Abstract
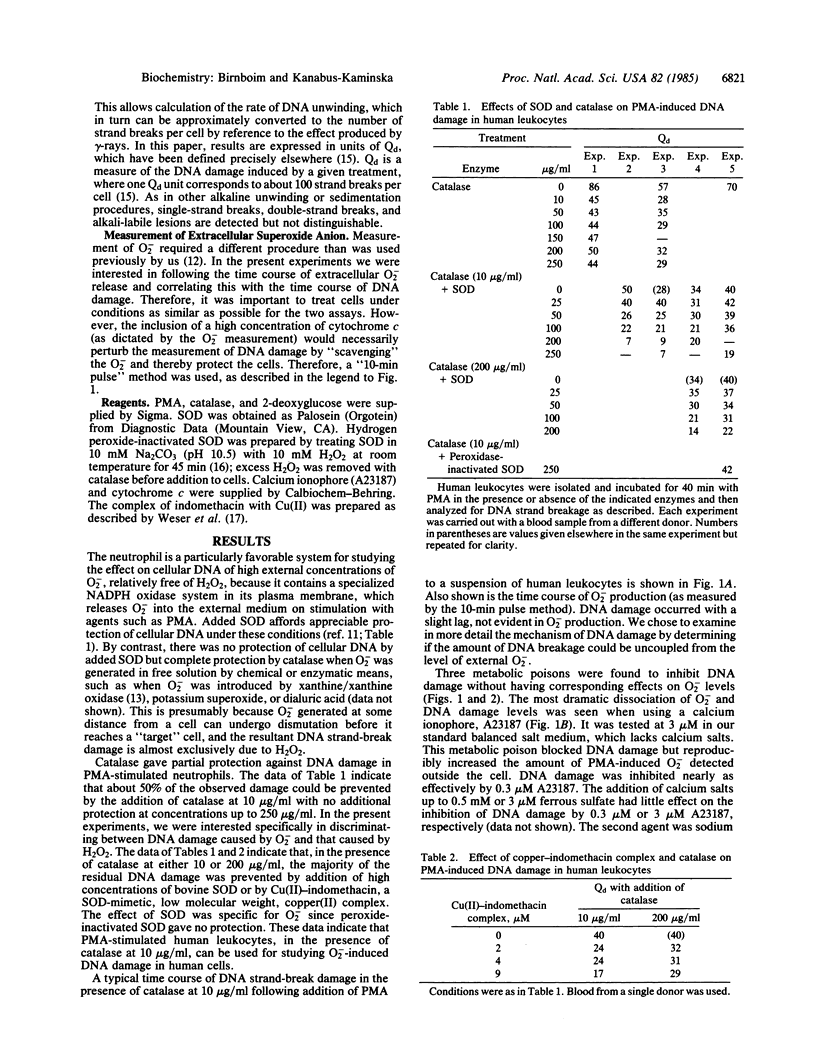
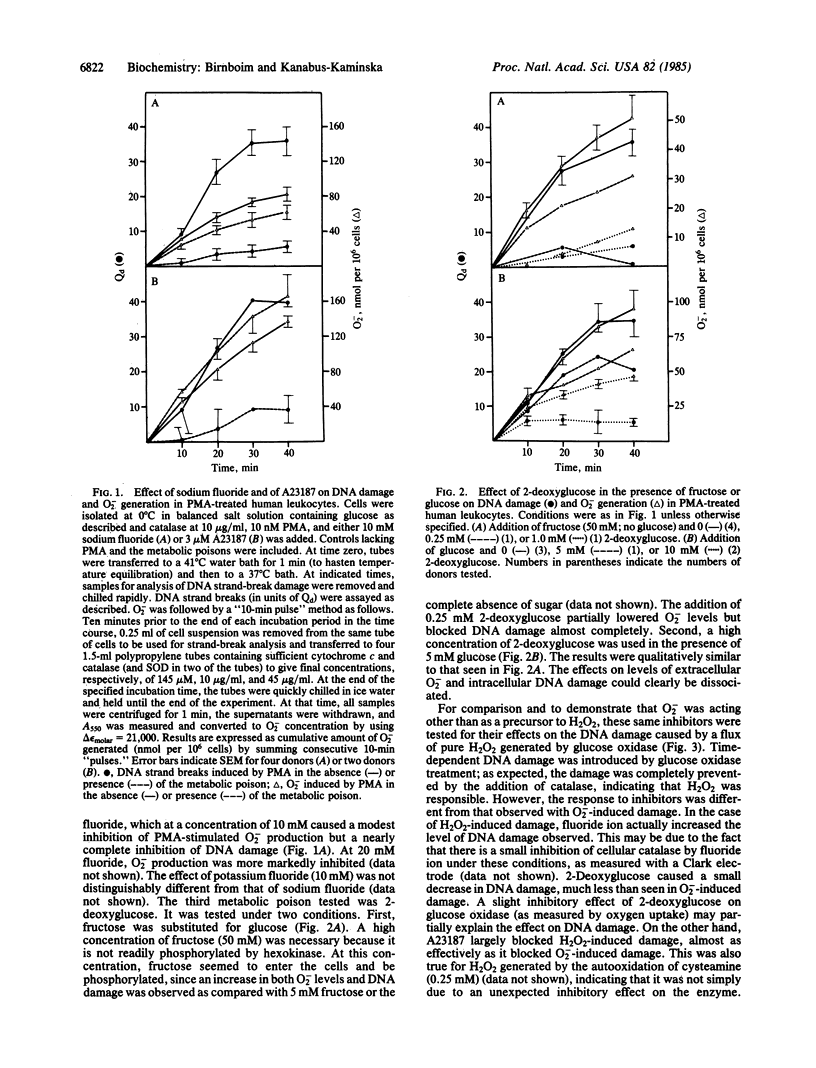
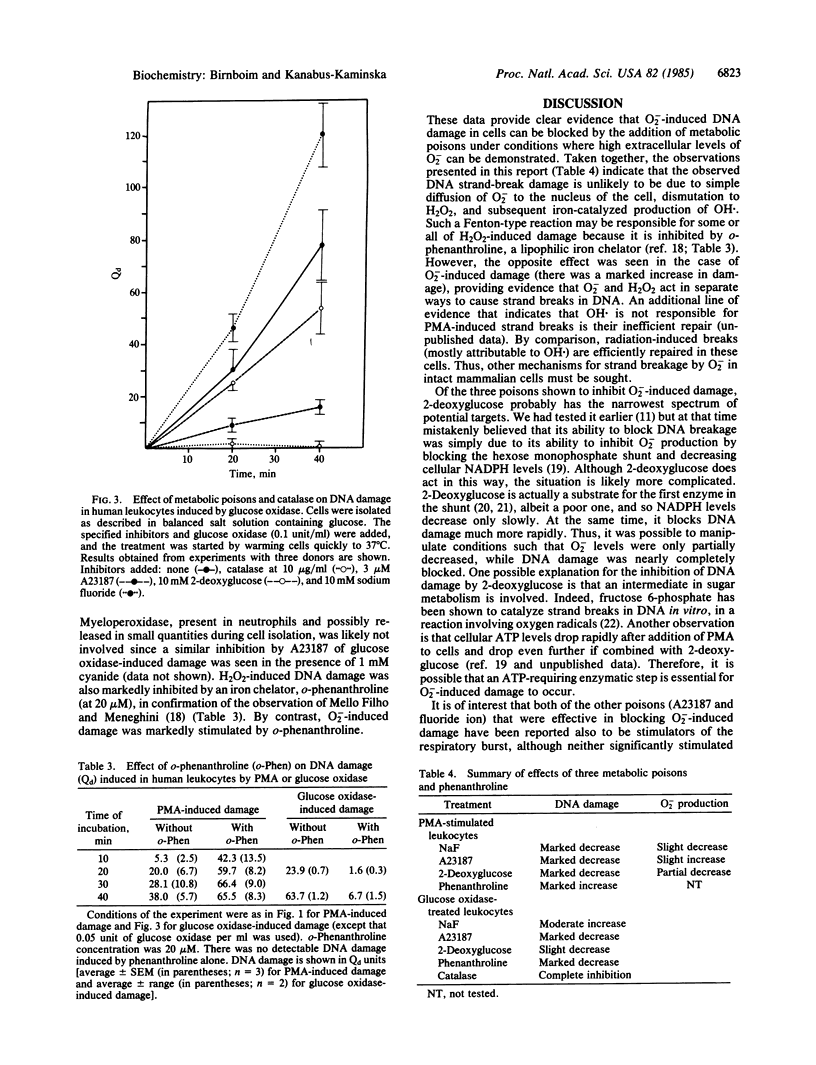
H2O2 is known to be capable of inducing strand-break damage in intracellular DNA, but whether O2- also can do so in the absence of H2O2 is uncertain. The difficulty in distinguishing the effects of the two is that, under physiological conditions, dismutation of O2- to H2O2 can readily occur. When human leukocytes are stimulated with phorbol 12-myristate 13-acetate (PMA), they release O2-, and within a few minutes strand breakage in intracellular DNA can be observed. We have attempted to determine whether the O2- produced is itself capable of causing DNA damage or whether H2O2 alone, or in combination with O2-, is responsible for the observed damage. Addition of catalase (up to 250 micrograms/ml) to remove H2O2 prevented no more than about 50% of the DNA damage. The majority of the remaining damage could be blocked, in a dose-dependent manner, by superoxide dismutase (SOD) or a SOD-mimetic copper complex, identifying a fraction of damage to intracellular DNA dependent upon extracellular O2-. We studied this O2(-)-specific fraction through the use of three metabolic poisons (fluoride, 2-deoxyglucose, and A23187). These agents largely blocked DNA damage, while affecting extracellular O2- levels only slightly. For comparison, H2O2-induced DNA damage was studied with glucose oxidase to generate a flux of H2O2. The first two metabolic poisons had little effect, whereas A23187 did inhibit H2O2-induced DNA damage. We conclude that O2(-)-induced damage occurs through a mechanism that differs, at least in part, from the H2O2 damage pathway and that the former may involve one or more metabolic steps.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Armato U., Andreis P. G., Romano F. Exogenous Cu,Zn-superoxide dismutase suppresses the stimulation of neonatal rat hepatocytes' growth by tumor promoters. Carcinogenesis. 1984 Dec;5(12):1547–1555. doi: 10.1093/carcin/5.12.1547. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. DNA strand breakage in human leukocytes exposed to a tumor promoter, phorbol myristate acetate. Science. 1982 Mar 5;215(4537):1247–1249. doi: 10.1126/science.6276978. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. Factors which affect DNA strand breakage in human leukocytes exposed to a tumor promoter, phorbol myristate acetate. Can J Physiol Pharmacol. 1982 Nov;60(11):1359–1366. doi: 10.1139/y82-203. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Jevcak J. J. Fluorometric method for rapid detection of DNA strand breaks in human white blood cells produced by low doses of radiation. Cancer Res. 1981 May;41(5):1889–1892. [PubMed] [Google Scholar]
- Borek C., Troll W. Modifiers of free radicals inhibit in vitro the oncogenic actions of x-rays, bleomycin, and the tumor promoter 12-O-tetradecanoylphorbol 13-acetate. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1304–1307. doi: 10.1073/pnas.80.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Copeland E. S. A National Institutes of Health Workshop report. Free radicals in promotion--a chemical pathology study section workshop. Cancer Res. 1983 Nov;43(11):5631–5637. [PubMed] [Google Scholar]
- DeChatelet L. R., Campbell T. L., McCall C. E., Shirley P. S., Swendsen C. L. Oxidation of 2-deoxyglucose by human polymorphonuclear leukocytes. Inflammation. 1980 Sep;4(3):249–259. doi: 10.1007/BF00915026. [DOI] [PubMed] [Google Scholar]
- Emerit I., Keck M., Levy A., Feingold J., Michelson A. M. Activated oxygen species at the origin of chromosome breakage and sister-chromatid exchanges. Mutat Res. 1982 Feb;103(2):165–172. doi: 10.1016/0165-7992(82)90024-0. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry. 1975 Dec 2;14(24):5294–5299. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R., Troll W., Little J. B. Role of free radicals in the initiation and promotion of radiation transformation in vitro. Carcinogenesis. 1984 Oct;5(10):1213–1218. doi: 10.1093/carcin/5.10.1213. [DOI] [PubMed] [Google Scholar]
- Kensler T. W., Trush M. A. Role of oxygen radicals in tumor promotion. Environ Mutagen. 1984;6(4):593–616. doi: 10.1002/em.2860060412. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Fishman M. C., Karnovsky M. J. Ionophore A23187 disrupts membrane structure by modifying protein-lipid interactions. Nature. 1979 Sep 6;281(5726):82–83. doi: 10.1038/281082a0. [DOI] [PubMed] [Google Scholar]
- McWilliams R. S., Cross W. G., Kaplan J. G., Birnboim H. C. Rapid rejoining of DNA strand breaks in resting human lymphocytes after irradiation by low doses of 60Co gamma rays or 14.6-MeV neutrons. Radiat Res. 1983 Jun;94(3):499–507. [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Nagasawa H., Little J. B. Factors influencing the induction of sister chromatid exchanges in mammalian cells by 12-O-tetradecanoyl-phorbol-13-acetate. Carcinogenesis. 1981;2(7):601–607. doi: 10.1093/carcin/2.7.601. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Colburn N. H., Gindhart T. D. Role of reactive oxygen in tumor promotion: implication of superoxide anion in promotion of neoplastic transformation in JB-6 cells by TPA. Carcinogenesis. 1985 Feb;6(2):229–235. doi: 10.1093/carcin/6.2.229. [DOI] [PubMed] [Google Scholar]
- Nelson E. M., Tewey K. M., Liu L. F. Mechanism of antitumor drug action: poisoning of mammalian DNA topoisomerase II on DNA by 4'-(9-acridinylamino)-methanesulfon-m-anisidide. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1361–1365. doi: 10.1073/pnas.81.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburger P. E., Chovaniec M. E., Cohen H. J. Activity and activation of the granulocyte superoxide-generating system. Blood. 1980 Jan;55(1):85–92. [PubMed] [Google Scholar]
- Potmesil M., Israel M., Silber R. Two mechanisms of adriamycin-DNA interaction in L1210 cells. Biochem Pharmacol. 1984 Oct 15;33(20):3137–3142. doi: 10.1016/0006-2952(84)90069-8. [DOI] [PubMed] [Google Scholar]
- Robinson J. M., Badwey J. A., Karnovsky M. L., Karnovsky M. J. Superoxide release by neutrophils: synergistic effects of a phorbol ester and a calcium ionophore. Biochem Biophys Res Commun. 1984 Jul 31;122(2):734–739. doi: 10.1016/s0006-291x(84)80095-9. [DOI] [PubMed] [Google Scholar]
- Tewey K. M., Rowe T. C., Yang L., Halligan B. D., Liu L. F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984 Oct 26;226(4673):466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- Troll W., Frenkel K., Wiesner R. Protease inhibitors as anticarcinogens. J Natl Cancer Inst. 1984 Dec;73(6):1245–1250. [PubMed] [Google Scholar]
- Weser U., Sellinger K. H., Lengfelder E., Werner W., Strähle J. Structure of Cu2(indomethacin)4 and the reaction with superoxide in aprotic systems. Biochim Biophys Acta. 1980 Aug 13;631(2):232–245. doi: 10.1016/0304-4165(80)90298-6. [DOI] [PubMed] [Google Scholar]
- Young S. P., Gomperts B. D. Mobile carrier ionophores for Fe(II). Biochim Biophys Acta. 1977 Sep 19;469(3):281–291. doi: 10.1016/0005-2736(77)90164-x. [DOI] [PubMed] [Google Scholar]
- Zabos P., Kyner D., Mendelsohn N., Schreiber C., Waxman S., Christman J., Acs G. Catabolism of 2-deoxyglucose by phagocytic leukocytes in the presence of 12-O-tetradecanoyl phorbol-13-acetate. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5422–5426. doi: 10.1073/pnas.75.11.5422. [DOI] [PMC free article] [PubMed] [Google Scholar]



