Abstract
Objective
To examine predictive factors associated with onset of depression among individuals diagnosed with Parkinson’s disease (PD).
Background
Depression may precede or follow symptomatic parkinsonism in PD. It is frequently treatable but often overlooked.
Methods
The clinical series comprised 685 individuals who were diagnosed with PD and followed by one neurologist (RJU) from 1994 to 2007. The primary outcome was time to depression following the onset of PD. Diagnosis of depression was based on clinical assessment of depressive symptoms from patients (and spouse/family/caregiver) and antidepressant usage. A number of demographic, historical and clinical predictive factors were examined, including gender, age at symptomatic onset, disease duration, onset characteristics, clinical ratings, antiparkinsonian medications, cognitive status, depression history, and familial history of PD and other neurodegenerative disorders.
Results
Seventy-two percent of patients developed depression within ten years of symptomatic PD onset, and the mean time to depression was 7.9 years (median: 5.7 years). Factors associated with depression included longer PD duration, greater impairment in activities of daily living, and positive family history of motor neuron disease (MND).
Conclusions
A high rate of individuals with PD develop depressive symptoms during the course of the disease. Based on first clinic visit characteristics, most factors examined were not helpful in identifying individuals with an increased risk of depression. However, disease duration, functional limitations and family history of MND should lead clinicians to an increased vigilance for identifying depression.
Parkinson’s disease (PD) is a chronic, progressive neuro-degenerative disorder, characterized by cardinal motor symptoms, such as bradykinesia, resting tremor, rigidity, and postural instability. The prevalence of psychopathology is estimated to be two to three times higher in PD patients than in the age-matched elderly population1–3, with depression being identified in as many as 50% of individuals with PD. Depression may be a part of PD itself, and depressive symptoms may precede the development of motor signs, potentially representing the first manifestations of PD in some patients4,5; however, depression may occur at any time during the course of PD.
Although depression in PD is usually mild or moderate in severity with a low rate of suicide,3,6 the results of previous studies demonstrate that depression in PD is frequent, and associated with poorer clinical outcome, increased motor-related functional disability,7–10 and significantly worse quality of life.11–13 Moreover, depression in PD often remains underdiagnosed and undertreated.14–16 All of these evidences strongly underscore the need for better tools to improve identification of depression in PD. The current study evaluates potential predictive factors associated with the occurrence of depressive symptoms from the onset of PD, using a large, clinical cohort assessed longitudinally by a single clinician.
Methods
Patients and procedures
Our study population was a clinical cohort of 685 outpatients diagnosed with PD by one movement disorders neurologist (RJU) at the Mayo Clinic, Jacksonville, Florida, from July, 1994 to December, 2002, and followed through December 2007. This allowed at least five years of follow-up and provided a mean follow-up length from symptomatic PD onset of 10.3 years. The Mayo Clinic serves as a tertiary referral clinic and the studied cohort was clinic-based. However, previous analyses of our database demonstrate the demographics and clinical characteristics are very similar to population-based cohorts.17
The diagnosis of idiopathic PD was made by virtue of patients having at least two of the following features: bradykinesia, resting tremor, rigidity, and postural instability, without any other explanation for parkinsonism (e.g. drug-induced parkinsonism)18 or other features of an atypical parkinsonian syndrome (e.g. vertical gaze palsy, significant autonomic dysfunction, dementia, ataxia).19
Demographic, historical, and clinical examination data including the presence of depressive symptoms were collected prospectively on every patient in a consistent manner by the same neurologist (RJU) and all data were entered into an electronic database in accordance with an IRB (Institutional Review Board) approved protocol as has been previously described.17
In short, demographic and historical data including gender, age at first clinic visit, age at symptomatic PD onset, mean symptomatic PD duration, type of initial predominant PD symptoms, initial PD symptoms, handedness, antiparkinsonian medication usage, personal history of depression, and familial history of PD, Alzheimer’s disease (AD), motor neuron disease (MND), and other neurodegenerative disorders (NDD) were determined by interview and reviewing of medical records. A levodopa equivalent dose (LED) was calculated using a combination of previously published reports20–22 (LED = (regular levodopa dose × 1) + (levodopa CR dose × 0.75) + (pramipexole dose × 67) + (ropinirole dose × 16.67) + (pergolide dose × 100) + (bromocriptine dose × 10) + [(regular levodopa dose + (CR levodopa dose × 0.75)] × 0.25 (if on COMT inhibitors) for all individuals taking antiparkinsonian medications).
Each patient was administered a standardized assessment battery at each clinic visit using a semi-structured interview format.17 The assessment battery consisted of patient- and clinician-based symptoms’ ratings, medication usage and side effects recording. The battery also included clinician-based ratings of the intellectual impairment, activity of daily living (ADL), the Unified Parkinson’s Disease Rating Scale (UPDRS)23 - the modified motor score, the Hoehn & Yahr scale (H&Y)24, handwriting, and the most prominent symptoms and body location.
More specifically, the intellectual impairment was assessed on the basis of UPDRS Part I, item 1 “intellectual impairment”. Activity of daily living (ADL) was estimated semi-quantitatively as an overall ADL capacity (no impairment = 0, mild impairment = 1, moderate impairment = 2, marked impairment = 3, severe impairment = 4). The modified UPDRS motor score consisted of UPDRS Part III except item 24 and with an additional item “arm swing impairment” (normal = 0, slight reduction = 1, moderate reduction = 2, marked reduction = 3, severe reduction = 4).
Depression was defined by inquiring patient and spouse / other family member / caregiver regarding the presence of depressive symptoms, antidepressive medications usage, and clinician-based assessment of symptoms consistent with the DSM symptom checklist. When antidepressive medications were prescribed, patients were asked if the reason for prescription was depressive symptoms. Only when the answer was “yes” was this item recorded as “supportive”. Depressed mood or anhedonia plus at least one other depressive symptom (e.g. insomnia or hypersomnia, loss or gain in appetite / weight, psychomotor agitation or retardation, fatigue or loss of energy, feelings of worthlessness / guilt, diminished ability to think or concentrate / indecisiveness, recurrent thoughts of death / suicidal ideation) were required for a diagnosis of depression. Evaluation of depressive symptoms was performed in an “inclusive” manner, regardless of their potential overlap or causal attribution. Diagnosis of clinically significant depressive symptoms was qualified as “present” or “not present” at each time point for the purpose of this study.
All clinical ratings were performed by one physician (RJU). Medical history was updated on an ongoing basis with every clinical contact.
Predictive Factors
Potential predictive factors considered included gender, age at first clinic visit, age at symptomatic PD onset, PD duration (from symptomatic, extrapyramidal onset to first clinic visit), initial predominant symptoms and body location, predominant symptoms and their location at first clinic visit, handedness, antiparkinsonian medication usage, LED, ADL capacity, UPDRS subscale scores (including intellectual impairment item), H&Y stage, depression history, and familial history of PD, AD, MND, and other NDD. Clinical characteristics from the first (baseline) clinic visit were used for analyses.
Statistical analyses
Analyses were carried out using the computer package SPSS version 11.0 for Windows (SPSS Inc., Chicago, IL). The primary end point was presence of depression. An event was defined as the date of the clinic visit when the first clinical assessment indicating depression occurred. Survival curves were constructed according to the Kaplan-Meier method and the estimated mean and median cumulative survival time to depression after PD onset were calculated. For this aim, the length of survival was defined as the time from symptomatic PD onset to clinical assessment of depression. For censored events (i.e. patients who were never judged to be depressed) the date of the last clinic visit was used to determine the length of observation. The proportion of patients “surviving” at each time point was the proportion of patients who were still free from depressive symptoms at that time point.
To evaluate predictors of depression Cox regression analysis was performed in two steps. First, the predictive utility of each factor was examined on a univariate basis. Second, those factors with a p-value of .15 or less on a univariate basis were included into stepwise regression analysis. The multivariate regression model included gender and age in the first block using an Enter method to control for these factors, and the subsequent factors that passed the first step were included in the second block using a Step-wise Enter method.
Results
The overall sample of 685 was predominantly male (68%), right-handed (91%), had a mean age of 64 years at symptomatic PD onset, and a mean age of 71 at the time of first clinic visit. Average follow-up length was 10.3 (SD 6.1) years after symptomatic onset and 3.9 (SD 3.1) years after first clinic visit. Patients were seen several times between first and last follow-up visits, with a mean inter-visit interval of 11.1 (SD 10.4) months. The demographic, historical, and clinical characteristics of our cohort are presented in Table 1.
Table 1.
Demographic, historical and clinical characteristics of patients with PD at baseline
| Mean (SD) | ||
|---|---|---|
|
| ||
| Age at baseline (years) | 70.8 (9.1) | |
| Age at symptomatic PD onset (years) | 64.5 (10.5) | |
| Follow-up from initial visit (years) | 3.9 (3.1) | |
| Follow-up from symptomatic PD onset (years) | 10.3 (6.1) | |
| Modified UPDRS motor score | 30.2 (14.6) | |
| H&Y stage | 2,3 | |
| LED (mg/day) | 526.2 (343.9) | |
|
| ||
| N (%) | ||
|
| ||
| Patients on antiparkinsonian medication | 421 (62) | |
| Gender | ||
| Female | 221 (32) | |
| Male | 464 (68) | |
| Handedness | ||
| Right | 627 (91) | |
| Left | 45 (7) | |
| Ambidextrous | 13 (2) | |
| History of depression | ||
| No | 457 (67) | |
| Yes | 228 (33) | |
| Family history of PD | ||
| No | 581 (85) | |
| Yes | 104 (15) | |
| Family history of AD | ||
| No | 638 (93) | |
| Yes | 47 (7) | |
| Family history of MND | ||
| No | 680 (99) | |
| Yes | 5 (1) | |
| Family history of other MD / NDD | ||
| No | 637 (93) | |
| Yes | 48 (7) | |
PD=Parkinson Disease; UPDRS=Unified Parkinson Disease Rating Scale; H&Y=Hoehn and Yahr Scale; LED=Levodopa Equivalent Dose; MD=Movement Disorder; NDD=Neurodegenerative Disorder; AD=Alzheimer Disease; MND=Motor Neuron Disease; SD=Standard Deviation
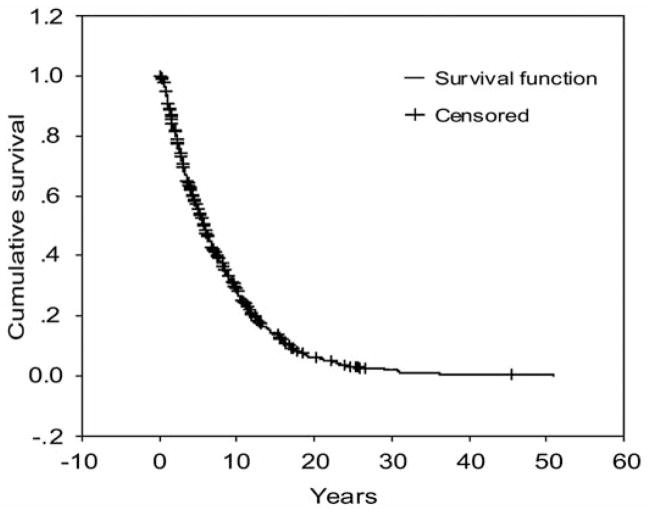
About 18% of PD patients were depressed at first clinic visit. Moreover, 42% of the entire cohort of PD patients developed depression within five years and 72% within ten years of symptomatic PD onset (Figure). The mean time from symptomatic onset to depression was 7.9 years and the median time to depression was 5.7 years (Table 2). Given that onset of depression was defined based on the visit when it was diagnosed, time to depression may have been overestimated as depressive episodes started before the visit. In addition, patients may have presented a short depressive episode between visits and have recovered by next follow-up visit. However, because the mean time between visits was relatively short (mean < 12 months), this is unlikely to have occurred in a significant number of patients.
Figure.
Cumulative survival time from symptomatic PD onset to occurrence of depression. Patients without depression when an observation time ends were censored.
Table 2.
Time from symptomatic PD onset to depression
| Meana | Median | ||||||
|---|---|---|---|---|---|---|---|
| Estimate (years) | Std. Error | 95% Confidence Interval | Estimate (years) | Std. Error | 95% Confidence Interval | ||
| Lower Bound (years) | Upper Bound (years) | Lower Bound (years) | Upper Bound (years) | ||||
| 7.86 | 0.23 | 7.40 | 8.32 | 5.69 | 0.22 | 5.26 | 6.12 |
PD=Parkinson’s disease;
Estimation is limited to the largest survival time if it is censored.
On a univariate basis, depression was predicted (with p<0.15) by a longer disease duration, a more advanced stage of the disease, a worse ADL score, usage of antiparkinsonian medications and a higher LED, initial and baseline symptoms’ location other than in extremities (i.e. axial symptoms), as well as a family history of MND. However, following multivariate stepwise Cox regression analysis, a longer symptomatic PD duration (p=0.007), increased impairment in ADL (p=0.001), and a positive family history of MND (p=0.043) at first clinic visit were the only significant predictors for depression (Table 3).
Table 3.
Cox regression analysis of time to depression
| Variable Description | Univariatea | Multivariate |
|---|---|---|
|
| ||
| P-value | P-value | |
|
| ||
| Gender | .417 | .250 |
| Age* (years) | .320 | .205 |
| Disease duration* (months) | .056 | .007 |
| Family history of MND | .002 | .043 |
| Initial symptoms’ location at: | ||
| Midline | .081 | .127 |
| Symptoms’ location*: | ||
| Other than in extremities (i.e. axial) | .072 | .215 |
| H&Y stage* | .093 | .330 |
| ADL overall capacity* | .063 | .001 |
| Current MD medications* | .060 | .064 |
| LED* (mg) | .106 | .271 |
Gender and age + only significanta predictors are presented;
Univariate features’ significance of p < .15 was used as criterion for inclusion in multivariate stepwise Cox regression model;
as assessed at first clinic visit; MND=Motor Neuron Disease; H&Y=Hoehn and Yahr Scale; ADL=Activity of Daily Living; MD=Movement Disorders; LED=Levodopa Equivalent Dose
Discussion
The present study determined the cumulative survival time to depression in a prospectively studied clinical series of patients with PD, estimated the proportion of patients developing depression, and identified characteristics at first clinic visit that predict depression in PD.
The mean cumulative survival time to depression was 7.86 years (95% CI: 7.40 – 8.32) from symptomatic extrapyramidal onset of PD, while the median survival time was 5.69 years (95% CI: 5.26 – 6.12). To our knowledge, the survival time to depression has not been systematically assessed or reported in patients with PD.
Rojo et al analyzed outcome of depressive symptoms within 2.7±1.6 years from an initial visit in 184 parkinsonian patients. The study showed that 25% of patients assessed at initial visit as “normal” (i.e. without depression) worsened eventually to the depressive level at follow-up visit according to the Geriatric Depression Scale (GDS) score.25 In our study, 42% of patients developed depression within five years and 72% within ten years of symptomatic PD onset according to conducted survival analysis. Moreover, 33% of studied patients had a positive history of depression as recorded at first clinic visit while 18% of patients were depressed at that time point. Interestingly, neither history of depression nor presence of depressive symptoms at first clinic visit was identified as a predictor for future diagnosis of depression in our study.
Personal history of depression was established as a risk factor for depression in the general population.26,27 In contrast, as reported by Leentjens et al, personal history as an individual variable was not statistically significant, neither in bivariate nor multivariate models in PD.28 However, the five general risk factors as a group, including personal history of depression, were predictors of depression in PD in the same study. The association between higher initial GDS score and subsequent development of depression in PD was previously reported by others.25
In our study, based on first clinic visit characteristics, most of the factors examined were not helpful to identify individuals with an increased risk of depression. However, the development of depression was predicted by a greater impairment in ADL, a longer symptomatic PD duration, and a family history of MND.
Previous cross-sectional studies showed a relationship between ADL and depression.9,29 Similarly, Rojo et al confirmed significance and independence of the UPDRS Part II score in predicting the GDS score. However, the prospectively designed part of the same study did not reveal any relation between functional impairment and outcome of depressive symptoms.25 Although a prospective study by Brown et al showed depression and disability in ADL being closely associated at a single assessment, the dynamic relationship between changes in both variables remained more complex.30 It should be emphasized that both referred prospective studies did not look directly for predictors of a novel depression episode.
As with previous studies,3,25 we did not identify age and age at symptomatic PD onset as predictive factors for future development of depression. However, longer symptomatic PD duration was a characteristic that predicted depression. Similar results were reported by some,31,32 but not all investigators.25,33
Family history of MND was a predictor for development of depression during the course of PD in our cohort. To our knowledge, such a relationship has not been reported before, potentially because the family history of neurodegenerative disorders was not collected or analyzed in detail. It is speculated that a diagnosis of MND in one of the family members may induce depression even in distant relatives with PD34, given the devastating and fatal nature of MND and the higher predisposition of parkinsonian patients to develop depression.
Although previous studies have reported a number of other factors associated with depression in patients with PD including higher UPDRS score, higher requirement of levodopa, motor fluctuations, severe motor disability, akinesia, right side predominance of parkinsonian symptoms, axial bradykinesia, gait and balance problems, early onset of disease, anxiety, psychosis, and cognitive impairment,2,9,29,32,33,35–38 predictors for depression in PD remain only partially established. The predictive factors that emerge across studies have been inconsistent due to a number of methodological limitations (e.g. small sample size, varied inclusion criteria, and an inconsistent operational definition of depressive symptomatology).
Our study has several limitations. First, the detection of depression was the result of an active inquiry regarding the presence of depressive symptoms and antidepressant usage, as well as the clinician-based assessment of symptoms. We admit that a broader approach to assessment of the symptoms might have improved sensitivity although presumably with loss of specificity. On the other hand, we conducted the evaluation of depressive symptoms in an “inclusive” manner, regardless of their potential overlap or causal attribution. Such an “inclusive” rather than “etiologic-exclusive” approach was recently recommended by the NINDS/NIHM Work Group on Depression and Parkinson’s disease.39 An inclusive approach gives a higher chance to identify the majority of cases with significant depression, recognizing the broad spectrum of depressive disturbances present in PD. We admit that this strategy may have led to partially inflated rates of positive diagnosis, especially in cases with non-major depression. However, we required the presence of depressed mood or anhedonia (but not decreased interest as a core symptom), which should have eliminated a diagnostic overlap with apathy and cognitive impairment. Moreover, there is still no established gold standard guideline for recognition of depression in PD and no reliable diagnostic method for PD-depression being both specific and sensitive for the full spectrum of depressive syndromes in PD. Given that, we used a considered diagnostic interview to define diagnosis of depression in our cohort.
The aim and design of this study did not focus on predictive factors for distinct, homogenous subgroups of PD-depression. Therefore, we did not determine the data for major and minor depression, dysthymia or subsyndromal depression separately. We did not separate the subgroups of PD-depression because it is not firmly established whether dysthymia and major depression are distinct problems or parts of a depressive continuum. We did not explore some unmeasured effects of both disease (e.g. personal attitudes to disability, personal experiences related to primary disease, health beliefs) and environment (e.g. family or society support). Furtheremore, family history of depression or other psychiatric diseases, which may be important factors to consider, were not collected and included in our analysis.
We also acknowledge that this is a clinic-based cohort. However, previous analyses of this database demonstrate the demographics and clinical characteristics are very similar to, albeit smaller, population-based cohorts.8 Nevertheless, the patient cohort may have different risks for depression than those from a population-based study, although no large scale PD population-based studies have investigated depression. Finally, since the follow-up was not strictly regular for some patients, the onset of depressive symptoms was defined as the date of the first occurrence of clinical assessment (by RJU) indicating depression.
Strengths of our study include follow-up with survival time to depression, the percentage of patients developing depression and the predictive factors for depression in PD being investigated simultaneously. Our data were collected prospectively and in an extensive parkinsonian cohort having at least one subsequent visit following first clinic visit, with the average follow-up length of ten years from extrapyramidal symptoms’ onset. Additionally, numerous demographic, historical and clinical factors were recorded and analyzed as potential predictors for depression in PD.
In conclusion, while it has been recognized that depression may be a preliminary symptom heralding a diagnosis of Parkinson’s disease, our study emphasizes the need for ongoing scrutiny for depression throughout the course of illness. In particular, those with greater impairment in ADL, longer symptomatic PD duration and familial history of MND are at greater risk for development of depression. Given that disease duration and familial history are not modifiable risk factors, concerted efforts towards early identification and ADL improvement are critical for timely treatment and better quality of life.
Acknowledgments
The authors thank all patients for their collaboration in the study. RJU and ZKW are supported by the NIH/NINDS Morris K. Udall Center of Exellence for PD Research (P50-NS40256) and the Pacific Alzheimer Research Foundation (PARF) C06-01 grant. RJU also receives research funding from Advanced Neuromodulation Systems, Inc. ZKW is also supported by the NIH/NINDS NS 057567, NIH/NIA P01-AG017216, NIH/NIA R01-AG015866, and the Mayo Clinic Florida CR 90052025 grant. CW is supported by the Swiss National Science Foundation (PASMP3-123268/1). BJ-M is supported by the Medical University of Silesia, Poland, the Robert and Clarice Smith Fellowship Program and partially by the Pacific Alzheimer Research Foundation (PARF) C06-01 grant.
References
- 1.Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord. 2008;23(2):183–9. doi: 10.1002/mds.21803. quiz 313. [DOI] [PubMed] [Google Scholar]
- 2.Tandberg E, Larsen JP, Aarsland D, Cummings JL. The occurrence of depression in Parkinson’s disease. A community-based study. Arch Neurol. 1996;53(2):175–9. doi: 10.1001/archneur.1996.00550020087019. [DOI] [PubMed] [Google Scholar]
- 3.Cummings JL. Depression and Parkinson’s disease: a review. Am J Psychiatry. 1992;149(4):443–54. doi: 10.1176/ajp.149.4.443. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson FM, Kessing LV, Bolwig TG. Increased risk of developing Parkinson’s disease for patients with major affective disorder: a register study. Acta Psychiatr Scand. 2001;104(5):380–6. doi: 10.1034/j.1600-0447.2001.00372.x. [DOI] [PubMed] [Google Scholar]
- 5.Leentjens AF, Van den Akker M, Metsemakers JF, Lousberg R, Verhey FR. Higher incidence of depression preceding the onset of Parkinson’s disease: a register study. Mov Disord. 2003;18(4):414–8. doi: 10.1002/mds.10387. [DOI] [PubMed] [Google Scholar]
- 6.Lemke MR, Fuchs G, Gemende I, Herting B, Oehlwein C, Reichmann H, et al. Depression and Parkinson’s disease. J Neurol. 2004;251(Suppl 6):VI/24–7. doi: 10.1007/s00415-004-1606-6. [DOI] [PubMed] [Google Scholar]
- 7.Ravina B, Camicioli R, Como PG, Marsh L, Jankovic J, Weintraub D, et al. The impact of depressive symptoms in early Parkinson disease. Neurology. 2007;69(4):342–7. doi: 10.1212/01.wnl.0000268695.63392.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson’s disease. J Am Geriatr Soc. 2004;52(5):784–8. doi: 10.1111/j.1532-5415.2004.52219.x. [DOI] [PubMed] [Google Scholar]
- 9.Cole SA, Woodard JL, Juncos JL, Kogos JL, Youngstrom EA, Watts RL. Depression and disability in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1996;8(1):20–5. doi: 10.1176/jnp.8.1.20. [DOI] [PubMed] [Google Scholar]
- 10.Starkstein SE, Mayberg HS, Leiguarda R, Preziosi TJ, Robinson RG. A prospective longitudinal study of depression, cognitive decline, and physical impairments in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1992;55(5):377–82. doi: 10.1136/jnnp.55.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuopio AM, Marttila RJ, Helenius H, Toivonen M, Rinne UK. The quality of life in Parkinson’s disease. Mov Disord. 2000;15(2):216–23. doi: 10.1002/1531-8257(200003)15:2<216::aid-mds1003>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2000;69(3):308–12. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slawek J, Derejko M, Lass P. Factors affecting the quality of life of patients with idiopathic Parkinson’s disease--a cross-sectional study in an outpatient clinic attendees. Parkinsonism Relat Disord. 2005;11(7):465–8. doi: 10.1016/j.parkreldis.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Hughes TA, Ross HF, Mindham RH, Spokes EG. Mortality in Parkinson’s disease and its association with dementia and depression. Acta Neurol Scand. 2004;110(2):118–23. doi: 10.1111/j.1600-0404.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 15.Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Recognition and treatment of depression in Parkinson’s disease. J Geriatr Psychiatry Neurol. 2003;16(3):178–83. doi: 10.1177/0891988703256053. [DOI] [PubMed] [Google Scholar]
- 16.Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2002;8(3):193–7. doi: 10.1016/s1353-8020(01)00015-3. [DOI] [PubMed] [Google Scholar]
- 17.Uitti RJ, Baba Y, Wszolek ZK, Putzke DJ. Defining the Parkinson’s disease phenotype: initial symptoms and baseline characteristics in a clinical cohort. Parkinsonism Relat Disord. 2005;11(3):139–45. doi: 10.1016/j.parkreldis.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 18.de Rijk MC, Rocca WA, Anderson DW, Melcon MO, Breteler MM, Maraganore DM. A population perspective on diagnostic criteria for Parkinson’s disease. Neurology. 1997;48(5):1277–81. doi: 10.1212/wnl.48.5.1277. [DOI] [PubMed] [Google Scholar]
- 19.Ward CD, Gibb WR. Research diagnostic criteria for Parkinson’s disease. Adv Neurol. 1990;53:245–9. [PubMed] [Google Scholar]
- 20.Rodriguez-Oroz MC, Gorospe A, Guridi J, Ramos E, Linazasoro G, Rodriguez-Palmero M, et al. Bilateral deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. Neurology. 2000;55(12 Suppl 6):S45–51. [PubMed] [Google Scholar]
- 21.Moro E, Scerrati M, Romito LM, Roselli R, Tonali P, Albanese A. Chronic subthalamic nucleus stimulation reduces medication requirements in Parkinson’s disease. Neurology. 1999;53(1):85–90. doi: 10.1212/wnl.53.1.85. [DOI] [PubMed] [Google Scholar]
- 22.Krause M, Fogel W, Heck A, Hacke W, Bonsanto M, Trenkwalder C, et al. Deep brain stimulation for the treatment of Parkinson’s disease: subthalamic nucleus versus globus pallidus internus. J Neurol Neurosurg Psychiatry. 2001;70(4):464–70. doi: 10.1136/jnnp.70.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahn S, Elton RL. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne D, editors. Recent developments in Parkinson’s disease. Vol. 2. Florham Park, NJ: Macmillan; 1987. pp. 153–63. [Google Scholar]
- 24.Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–32. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 25.Rojo A, Aguilar M, Garolera MT, Cubo E, Navas I, Quintana S. Depression in Parkinson’s disease: clinical correlates and outcome. Parkinsonism Relat Disord. 2003;10(1):23–8. doi: 10.1016/s1353-8020(03)00067-1. [DOI] [PubMed] [Google Scholar]
- 26.Schoevers RA, Beekman AT, Deeg DJ, Geerlings MI, Jonker C, Van Tilburg W. Risk factors for depression in later life; results of a prospective community based study (AMSTEL) J Affect Disord. 2000;59(2):127–37. doi: 10.1016/s0165-0327(99)00124-x. [DOI] [PubMed] [Google Scholar]
- 27.Beekman AT, Deeg DJ, van Tilburg T, Smit JH, Hooijer C, van Tilburg W. Major and minor depression in later life: a study of prevalence and risk factors. J Affect Disord. 1995;36(1–2):65–75. doi: 10.1016/0165-0327(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 28.Leentjens AF, Lousberg R, Verhey FR. Markers for depression in Parkinson’s disease. Acta Psychiatr Scand. 2002;106(3):196–201. doi: 10.1034/j.1600-0447.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 29.Tandberg E, Larsen JP, Aarsland D, Laake K, Cummings JL. Risk factors for depression in Parkinson disease. Arch Neurol. 1997;54(5):625–30. doi: 10.1001/archneur.1997.00550170097020. [DOI] [PubMed] [Google Scholar]
- 30.Brown RG, MacCarthy B, Gotham AM, Der GJ, Marsden CD. Depression and disability in Parkinson’s disease: a follow-up of 132 cases. Psychol Med. 1988;18(1):49–55. doi: 10.1017/s0033291700001872. [DOI] [PubMed] [Google Scholar]
- 31.Palhagen SE, Carlsson M, Curman E, Walinder J, Granerus AK. Depressive illness in Parkinson’s disease--indication of a more advanced and widespread neurodegenerative process? Acta Neurol Scand. 2008;117(5):295–304. doi: 10.1111/j.1600-0404.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- 32.Chia LG, Cheng LJ, Chuo LJ, Cheng FC, Cu JS. Studies of dementia, depression, electrophysiology and cerebrospinal fluid monoamine metabolites in patients with Parkinson’s disease. J Neurol Sci. 1995;133(1–2):73–8. doi: 10.1016/0022-510x(95)00146-s. [DOI] [PubMed] [Google Scholar]
- 33.Cubo E, Bernard B, Leurgans S, Raman R. Cognitive and motor function in patients with Parkinson’s disease with and without depression. Clin Neuropharmacol. 2000;23(6):331–4. doi: 10.1097/00002826-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Houde SC, Mangolds V. Amyotrophic lateral sclerosis: a team approach to primary care. Clin Excell Nurse Pract. 1999;3(6):337–45. [PubMed] [Google Scholar]
- 35.Oertel WH, Hoglinger GU, Caraceni T, Girotti F, Eichhorn T, Spottke AE, et al. Depression in Parkinson’s disease. An update. Adv Neurol. 2001;86:373–83. [PubMed] [Google Scholar]
- 36.Tom T, Cummings JL. Depression in Parkinson’s disease. Pharmacological characteristics and treatment. Drugs Aging. 1998;12(1):55–74. doi: 10.2165/00002512-199812010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayeux R, Stern Y, Rosen J, Leventhal J. Depression, intellectual impairment, and Parkinson disease. Neurology. 1981;31(6):645–50. doi: 10.1212/wnl.31.6.645. [DOI] [PubMed] [Google Scholar]
- 38.Wichowicz HM, Slawek J, Derejko M, Cubala WJ. Factors associated with depression in Parkinson’s disease: a cross-sectional study in a Polish population. Eur Psychiatry. 2006;21 (8):516–20. doi: 10.1016/j.eurpsy.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Marsh L, McDonald WM, Cummings J, Ravina B. Provisional diagnostic criteria for depression in Parkinson’s disease: report of an NINDS/NIMH Work Group. Mov Disord. 2006;21(2):148–58. doi: 10.1002/mds.20723. [DOI] [PubMed] [Google Scholar]