Abstract
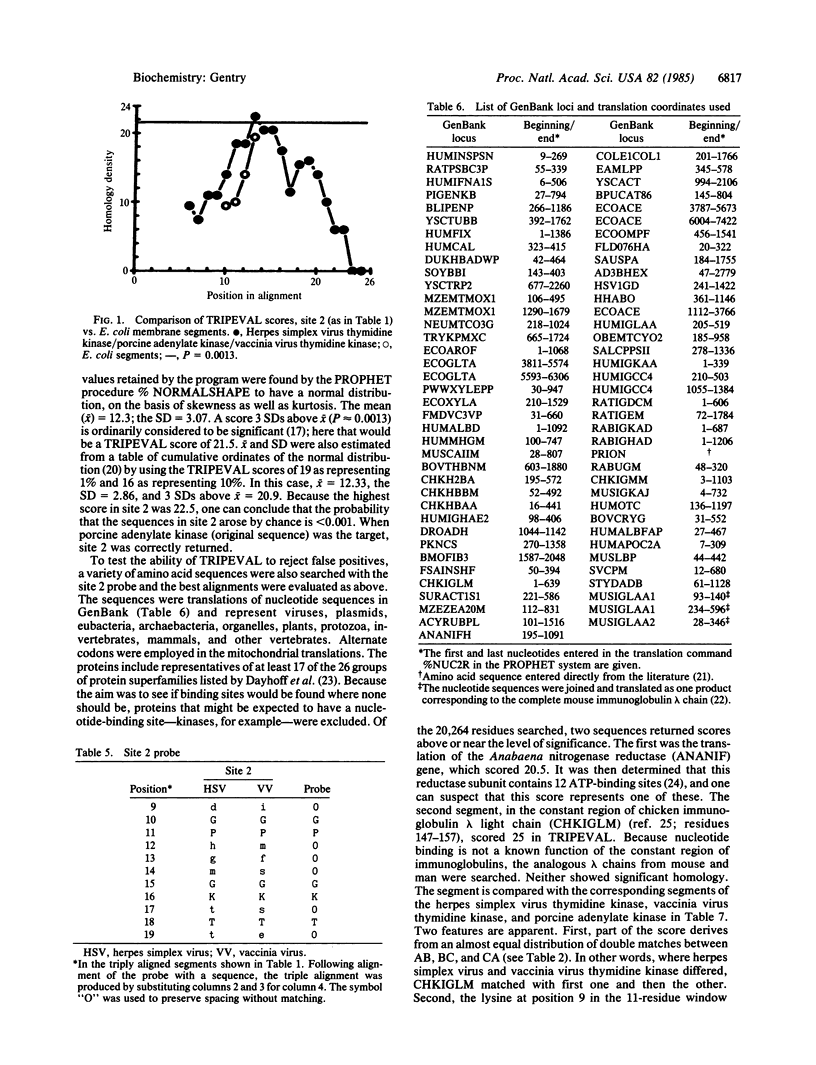
Computer techniques were used to locate related segments of amino acid sequences in the thymidine kinases of vaccinia virus and of herpes simplex virus type 1 and in porcine adenylate kinase. As determined by a procedure that evaluates triply aligned sequences, the probability that the similarities among the segments described here arose by chance was no greater than 0.001. Because the sequence in porcine adenylate kinase is a nucleotide phosphate-binding site it is concluded that the segments in the vaccinia virus and herpes simplex virus thymidine kinases perform similar functions. The segments are residues 16-23 in porcine adenylate kinase, 11-19 in vaccinia virus thymidine kinase, and 56-64 in herpes simplex virus thymidine kinase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. M., Zucker F. H., Steitz T. A. Space-filling models of kinase clefts and conformation changes. Science. 1979 Apr 27;204(4391):375–380. doi: 10.1126/science.220706. [DOI] [PubMed] [Google Scholar]
- Bernard O., Hozumi N., Tonegawa S. Sequences of mouse immunoglobulin light chain genes before and after somatic changes. Cell. 1978 Dec;15(4):1133–1144. doi: 10.1016/0092-8674(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Bradshaw H. D., Jr, Deininger P. L. Human thymidine kinase gene: molecular cloning and nucleotide sequence of a cDNA expressible in mammalian cells. Mol Cell Biol. 1984 Nov;4(11):2316–2320. doi: 10.1128/mcb.4.11.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Gentry G. A., Allen G. P., Holton R., Nevins R. B., McGowan J. J., Veerisetty V. Thymine salvage, mitochondria, and the evolution of the herpesviruses. Intervirology. 1983;19(2):67–76. doi: 10.1159/000149340. [DOI] [PubMed] [Google Scholar]
- Halpern M. E., Smiley J. R. Effects of deletions on expression of the herpes simplex virus thymidine kinase gene from the intact viral genome: the amino terminus of the enzyme is dispensable for catalytic activity. J Virol. 1984 Jun;50(3):733–738. doi: 10.1128/jvi.50.3.733-738.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil A., Müller G., Noda L., Pinder T., Schirmer H., Schirmer I., von Zabern I. The amino-acid sequence of sarcine adenylate kinase from skeletal muscle. Eur J Biochem. 1974 Mar 15;43(1):131–144. doi: 10.1111/j.1432-1033.1974.tb03393.x. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Guarino L. A. Novel codon utilization within the vaccinia virus thymidine kinase gene. Virus Res. 1984;1(4):315–320. doi: 10.1016/0168-1702(84)90020-0. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. Acquisition of thymidine kinase activity by herpes simplex-infected mouse fibroblast cells. Biochem Biophys Res Commun. 1963 Apr 2;11:55–59. doi: 10.1016/0006-291x(63)90027-5. [DOI] [PubMed] [Google Scholar]
- KIT S., PIEKARSKI L. J., DUBBS D. R. Induction of thymidine kinase by vaccinia-infected mouse fibroblasts. J Mol Biol. 1963 Jan;6:22–33. doi: 10.1016/s0022-2836(63)80078-9. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. On the use of sequence homologies to predict protein structure: identical pentapeptides can have completely different conformations. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1075–1078. doi: 10.1073/pnas.81.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwoh T. J., Engler J. A. The nucleotide sequence of the chicken thymidine kinase gene and the relationship of its predicted polypeptide to that of the vaccinia virus thymidine kinase. Nucleic Acids Res. 1984 May 11;12(9):3959–3971. doi: 10.1093/nar/12.9.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson L. E., Thorneley R. N. Structure and function of nitrogenase. Annu Rev Biochem. 1979;48:387–418. doi: 10.1146/annurev.bi.48.070179.002131. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Wu H. C. Amino acid sequence homology among the major outer membrane proteins of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1048–1052. doi: 10.1073/pnas.81.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Otsuka H., Kit S. Nucleotide sequence of the marmoset herpesvirus thymidine kinase gene and predicted amino acid sequence of thymidine kinase polypeptide. Virology. 1984 Jun;135(2):316–330. doi: 10.1016/0042-6822(84)90189-2. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Sachsenheimer W., Schirmer R. H., Schulz G. E. Substrate positions and induced-fit in crystalline adenylate kinase. J Mol Biol. 1977 Jul;114(1):37–45. doi: 10.1016/0022-2836(77)90281-9. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Dahan A., Weill J. C. Complete sequence of a chicken lambda light chain immunoglobulin derived from the nucleotide sequence of its mRNA. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4099–4103. doi: 10.1073/pnas.80.13.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachsenheimer W., Schulz G. E. Two conformations of crystalline adenylate kinase. J Mol Biol. 1977 Jul;114(1):23–36. doi: 10.1016/0022-2836(77)90280-7. [DOI] [PubMed] [Google Scholar]
- Schwartz R. M., Dayhoff M. O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science. 1978 Jan 27;199(4327):395–403. doi: 10.1126/science.202030. [DOI] [PubMed] [Google Scholar]
- Swain M. A., Galloway D. A. Nucleotide sequence of the herpes simplex virus type 2 thymidine kinase gene. J Virol. 1983 Jun;46(3):1045–1050. doi: 10.1128/jvi.46.3.1045-1050.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M. J., Sharp J. A., Summers W. C. Nucleotide sequence of the thymidine kinase gene of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1441–1445. doi: 10.1073/pnas.78.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G., Noda L. Amino acid sequences aroung the thiol groups of myokinase. Biochem J. 1968 Mar;107(2):311–312. doi: 10.1042/bj1070311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Nucleotide sequence of the vaccinia virus thymidine kinase gene and the nature of spontaneous frameshift mutations. J Virol. 1983 May;46(2):530–537. doi: 10.1128/jvi.46.2.530-537.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



