Abstract
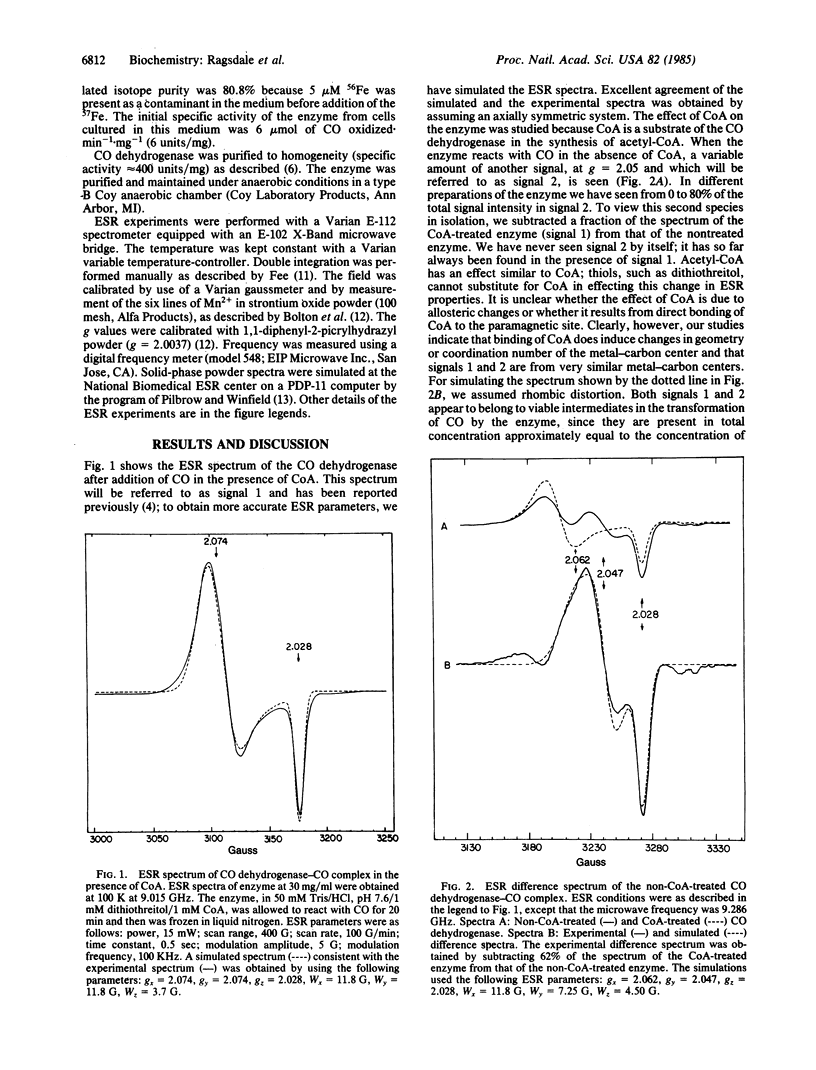
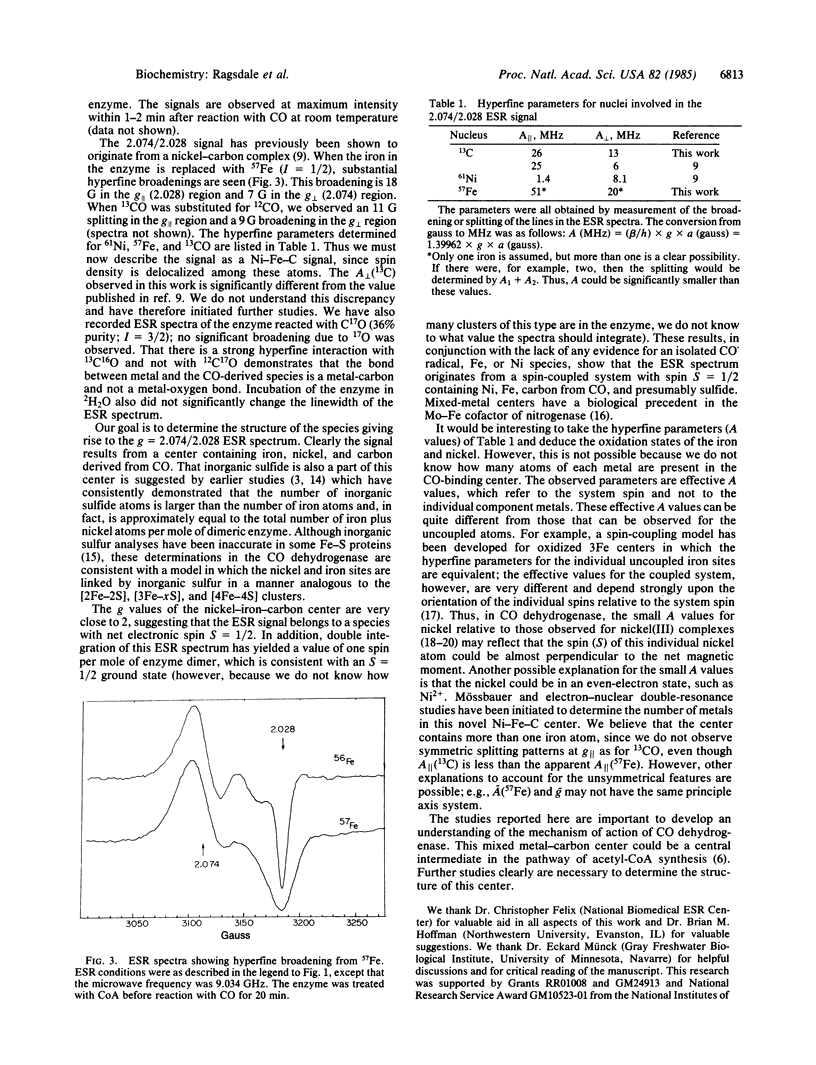
The interaction between carbon monoxide and the CO dehydrogenase from Clostridium thermoaceticum was studied by electron spin resonance (ESR) techniques. When the enzyme reacts with CO, a paramagnetic complex is formed which previously was shown, by isotope substitution, to be due to a nickel-carbon species. In this paper, we demonstrate that iron is also a component of this ESR-detectable complex. When the iron in the enzyme is replaced with 57Fe, a broadening of 18 G in the g parallel and 7 G in the g perpendicular region is seen. This hyperfine interaction is probably due to more than one iron atom in the complex. Coenzyme A influences this ESR spectrum. In the absence of CoA, the ESR spectrum consists of two superimposed signals, which were simulated using the following ESR parameters: signal 1, with g = 2.074 and g = 2.028, and signal 2 with gx = 2.062, gy = 2.047, and gz = 2.028. CoA converts signal 2 into signal 1. Since iron, nickel, and carbon all are part of this ESR-detectable complex, we propose that these atoms exist in a spin-coupled complex with net spin = 1/2, analogous to other iron-sulfur centers in which the metals are bridged by acid-labile sulfide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark J. E., Ragsdale S. W., Ljungdahl L. G., Wiegel J. Levels of enzymes involved in the synthesis of acetate from CO2 in Clostridium thermoautotrophicum. J Bacteriol. 1982 Jul;151(1):507–509. doi: 10.1128/jb.151.1.507-509.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekert G. B., Thauer R. K. Carbon monoxide oxidation by Clostridium thermoaceticum and Clostridium formicoaceticum. J Bacteriol. 1978 Nov;136(2):597–606. doi: 10.1128/jb.136.2.597-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee J. A. Transition metal electron paramagnetic resonance related to proteins. Methods Enzymol. 1978;49:512–528. doi: 10.1016/s0076-6879(78)49022-6. [DOI] [PubMed] [Google Scholar]
- Hu S. I., Drake H. L., Wood H. G. Synthesis of acetyl coenzyme A from carbon monoxide, methyltetrahydrofolate, and coenzyme A by enzymes from Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):440–448. doi: 10.1128/jb.149.2.440-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent T. A., Huynh B. H., Münck E. Iron-sulfur proteins: spin-coupling model for three-iron clusters. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6574–6576. doi: 10.1073/pnas.77.11.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl L. G., Andreesen J. R. Formate dehydrogenase, a selenium--tungsten enzyme from Clostridium thermoaceticum. Methods Enzymol. 1978;53:360–372. doi: 10.1016/s0076-6879(78)53042-5. [DOI] [PubMed] [Google Scholar]
- Pezacka E., Wood H. G. Role of carbon monoxide dehydrogenase in the autotrophic pathway used by acetogenic bacteria. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6261–6265. doi: 10.1073/pnas.81.20.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale S. W., Clark J. E., Ljungdahl L. G., Lundie L. L., Drake H. L. Properties of purified carbon monoxide dehydrogenase from Clostridium thermoaceticum, a nickel, iron-sulfur protein. J Biol Chem. 1983 Feb 25;258(4):2364–2369. [PubMed] [Google Scholar]
- Ragsdale S. W., Ljungdahl L. G., DerVartanian D. V. 13C and 61Ni isotope substitutions confirm the presence of a nickel (III)-carbon species in acetogenic CO dehydrogenases. Biochem Biophys Res Commun. 1983 Sep 15;115(2):658–665. doi: 10.1016/s0006-291x(83)80195-8. [DOI] [PubMed] [Google Scholar]
- Ragsdale S. W., Ljungdahl L. G., DerVartanian D. V. EPR evidence for nickel-substrate interaction in carbon monoxide dehydrogenase from Clostridium thermoaceticum. Biochem Biophys Res Commun. 1982 Sep 30;108(2):658–663. doi: 10.1016/0006-291x(82)90880-4. [DOI] [PubMed] [Google Scholar]
- Ragsdale S. W., Ljungdahl L. G., DerVartanian D. V. Isolation of carbon monoxide dehydrogenase from Acetobacterium woodii and comparison of its properties with those of the Clostridium thermoaceticum enzyme. J Bacteriol. 1983 Sep;155(3):1224–1237. doi: 10.1128/jb.155.3.1224-1237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale S. W., Wood H. G. Acetate biosynthesis by acetogenic bacteria. Evidence that carbon monoxide dehydrogenase is the condensing enzyme that catalyzes the final steps of the synthesis. J Biol Chem. 1985 Apr 10;260(7):3970–3977. [PubMed] [Google Scholar]
- Sugiura Y., Kuwahara J., Suzuki T. ESR characteristics of sulfhydryl-containing peptide-nickel (III) complexes: implication for nickel (III) center of hydrogenases. Biochem Biophys Res Commun. 1983 Sep 30;115(3):878–881. doi: 10.1016/s0006-291x(83)80016-3. [DOI] [PubMed] [Google Scholar]
- Wahl R. C., Rajagopalan K. V. Evidence for the inorganic nature of the cyanolyzable sulfur of molybdenum hydroxylases. J Biol Chem. 1982 Feb 10;257(3):1354–1359. [PubMed] [Google Scholar]



