | 8% (n6/71) | 12% (n11/89) | 14% (n12/83) | 11% (n10/87) | |
| Class I+ | 8% (n36) | 11% (n8) | 7% (n28) | 0.2214 | 7% (n5) | 6% (n4) | 6% (n5) | 7% (n6) | 9% (n8) | |
| Class II+ | 9% (n41) | 7% (n5) | 9% (n36) | 0.5762 | 14% (n10) | 3% (n2) | 11% (n10) | 11% (n9) | 6% (n5) | |
| 1 year 24 Hour Urine Protein | 40% (n238) | 58% (n46) | 38% (n192) | 0.0005 | 39% (n38) | 37% (n39) | 35% (n34) | 38% (n41) | 38% (n40) | |
| 1 year histology | ||||||||||
| Normal (cg, i, ci = 0) | 47% (n289) | 23% (n19) | 51% (n270) | <0.0001 | 47% (n47) | 49% (n51) | 49% (n52) | 55% (n64) | 57% (n56) | |
| IF alone (ci>0, i & cg=0) | 38% (n231) | 51% (n43) | 36% (n188) | 0.0062 | 33% (n33) | 39% (n41) | 42% (n44) | 34% (n40) | 30% (n30) | |
| IF+I (ci & i>0, cg=0) | 11% (n68) | 23% (n19) | 9% (n49) | 0.0003 | 13% (n13) | 11% (n11) | 7% (n7) | 8% (n9) | 9% (n9) | |
| Txp Glomerulopathy (cg>0) | 4% (n22) | 4% (n3) | 4% (n19) | 0.9919 | 7% (n7) | 1% (n1) | 3% (n3) | 3% (n4) | 4% (n4) | |
Abbreviations: CI = Calcineurin Inhibitor; DSA = Donor Specific Antibody; IF = Interstitial Fibrosis; IF+I = Interstitial Fibrosis with Inflammation.
Plotting trends in kidney transplant function from 1 to 5 years after transplantation
Trends in renal function for the High GFR group were next analyzed between 1 and 5 years post-transplant by plotting mean eGFRMDRD for sequential 6-month intervals. For the entire group, a broad distribution of renal function values was observed across all time intervals with the mean eGFRMDRD remaining constant throughout (Supplementary Figure S1B). The slope of eGFRMDRD was then calculated for each individual High GFR transplant (see Methods). The mean eGFRMDRD slope for all 675 High GFR transplants was −1.7 ± 9.0%, corresponding to a change in eGFRMDRD of −1.0 ± 5.3 ml/min/yr (range: +18 to −41). However, when the group was subdivided into quintiles based on the distribution of eGFRMDRD slopes (Table 1C), only two of five quintiles had declining eGFRMDRD (slopes of −15 ± 10% and −3.4 ± 1.3%) while three demonstrated either increasing or stable eGFRMDRD over time (slopes of 0.0 ± 0.7%, +2.5 ± 0.8% and +7.4 ± 4.1%). The quintile with the largest decrease in eGFRMDRD had a mean change of −8.7 ± 6.2 ml/min/yr. Strikingly, 36 of the 37 allograft failures that occurred < 5-years post-transplant in the High GFR group and 42 of the 48 failures during the entire follow-up period were contained within in this quintile. In contrast, the quintile with the second greatest declining slope experienced only 3 graft losses, each occurring >5-years post-transplant. Among the remaining quintiles, only 1 graft loss occurred which was also >5-years post-transplant.
There were no notable differences in follow-up time, donor source or age across quintiles (Table 1C). In addition, while the frequency of abnormal proteinuria (>150 mg/24 hours) at 1 year post-transplant was higher among Low GFR compared to High GFR groups (58% vs 38%), there was no difference in the frequency of abnormal proteinuria at 1 year among the quintiles (Table 1B and 1C). The availability of 1-year surveillance biopsies for the majority of transplants within the total High GFR cohort also allowed for comparison of histological abnormalities among quintiles. The quintile with the greatest decline in eGFRMDRD did have higher proportions of biopsies with some grade of transplant glomerulopathy or interstitial fibrosis with inflammation. However, no quintile had <80% of 1-year biopsies with normal histology or interstitial fibrosis alone. Therefore, it was concluded that the progressive decline in renal function among allografts with apparently good function at 1-year could not be largely accounted for by obvious baseline characteristics, increased rate of development of abnormal proteinuria or histological abnormalities during the first post-transplant year.
Further defining and examining the clinical characteristics of High GFR transplants with progressive loss of function
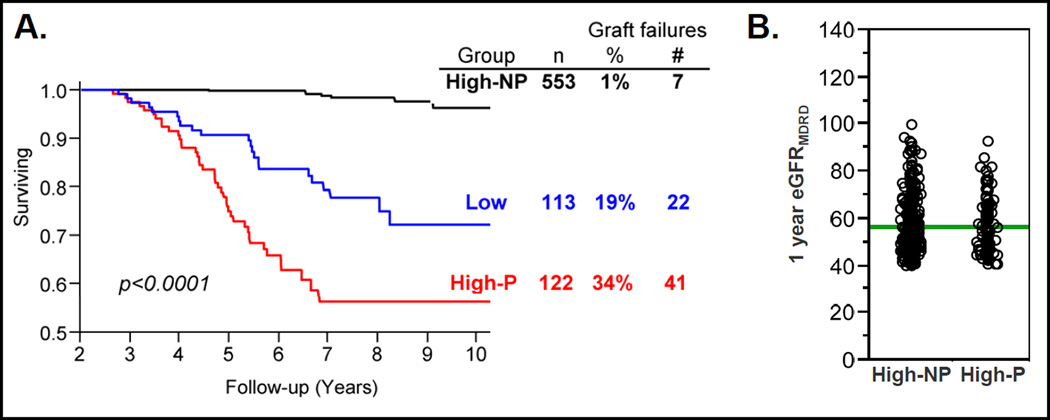
To further characterize High GFR transplants that subsequently “progressed” to poor function, 13 allografts were excluded from the quintile with the greatest eGFR decline. These had eGFRMDRD ≥ 60 ml/min throughout follow-up (n=3) and/or had <20% absolute reduction in eGFRMDRD over time (n=10). The remaining 122 grafts were termed “High GFR Progressors” (High-P) and were compared to all other High eGFR grafts (High eGFR Non-Progressors, High-NP, n=553) (Table 2A). Predictably, the High-P group had more graft failures (n=41, 34%) than the High-NP group (1%, p<0.0001). In addition, the rate of graft failure among the High-P group was higher than that of the Low GFR group during this time-frame (19%, p<0.0021) (Figure 3A). Similar to the initial quintiles analysis, the mean 1-year eGFRMDRD of the High-P and High-NP groups did not differ (Figure 3B).
Table 2.
High GFR Non-Progressor (High-NP) and Progressors (High-P) transplants comparison by univariate (A) and multivariate (B) analyses
| A. | ||||
|---|---|---|---|---|
| High-NP | High-P | P-value | ||
| # Patients | 553 | 122 | ||
| # Serum Creatinine / patient | 35.2 ± 27.4 | 49.7 ± 32.2 | <0.0001 | |
| Slope of line | 1.4 ± 4.7% | −16 ± 10.2% | <0.0001 | |
| Graft loss (death-censored) | 1% (n7) | 34% (n41) | <0.0001 | |
| Graft failure <5 yrs | 1 | 36 | ||
| Graft failure >5 yrs | 6 | 5 | ||
| Mean follow-up (days) | 2525 ± 627 | 2069 ± 626 | <0.001 | |
| Range of follow-up (days) | [1075 – 3879] | [968 – 3897] | ||
| Demographics | ||||
| Recipient age at transplant | 51.9 ± 13.9 | 48.9 ± 14.2 | 0.0381 | |
| Donor age at transplant | 40.1 ± 12.6 | 41 ± 12.8 | 0.3433 | |
| Total # transplants | 1.1 ± 0.4 | 1.3 ± 0.6 | <0.0001 | |
| Recipient race (%caucasian) | 93% (n510) | 98% (n118) | 0.0477 | |
| Recipient gender (% female) | 37% (n207) | 48% (n58) | 0.0241 | |
| Donor type (% deceased) | 21% (n118) | 20% (n24) | 0.6809 | |
| % HLA A-B-DR identical | 8% (n45) | 7% (n9) | 0.7773 | |
| Pre-1 year data | ||||
| Induction (% Thymoglobulin) | 84% (n466) | 75% (n91) | 0.0274 | |
| Primary Immunosup (%CI) | 78% (n429) | 77% (n94) | 0.6743 | |
| Pre-transplant DSA+ | 12% (n39/339) | 21% (n13/63) | 0.0606 | |
| Class I+ | 7% (n23) | 8% (n5) | 0.7456 | |
| Class II+ | 8% (n26) | 16% (n10) | 0.0515 | |
| ≤1 year BK+ | 4% (n23) | 2% (n2) | 0.1435 | |
| ≤1 year Recurrent disease | 4% (n20) | 7% (n9) | 0.0837 | |
| ≤1 year Post-txp DSA+ | 7% (n6/87) | 15% (n3/20) | 0.2368 | |
| ≤1 year Non-protocol biopsy | 23% (n125) | 24% (n29) | 0.9685 | |
| 1 year data | ||||
| eGFRMDRD | 56.5 ± 10.9 | 56.6 ± 11.3 | 0.9530 | |
| 24-hr urine protein (% >150 mg) | 37% (n155) | 42% (n37) | 0.3438 | |
| Tacrolimus level | 8.6 ± 3.3 (86%) | 7.9 ± 2.6 (86%) | 0.0654 | |
| Histology | ||||
| Normal (cg, i, ci = 0) | 52% (n226) | 49% (n44) | 0.6577 | |
| IF alone (ci>0, i & cg=0) | 37% (n162) | 29% (n26) | 0.2253 | |
| IF+I (ci & i>0, cg=0) | 8% (n37) | 13% (n12) | 0.1619 | |
| Txp Glomerulopathy (cg>0) | 3% (n11) | 9% (n8) | 0.0346 | |
| Last Lab value (<1 yr from last eGFR) | ||||
| Tacrolimus level | 6.8 ± 2.7 (86%) | 7 ± 3.1 (88%) | 0.4512 | |
| 24-hr urine protein (% >150 mg) | 30% (n112) | 66% (n49) | <0.0001 | |
| DSA CI+ | 7% (n17) | 9% (n4) | 0.5980 | |
| DSA CII+ | 16% (n39) | 27% (n12) | 0.0728 | |
| B. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Proportion of High-P grafts |
Univariate analysis | Multivariate analysis | ||||||
| Variable |
WITH variable |
WITHOUT variable |
Odds ratio |
CI 95% | P-value | Odds ratio |
CI 95% | P-value |
| Txp Glomerulopathy (cg>0) | 42% (n8/19) | 16% (n83/516) | 3.00 | 1.09 – 7.69 | 0.0346 | 2.05 | 0.4 – 9.41 | 0.3751 |
| Recipient age at transplant | - | - | 0.99 | 0.97 – 1 | 0.0381 | 0.99 | 0.97 – 1.02 | 0.6094 |
| Total # transplants | - | - | 2.27 | 1.51 – 3.41 | <0.0001 | 4.39 | 2.18 – 9.03 | <0.0001 |
| Recipient race (%caucausian) | 19% (n118/628) | 8% (n3/40) | 2.85 | 1.01 – 11.96 | 0.0477 | 1.88 | 0.42 – 13.6 | 0.4346 |
| Recipient gender (% female) | 22% (n58/265) | 16% (n64/410) | 1.58 | 1.06 – 2.23 | 0.0241 | 2.91 | 1.53 – 5.64 | 0.001 |
| Induction (% Thymoglobulin) | 16% (n91/557) | 26% (n31/118) | 0.58 | 0.37 – 0.94 | 0.0274 | 0.36 | 0.17 – 0.75 | 0.0068 |
| Pre-transplant DSA+ | 25% (n13/52) | 14% (n50/350) | 2.00 | 0.97 – 3.93 | 0.0606 | - | ||
| Pre-transplant DSA+Class II+ | 28% (n10/36) | 14% (n53/366) | 2.27 | 0.99 – 4.86 | 0.0515 | - | ||
| Last Lab value (<1 yr from last eGFR) | ||||||||
| 24-hr urine protein (% >150 mg) | 30% (n49/161) | 9% (n25/285) | 4.55 | 2.7 – 7.83 | <0.0001 | 4.24 | 2.27 – 8.08 | <0.0001 |
| DSA+Class II+ | 24% (n12/51) | 13% (n32/243) | 2.03 | 0.93 – 4.2 | 0.0728 | - | ||
Abbreviations: DSA = Donor Specific Antibody; IF = Interstitial Fibrosis; IF+I = Interstitial Fibrosis with Inflammation; DSA CI = DSA against Class I MHC, DSA CII = DSA against Class II MHC.
Figure 3.
A. Graft survival curves from 2.5 years post-transplant of groups of kidney transplant categorized as High-NP, High-P and Low GFR. Total group numbers and %/number of graft failures during follow-up per group as tabulated to the right. B. 1-year eGFRMDRD values for kidney transplant categorized as High-NP and High-P. Values for individual transplants are shown as open circles and group mean values as horizontal green lines.
To determine whether the High-P and High-NP subgroups differed for relevant clinical, histological and laboratory characteristics either at baseline or during subsequent follow-up, univariate and multivariate analyses were conducted. As shown in Table 2A, univariate analysis indicated associations between High-P status and younger recipient age, higher number of transplants, Caucasian recipient race, female recipient gender, non-use of Thymoglobulin induction, transplant glomerulopathy on 1-year surveillance biopsy and abnormal proteinuria within 1 year of the most recent eGFR measurement. There were also trends toward associations of High-P status with pre-transplant anti-Class II donor specific antibody (DSA) and with anti-Class II DSA within 1 year of the most recent eGFR measurement which did not reach significance (although post-transplant DSA data was available for only a limited number of study subjects). In multivariate analysis (Table 2B), the associations with higher transplant number, female recipient gender, non-use of Thymoglobulin induction and abnormal proteinuria within 1 year of the most recent eGFR measurement remained significantly associated with High-P status.
Alternative approaches to assessing renal function trends
Comparisons were performed between the eGFRMDRD 6m interval approach and alternative methods for estimating or measuring GFR (Supplemental Digital Content, Table S1A and S1B). The slope cut-off for Progressor status was independently determined for each method. The proportion of failed grafts considered to be progressors was similar for each method (83–88%; Supplemental Digital Content, Table S1A). However, the formula-based eGFR methods identified higher proportions of progressor grafts that failed during follow-up compared to iothalamate clearance (34–40% vs 17%). Overall the similarity between eGFRMDRD 6m interval and other methods was 88–96% (Supplemental Digital Content, Table S1B).
DISCUSSION
Our results agree with existing literature indicating that renal allografts with low eGFRMDRD ≤1-year post-transplant have inferior subsequent graft survival (3, 9, 11), in the first few years after transplantation. However, in the current study, the majority of allograft failures between 2.5 and 7 years post-transplant had a 1-year eGFRMDRD ≥40 ml/min (65%; 53/81). Thus, a low 1-year eGFR appears to contribute most of its predictive value during the first few post-transplant years. Therefore categorizing transplant recipients into low and high risk groups on the basis of a single early GFR estimate, would fail to identify a substantial number of recipients who are destined for future graft failure beyond 2.5 years. We believe that this concept is an important and underappreciated finding and questions the validity of using a single measure of early renal function as a primary end-point for clinical trials in kidney transplantation [13–15]. We contend that the current study’s use of sequential eGFR values between 1–5 years to create an eGFR slope provides a fuller picture of the fate of renal allografts after transplantation and might provide a useful endpoint for clinical trials designed to improve long-term graft survival.
This study also extends our prior research in chronic injury which showed that not all renal allografts are affected by chronic injury in the first 5 years after transplantation [8]. Indeed, the majority of renal allografts with 1-year eGFRMDRD ≥40 ml/min have stable or improving function between 1 and 5 years. Even after excluding all patients with low 1-year eGFR and grafts with limited survival or follow-up during the first 2.5 years post-transplant, 43% of the entire starting population (405/953) would have had renal functional profiles comparable to healthy people up to 7 years post-transplant. These results are similar to findings reported from the United States Renal Data System (USRDS) and single centers using data from exclusively deceased donor recipients [16, 17]. Most notably, in a study by Gill et al, in which the rate of functional decline was assessed for grafts surviving at least 2-years, the overall decline in renal function was slow (−1.66 ml/min/1.73m2/yr) and 50% of recipients had no change or an improvement in eGFRMDRD [16].
We believe that these findings have important therapeutic implications. First, the fact that the majority of well-functioning grafts at 1 year have stable or improved function suggests that sweeping changes in immunosuppression are not needed to prevent chronic injury in the majority of allografts in the first 5-years after transplantation. In contrast, if we are to improve overall long-term graft survival, we cannot focus solely on improving 1-year GFR, but also must identify the causes of renal functional decline in grafts that have good function at 1 year.
For the 122 allografts identified as having progressive renal dysfunction, we investigated the possible causes using both univariate and multivariate analyses. Only a small proportion of Progressors exhibited specific characteristics previously associated with increased risk of graft failure such as overt complications (BK nephropathy, recurrent disease, calcineurin inhibitor), abnormal histology (glomerulopathy, fibrosis+inflammation) and deceased donor source [2, 4, 7]. The relatively low frequency of these well-defined risks among the High-P group suggests that different factors may contribute to the progressive loss of function in these grafts compared to Low GFR grafts. The significant associations with Progressor status observed included female gender, re-transplantation and lack of Thymoglobulin induction, perhaps implicating a role for anti-donor sensitization. Interestingly, despite a trend toward higher frequency of pre-transplant anti-Class II donor-specific antibody (DSA) among the High-P compared to High-NP group (16% vs 8%), this did not reach significance indicating that pre-transplant sensitization was not highly enriched among the Progressors. Although the amount of data available to interrogate the role of time-dependent post-transplant variables in this cohort was relatively limited, it is of interest that the most recent 24 hour urine protein measurements indicated that the frequency of abnormal proteinuria became higher over time in the High GFR Progressors having been no different to Non-Progressors at 1 year post-transplant. Clearly, more comprehensive, prospective analysis will be necessary to determine whether emergence of de novo proteinuria, DSA or other abnormalities occurs before, after or concurrent with declining functional measurements.
A limited number of prior studies have used multiple measures of renal function collected within the first 2-years post-transplant and have determined that the change in function between 2 measurements can be used to improve the association with eGFR and long-term survival [5, 9, 10]. Given the known variability in eGFR values [18] it is likely that the inclusion of additional data points, as we have done here, adds further accuracy to the estimation of the rate of functional change. For all subjects in the study, we used ≥5 data points (range 5–9), each representing the mean of all eGFRMDRD measurements available within the 6-month intervals between 1 and 5-years. On average, 40 (range 6–242) unique eGFRMDRD measurements were available for the study-eligible grafts. Given the ubiquitous use of frequent serum creatinine and formula-based eGFR measurements in the follow-up of kidney transplant recipients, we believe that this approach can be readily applied both retrospectively and prospectively to routine clinical practice as well as to clinical trials.
A concern for any long-term prospective study of kidney transplant recipients is the collection of functional measurements in the majority of patients. For example, 3 year follow-up of the Symphony study included eGFR data on only 45% of the original study population (710/1589) [19] and a 5-year analysis of the BENEFIT study included eGFR data on 52% (66/145) of patients originally randomized to belatacept [20]. The large proportions of missing subjects from these studies make it difficult to confidently interpret the results. In contrast, our approach resulted in the inclusion 76% of all adult conventional recipients transplanted from 2000 and 2005 (788/1039 if 86 grafts lost <1 year are included). To reach such a high inclusion rate in our cohort we used eGFRMDRD which is known to underestimate the rate of change in renal function when compared to iothalamate clearance [12]. Consistent with this, only 59% of the Progressors identified by uncorrected iothalamate were also identified by eGFRMDRD (as opposed to 95% of Non-Progressors; SDC Table 1B). However, the rate of graft failure among the iothalamate-defined progressors was lower than those identified by eGFRMDRD (17% vs 34%, SDC Table 1A) suggesting that a formula-based approach using a large number of sequential creatinine measurements has distinct value for identifying transplants at high risk for failure.
We conclude that a single GFR measurement at 1-year (or any time point), while associated with graft failure risk in the short-term, is insufficient to provide long-term risk stratification of renal transplant recipients. Instead, a combination of early and repeated estimates of GFR can be used to identify grafts at high risk for failure out to 7 or more years post-transplant. This approach also more accurately identifies the 40%–60% of all kidney transplant recipients who achieve good early function and maintain it for a prolonged period of time. The progressive decline in eGFR observed among a subset of grafts with good early function was associated with higher frequency of characteristics that are linked to immune-mediated injury. However, the poor outcome for this subset cannot be fully explained by these associations and investigation of other candidate factors such as patient compliance, late development of anti-donor antibody and genetic variability is merited [21–23]. Finally, we contend that to improve long-term renal allograft survival, attention must be focused on refining methods to accurately identify progressive loss of graft function as early as possible, with the goal of elucidating and treating the causes.
METHODS
Study subjects
The study protocol was approved by the Mayo Clinic Institutional Review Board. All adult recipients of kidney transplants performed at Mayo Clinic, Rochester, MN between 1/2000 and 12/2005 were identified. The following groups were excluded from further study: 1) Pediatric (<18 years). 2) Positive pre-transplant anti-donor T and/or B cell flow cytometric crossmatch. 3) ABO blood group incompatible. 4) Combined solid organ transplants. 4) Non-consent to research.
78% of study subjects received calcineurin inhibitor-based immunosuppression, 3% received mTOR inhibitor and 19% received a combination of both classes. 81% received induction with Thymoglobulin®.
One-year surveillance biopsies were obtained from 75% of study subjects. Biopsies were performed as previously described and were interpreted by a consultant renal pathologist using the Banff 97 classification [24]. Proteinuria was assessed by 24-hour urine total protein measurements at 1 year post-transplant and annually thereafter with abnormal proteinuria defined as >150 mg/24 h [25]. Pre-transplant donor-specific antibody screening was performed on stored serum samples by single-antigen bead assay as previously described [6]. Graft failure was defined as return to dialysis or eGFRMDRD consistently <20 ml/min for ≥ 6-months.
Assessment of renal function
Uncorrected iothalamate clearance and serum creatinine measurements were extracted from the Mayo Clinic Transplant Database for all study subjects. Serum creatinine values were converted to estimates of glomerular filtration rate using the MDRD equation (eGFRMDRD) as well as other formula-based calculations (see Supplementary Digital Content, Table S1). A total of 2,783 iothalamate clearances and 34,376 serum creatinine measurements between 1 and 5-years post-transplant were obtained for 953 subjects. Forty five percent of the creatinine measurements were performed by Mayo Clinic laboratories and 55% by external laboratories. For the Mayo laboratory data, both the pre-IDMS [26] and IDMS [27] equations were used whereas for external laboratory values only the pre-IDMS [26] formula was used.
Calculation of slope of renal function
Change in estimated renal function over time was determined by linear regression of all eGFRMDRD values between 1 and 5-years. To reduce variation across time, individual eGFRMDRD values were combined into means within 6-month post-transplant intervals (i.e. 1–1.5yr, 1.5–2yr, etc.). Data for a minimum of five 6-month intervals was required equating to a minimum renal transplant follow-up of 2.6 years. The log10 of the 6-month averaged eGFRMDRD values was plotted over time for each study subject and the slope of each plot calculated. Following this, the subjects were divided into quintiles based on the values for eGFRMDRD slope.
Subsequently, categorization as “Progressor” status was based on the following criteria: 1) Located in the quintile with the most negative eGFRMDRD slope. 2) Absolute eGFRMDRD decline of ≥20% between 1-year and most recent < 5-year eGFRMDRD. 3) Average eGFRMDRD < 60 ml/min for at least 1 6-month interval.
Statistical analyses
Results are expressed throughout as means ± SD. The proportions of nominal data were tested using chi-square (Pearson). Continuous variables were tested using Student’s t-test for parametric data and Wilcoxon for non-parametric data. Univariate and multivariate logistic regression analyses were used to analyze clinical variables associated with unstable graft function between 1 and 5-years and a ROC analysis was performed to determine if any combination of factors could predict outcomes. A p-value of <0.05 was considered statistically significant. The JMP® statistical software system was used to perform calculations.
Supplementary Material
ACKNOWLEDGEMENTS
The study was supported by NIH/NCRR CTSA Grant Number UL1 RR024150. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. MDG is supported by Science Foundation Ireland through grant numbers 06/IN.1/B652 and SFI 09/SRC/B1794.
Abbreviations
- eGFR
Estimated Glomerular Filtration Rate
- GFRiothal
GFR determined using iothalamate clearance
- eGFRMDRD
Estimated Glomerular Filtration Rate using the MDRD formula
- DWF
Deceased With Function
- OR
Odds Ratio
- High-NP
High-Non-Progressor
- High-P
High-Progressor
Footnotes
Author contributions:
WP, MG and MS participated in research design, performance of research, data analysis and writing of manuscript. TL participated in research design and data analysis.
DISCLOSURE
All the authors declared no competing interests.
References
- 1.Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4(8):1289. doi: 10.1111/j.1600-6143.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 2.Park WD, Griffin MD, Cornell LD, et al. Fibrosis with inflammation at one year predicts transplant functional decline. J Am Soc Nephrol. 2011;21(11):1987. doi: 10.1681/ASN.2010010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasiske BL, Israni AK, Snyder JJ, et al. The relationship between kidney function and long-term graft survival after kidney transplant. Am J Kidney Dis. 2011;57(3):466. doi: 10.1053/j.ajkd.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 4.Issa N, Cosio FG, Gloor JM, et al. Transplant glomerulopathy: risk and prognosis related to anti-human leukocyte antigen class II antibody levels. Transplantation. 2008;86(5):681. doi: 10.1097/TP.0b013e3181837626. [DOI] [PubMed] [Google Scholar]
- 5.Hariharan S, McBride MA, Cherikh WS, et al. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002;62(1):311. doi: 10.1046/j.1523-1755.2002.00424.x. [DOI] [PubMed] [Google Scholar]
- 6.Gloor JM, Sethi S, Stegall MD, et al. Transplant glomerulopathy: subclinical incidence and association with alloantibody. Am J Transplant. 2007;7(9):2124. doi: 10.1111/j.1600-6143.2007.01895.x. [DOI] [PubMed] [Google Scholar]
- 7.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9(3):527. doi: 10.1111/j.1600-6143.2008.02519.x. [DOI] [PubMed] [Google Scholar]
- 8.Stegall MD, Park WD, Larson TS, et al. The histology of solitary renal allografts at 1 and 5 years after transplantation. Am J Transplant. 2011;11(4):698. doi: 10.1111/j.1600-6143.2010.03312.x. [DOI] [PubMed] [Google Scholar]
- 9.Magott-Procelewska M, Boratynska M, Janczak D, et al. Estimated glomerular filtration rate evolution between 6 and 24 months predicts long-term kidney transplant survival among patients with inferior graft function. Transplant Proc. 2009;41(8):3028. doi: 10.1016/j.transproceed.2009.07.105. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Li H, Huang H, et al. Slope of changes in renal function in the first year post-transplantation and one-yr estimated glomerular filtration rate together predict long-term renal allograft survival. Clin Transplant. 2010;24(6):862. doi: 10.1111/j.1399-0012.2009.01186.x. [DOI] [PubMed] [Google Scholar]
- 11.Lenihan CR, O'Kelly P, Mohan P, et al. MDRD-estimated GFR at one year post-renal transplant is a predictor of long-term graft function. Ren Fail. 2008;30(4):345. doi: 10.1080/08860220801947686. [DOI] [PubMed] [Google Scholar]
- 12.Gera M, Slezak JM, Rule AD, et al. Assessment of changes in kidney allograft function using creatinine-based estimates of glomerular filtration rate. Am J Transplant. 2007;7(4):880. doi: 10.1111/j.1600-6143.2006.01690.x. [DOI] [PubMed] [Google Scholar]
- 13.Guba M, Pratschke J, Hugo C, et al. Renal function, efficacy, and safety of sirolimus and mycophenolate mofetil after short-term calcineurin inhibitor-based quadruple therapy in de novo renal transplant patients: one-year analysis of a randomized multicenter trial. Transplantation. 2010;90(2):175. doi: 10.1097/TP.0b013e3181e11798. [DOI] [PubMed] [Google Scholar]
- 14.Holdaas H, Rostaing L, Seron D, et al. Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24-month study. Transplantation. 2011;92(4):410. doi: 10.1097/TP.0b013e318224c12d. [DOI] [PubMed] [Google Scholar]
- 15.Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357(25):2562. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 16.Gill JS, Tonelli M, Mix CH, et al. The change in allograft function among long-term kidney transplant recipients. J Am Soc Nephrol. 2003;14(6):1636. doi: 10.1097/01.asn.0000070621.06264.86. [DOI] [PubMed] [Google Scholar]
- 17.Gourishankar S, Hunsicker LG, Jhangri GS, et al. The stability of the glomerular filtration rate after renal transplantation is improving. J Am Soc Nephrol. 2003;14(9):2387. doi: 10.1097/01.asn.0000085019.95339.f0. [DOI] [PubMed] [Google Scholar]
- 18.Buron F, Hadj-Aissa A, Dubourg L, et al. Estimating glomerular filtration rate in kidney transplant recipients: performance over time of four creatinine-based formulas. Transplantation. 2011;92(9):1005. doi: 10.1097/TP.0b013e3182301602. [DOI] [PubMed] [Google Scholar]
- 19.Ekberg H, Bernasconi C, Tedesco-Silva H, et al. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant. 2009;9(8):1876. doi: 10.1111/j.1600-6143.2009.02726.x. [DOI] [PubMed] [Google Scholar]
- 20.Vincenti F, Blancho G, Durrbach A, et al. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol. 2010;21(9):1587. doi: 10.1681/ASN.2009111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrissey PE, Flynn ML, Lin S. Medication noncompliance and its implications in transplant recipients. Drugs. 2007;67(10):1463. doi: 10.2165/00003495-200767100-00007. [DOI] [PubMed] [Google Scholar]
- 22.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003;3(6):665. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson PA, Schladt D, Israni A, et al. Genetic and Clinical Determinants of Early, Acute Calcineurin Inhibitor-Related Nephrotoxicity: Results From a Kidney Transplant Consortium. Transplantation. 2012;93(6):624. doi: 10.1097/TP.0b013e3182461288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 25.Amer H, Fidler ME, Myslak M, et al. Proteinuria after kidney transplantation, relationship to allograft histology and survival. Am J Transplant. 2007;7(12):2748. doi: 10.1111/j.1600-6143.2007.02006.x. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS GT, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11(155A) [Google Scholar]
- 27.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.