Abstract
To determine the effects of heat-killed VSL#3 (B. breve, B. longum and B. infantis; L. plantarum, L. bulgaricus, L. casei and L. acidophilus; S. salivarius subsp. thermophilus) therapy in the dextran sulfate sodium (DSS)-induced acute experimental colitis in rats. Acute experimental colitis was induced in rats by 5% DSS and freely drink for seven days. Beginning on Day 8, rats underwent gavage once daily for seven days with heat-killed probiotic VSL#3 (0.6 g/kg/day), colonic damage was evaluated histologically and biochemically seven days after gavage. Expression of inflammatory related mediators (STAT3, P-STAT3) and cytokines (IL-6, IL-23, TGFβ) in colonic tissue were detected. The results revealed that heat-killed and live VSL#3 have identical anti-inflammatory properties by the assessed DAI (disease activity index), colon length, histological tissue and MPO activity. Heat-killed and live VSL#3 results in reduced IL-6, IL-23, TGFβ, STAT3 and P-STAT3 expression in colonic tissue. Heat-killed and live VSL#3 have showed the similar anti-inflammatory activity by inhibiting IL-6/STAT3 pathway in the DSS-induced acute experimental colitis in rats.
Keywords: probiotic, ulcerative colitis, inflammatory bowel disease, experimental colitis, IL-6/STAT3
1. Introduction
Inflammatory bowel disease (IBD) is a set of chronic recurrent diseases, including ulcerative colitis (UC) and Crohn’s disease (CD). The exact pathogenic mechanism of IBD remains unclear but several possible mechanisms have been clarified. It has been shown that genetic factors, environmental factors, intestinal flora disturbance and immune dysregulation may involve in pathogenesis of IBD. More than four million people worldwide suffer from IBD. Currently, medical therapies for IBD include 5-aminosalicylic acid drugs, methotrexate, immunomodulators and biological therapies (mainly anti-tumor necrosis factor). The high cost, multiple adverse effects and limited effect prompt research and development of new treatment options.
Intestinal dysbacteriosis caused by bacterial invasion and changes in protective factors are involved in the pathogenesis of IBD. Changes in intestinal flora have been observed in dextran sulfate sodium (DSS)-induced acute experimental colitis, with the primary changes being increases in Bacteroides and Clostridia, suggesting that intestinal bacteria are involved in colitis in rats [1]. Moreover, transgenic or knockout methods result in immunodeficient animal models of IBD [2,3], however, in a sterile gut environment, IBD cannot develop in these models, nor can intestinal inflammation recur, suggesting that the composition of gut microbiota is necessary for the pathogenesis of IBD. However, many studies have shown that not all probiotic exert anti-inflammatory effects in rat model of colitis. Moreover, a feces enema was shown to ameliorate intestinal inflammation [4,5], and antibiotics have some beneficial effects in the treatment of IBD [6,7]. These relationships between IBD and intestinal bacteria suggest that normalizing intestinal microflora may be effective in the treatment of patients with IBD.
Probiotics are live microorganisms that provide beneficial effects to the host, and some studies demonstrated that probiotics ameliorate symptoms of IBD and relieve antibiotic-associate diarrhea. Moreover, probiotic VSL#3 was verified on successful induction and maintenance of the remission of ulcerative colitis. In addition to living bacteria, gamma-ray irradiated bacteria can play a useful role in experimental colitis. Similarly, extracted bacterial DNA and even the culture medium of bacteria can have the same effects, suggesting that the activity of probiotics may be mediated by their DNA [8]. However, the mechanisms by which heat-killed VSL#3 provoke beneficial effects are not yet fully understood. Moreover, VSL#3 treatment is not always effective for each patient of the intestinal inflammation, mainly because the efficacy of live probiotic may be influenced by the number probiotic that reach the intestinal lumen alive. In addition, the number of live probiotic may vary from one experiment to the next. Heat-killed probiotics can achieve stable effects because these are not required to colonize and maintain activity in the intestine. Therefore, we used heat-killed VSL#3 rather than live VSL#3 in our experiments.
To explore the effects of heat-killed VSL#3 on regular intestinal mucosal internal environment, we observed the level of the expression of IL-6/STAT3 trans-signaling related cytokines in large intestine from rats with colitis induced by dextran sulfate sodium. IL-6/STAT3 trans-signaling was shown to be activated in the intestinal mucosa of an IBD rat model, suggesting that signal transduction pathways play an important role in the pathogenesis of UC. Blocking this pathway may be useful in the treatment of UC. This study may provide a new strategy for the treatment of UC.
2. Results
2.1. Disease Activity Index, Colon Length, Histopathology and Score of Histopathological Lesions
DAI, colon length and histological score are shown in Table 1. In the DSS group, DAI decreased compared with those of mesalazine group, VSL#3 and heat-killed VSL#3 group. The DAIs of mesalazine and VSL#3 group was similar to heat-killed VSL#3 group (p > 0.05). In the live and heat-killed VSL#3 group, colon lengths were longer than those of the DSS group (p < 0.05) and were similar to mesalazine group (p > 0.05).
Table 1.
The effect of each group on DAI, colon length and histopathological score in DSS-induced acute experimental colitis.
| Group | DAI | Colon length | Histopathological score |
|---|---|---|---|
| Control | 0.12 ± 0.5 bc | 19.09 ± 0.41 bc | 0 bc |
| DSS | 7.75 ± 0.75 ac | 15.62 ± 0.39 ac | 8.50 ± 0.93 ac |
| DSS + normal saline | 7.12 ± 0.64 ac | 15.59 ± 0.41 ac | 8.75 ± 0.71 ac |
| Mesalazine | 6.00 ± 0.93 ab | 17.38 ± 0.47 ab | 6.38 ± 0.74 ab |
| VSL#3 | 6.25 ± 0.71 ab | 17.05 ± 0.33 ab | 6.50 ± 0.76 ab |
| Heat-killed VSL#3 | 6.12 ± 0.84 ab | 16.95 ± 0.47 ab | 6.38 ± 0.52 ab |
| Mesalazine + VSL#3 | 5.00 ± 0.76 abc | 18.05 ± 0.58 abc | 4.88 ± 0.84 abc |
p < 0.05 compared with the control group;
p < 0.05 compared with the model group;
p < 0.05 compared with the mesalazine group.
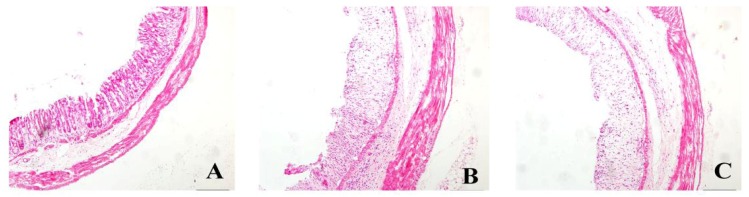
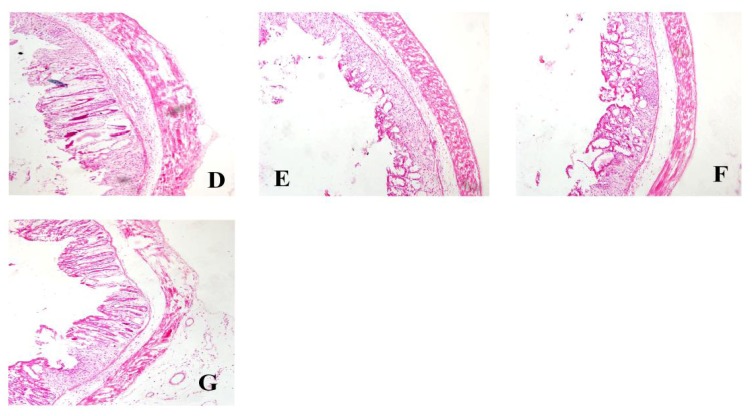
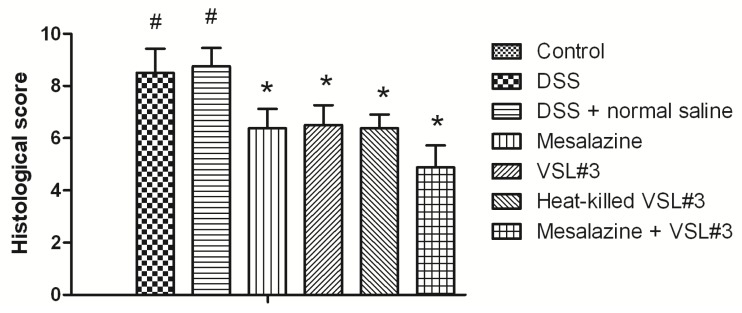
Histopathologic changes were assessed by HE stain (Figure 1). Colonic membrane structure was intact in the control group. Extensive lesions were observed in DSS group, including mucosa destruction, crypt destruction and glands separation. mesalazine group, VSL#3 group, heat-killed VSL#3 group and VSL#3 + mesalazine group had significantly less histological damage compared with DSS group. Heat-killed VSL#3 group have reduced mucosal injury in experimental colitis. There was no significant difference between heat-killed VSL#3 group and mesalazine group (p > 0.05) (Figure 2).
Figure 1.
HE staining of rat colonic tissue samples (×10) from the (A) control group and (B–G) rats treated with DSS and (C) normal saline; (D) mesalazine; (E) VSL#3; (F) heat-killed VSL#3; and (G) mesalazine plus VSL#3.
Figure 2.
Histology injury score (# p < 0.05 versus control group; * p < 0.05 versus DSS group).
2.2. MPO Activity
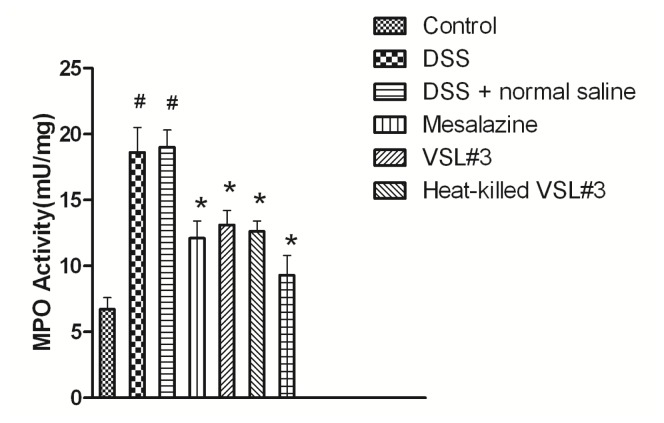
We assessed leukocyte infiltration in colonic tissue by determining the levels of MPO. The results of MPO are shown in Figure 3. MPO activity was significantly increased in the DSS group (18.6 ± 1.9 mU/mg) compared with the control group (6.7 ± 0.9 mU/mg) (p < 0.05). The MPO activity was significantly decreased by the mesalazine group (12.1 ± 1.3 mU/mg), VSL#3 group (13.1 ± 1.1 mU/mg), heat-killed VSL#3 group (12.6 ± 0.8 mU/mg) and VSL#3 + mesalazine group (9.3 ± 1.5 mU/mg) compared with DSS group (p < 0.05). Compared with mesalazine and VSL#3 group, MPO activity was similar to heat-killed VSL#3 group (p > 0.05).
Figure 3.
MPO activity of rats in each group (# p < 0.05 versus control group; * p < 0.05 versus DSS group).
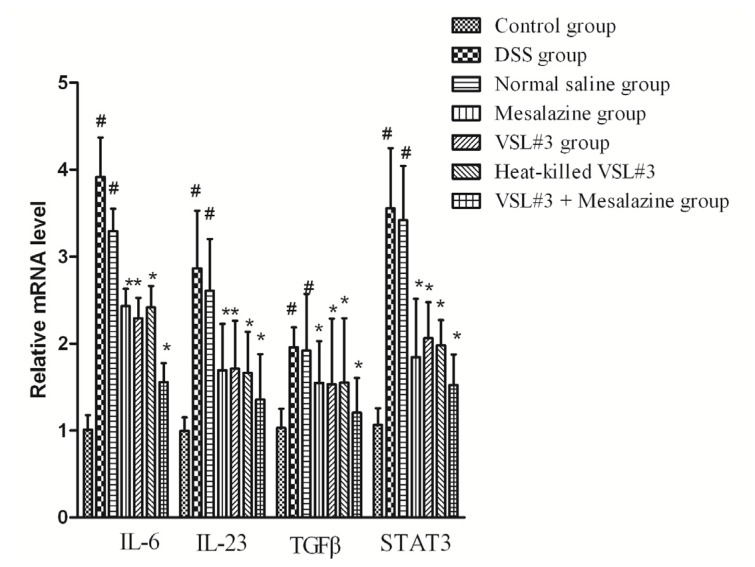
2.3. Expression of IL-6, IL-23, TGF-β, and STAT3 mRNA in Colonic Tissue
The mRNA expression levels of IL-6, IL-23, TGF-β, and STAT3 were examined using real-time PCR. The mRNA levels of these genes in the DSS group were significantly higher than in the control group (p < 0.05). Heat-killed VSL#3 group significantly reduced the mRNA levels in DSS-induced colons compared with the DSS group (p < 0.05). The mRNA levels of these genes in the heat-killed VSL#3 group were no significant differences in the mesalazine and VSL#3 group (p > 0.05) (Figure 4).
Figure 4.
Relative expression of IL-6, IL-23, TGF-β, and STAT3 mRNA in groups of rats. Expression was assessed by real-time RT-PCR. Groups of rats are defined in the text (# p < 0.05 versus control group; * p < 0.05 versus DSS group).
2.4. Western-Blot Analysis
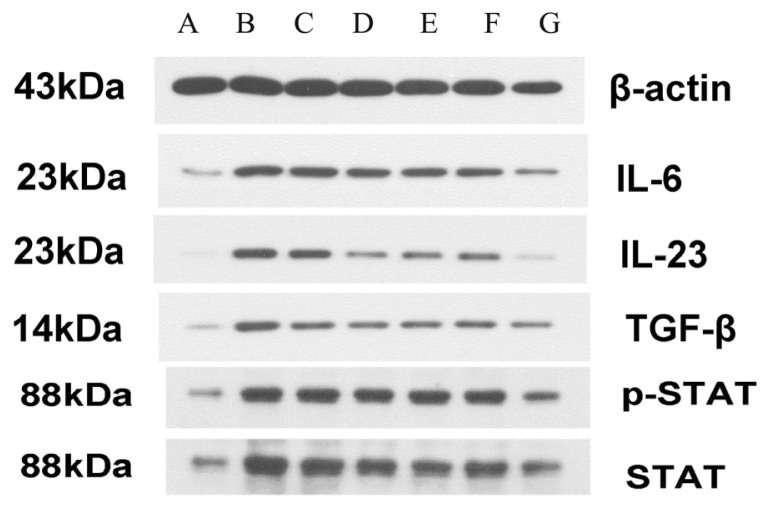
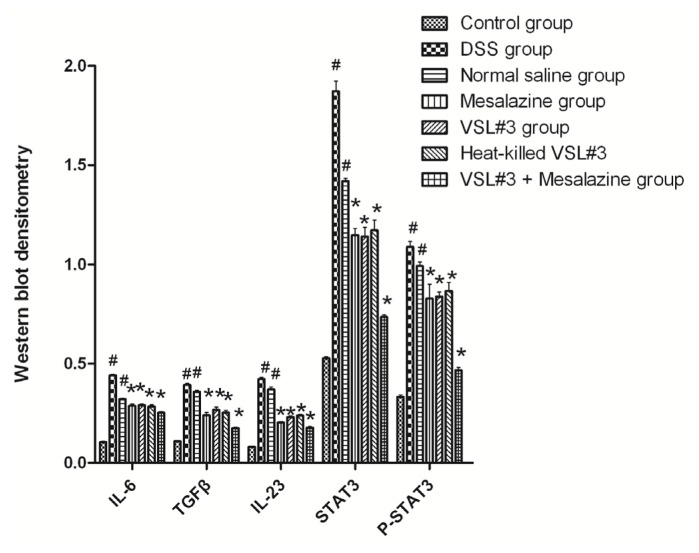
We examined the effect of probiotic VSL#3 and heat-killed VSL#3 on the protein expression of IL-6, IL-23, TGF-β, STAT3 and P-STAT3 proteins in the colons of rats of each group by Western-blot. In contrast to control group, the protein expression of IL-6, IL-23, TGF-β, STAT3 and P-STAT3 were significant increased in DSS group (p < 0.05). In contrast to DSS group, the protein of expression of heat-killed VSL#3 group were significant decreased (p < 0.05) and were similar to mesalazine and VSL#3 group (p > 0.05) (Figures 5 and 6).
Figure 5.
Western blots of IL-6, IL-23, TGF-β, STAT3, and P-STAT3 in each group (Lane A control group; Lane B DSS group; Lane C placebo group; Lane D Mesalazine group; Lane E VSL#3 group; Lane F Heat-killed VSL#3 group; Lane G VSL#3 + Mesalazine group).
Figure 6.
Western blot densitometry of the proteins IL-6, IL-23, TGF-β, STAT3, and P-STAT3 in colon tissue samples from groups of rats (# p < 0.05 versus control group; * p < 0.05 versus DSS group.
3. Discussion
Many chemical compositions have been used to induce experimental colitis, such as TNBS [9], acetic acid [10], OXZ [11] and DSS [12]. DSS-induced colitis is widely used to evaluate the effects of drugs because of its similarity to UC [13], and low mortality [14]. Therefore, DSS-induced colitis is adopted in our study.
In this study, we observed the effects of heat-killed VSL#3 on acute experimental colitis induced by DSS. Our results showed that heat-killed VSL#3 could reduce the colon injury, elevated shorter colon length, decrease DAI and MPO activity, as well as expression of IL-6, TGF-β, STAT3 and P-STAT3. Live VSL#3 are able to maintain the intestinal homeostasis as well as to ameliorate intestinal disorders. However, live VSL#3 must be colonize the intestinal lumen and maintain their activities when exerting its physiological functions, while heat-killed VSL#3 requires no colonization in the intestinal lumen. Therefore, heat-killed VSL#3 are hoped to be effective even though the intestinal condition is not conducive to colonizing and maintaining their activities.
Madsen et al. [15] have reported that oral administration of VSL#3 was effective as primary therapy in IL-10 gene-deficient mice, and had a direct effect on epithelial barrier function. Rachmilewitz et al. [8] have reported that the administration of heat-killed VSL#3 (100 °C for 30 min) had no effect on the severity of DSS induced colitis and this results are in contrast with ours (121 °C for 20 min). A heat-killed may cause structural or chemical change in the composition of immunomodulatory substance in VSL#3 which may lead to changes in the composition and function of cytokines. The different temperature and period may result in different results. Furthermore, strain of animal, dose of VSL#3, severity of colitis may also lead to different results.
Oxidative stress is believed to play an important role in the pathogenesis of colitis-related intestinal tissue injury [16]; MPO is a marker of oxidative stress produced mainly by polymorphonuclear leucocytes and is associated with the severity of colitis. The assessment of MPO activity is well established for evaluating intestinal inflammation [17,18]. Therefore, we assessed leukocyte infiltration in colonic tissue by detecting the levels of MPO. In our results, MPO activity was significantly decreased in heat-killed VSL#3 group compared with the DSS group. Therefore, heat-killed VSL#3 suppressed inflammation induced by DSS by suppressing the MPO-mediated activation of inflammatory cells.
The hyper-activation of immune cells involved in the pathogenesis of ulcerative colitis, known to produce high levels of pro-inflammatory cytokines such as IL-6. IL-6 is a multifunctional cytokines that plays an important role in the initiation and continuation of mucosal inflammation [19,20].
The study suggested that an increase serum-6 during active IBD [21]. Moreover, RT-PCR analysis showed that IL-6 mRNA levels are increased in active IBD patients [22]. In our study, IL-6 levels were associated with the severity of inflammation. IL-6 levels significantly increased in the DSS group. Compared with the DSS group, IL-6 levels significantly decreased in heat-killed VSL#3 group. Anti-IL-6 was proposed as anti-inflammatory agent in IBD has been reported [23]. The results of our study suggest the heat-killed VSL#3 decreased the expression of pro-inflammatory cytokines IL-6.
The IL-23 pathway has demonstrated significant advances in the UC and plays a key role in mediating intestinal inflammation, host defense and autoimmunity. IL-23 comes from a variety of cells during intestinal inflammation, including monocyte-derived cells [24]. Moreover, transgenic expression of IL-23 leads to severe intestinal inflammation [25]. IL-23 blockade or deficiency protects from intestinal inflammatory disease [26,27], including human IBD [28]. The association of IL-23 receptor mutations with CD in a genome-wide association study has suggested that IL-23 is a key role in human IBD [29]. In our study, IL-23 levels were associated with the severity of inflammation. IL-23 levels significantly decreased in heat-killed VSL#3 group compared with the DSS group. Anti-IL-23 was proposed as anti-inflammatory agent in IBD has been reported [23]. The current study has shown that heat-killed VSL#3 decrease the expression of IL-23.
TGFβ is a pleiotropic cytokine with important functions for the maintenance of immune balance. The study showed that TGFβ is very important to control T Cell periphery [30]. Recent investigations have demonstrated that the role of TGFβ in suppressing of T cell mediated autoimmune inflammation [31,32]. However, TGFβ and IL-6 can drive the initial differentiation of IL-17A and lead to colon inflammation [33]. Therefore, TGFβ can play both anti- and pro-inflammatory roles in the immune system. In our study, TGFβ levels were associated with the severity of colon inflammation. TGFβ levels significantly increased in the DSS group. TGFβ levels significantly decreased in VSL#3 group, heat-killed VSL#3 and VSL#3 + mesalazine group compared with the DSS group. TGFβ was proposed as pro-inflammatory agent in our experiment and the exact mechanism of its remains unclear.
Recent studied have revealed dextran sulfate sodium-induced colitis was mild in mice lacking STAT3 in macrophages and gut epithelial cells [34]. Moreover, the role of STAT3 in IBD has been documented by human IBD studies [35] and animal models of colitis [36]. Mudter et al. showed that an increase amount of STAT3 protein appeared in IBD compared to control cell [37]. Furthermore, higher expression of phosphorylated STAT3 was associated with the disease activity in animal models of colitis as well as in IBD patients [36].
The results of our study indicated STAT3 and phosphorylated STAT3 levels were associated with the severity of inflammation. STAT3 and phosphorylated STAT3 levels significantly increased in the DSS group. By contrast, STAT3 and phosphorylated STAT3 levels significantly decreased in heat-killed VSL#3 group. The results of our study suggest the therapeutic potential of heat-killed VSL#3 with increase STAT3 and phosphorylated STAT3 levels. The therapeutic effects of heat-killed VSL#3 on colitis may due to its effects on IL-6/STAT3 pathway. Furthermore, some possible mechanisms of intestinal protection by probiotics have been proposed, including the up-regulation of tight junction proteins ZO-1 expression [38], activated the p38MAPK pathway, regulated the production of proinammatory cytokines, improved the barrier function of the intestinal epithelia in the presence of oxidant stress by heat shock protein [39], increased production of IgA [40] and inhibited the growth of pathogenic bacteria as antibiotic [41].
Our study raises the following questions: (i) is heat-killed VSL#3 effective on other drug induced colitis, such as TNBS and OXZ-induced colitis? (ii) is heat-killed VSL#3 effective on other intestinal diseases, such as irritable bowel syndrome? (iii) what are the specific mechanisms of heat-killed VSL#3 when exerting its physiological functions? and (iv) are other probiotic species also efficacious? Further studies are needed to elaborate these questions.
4. Materials and Methods
4.1. Animals
Male SD rats (Eight-week-old), weighing 180 ± 10 g, were purchased from Experimental Animal Center of China Medical University (Shenyang, China), rats were housed in the SPF laboratory. The rats were allowed to adapt to their environment for 1 week, with temperature maintained at 21 ± 1 °C, with 12 h of light (08:00–20:00) and 12h of dark (20:00–08:00) per day and 60%–80% relative humidity. All animal experiments confirmed to the animal protection laws in China and were approved by the China Medical University Animals Committee (Shenyang, China).
4.2. Experimental Protocol
The VSL#3 mixture combines 8 strains of lactic acid producing bacteria, including three strains of Bifidobacterium (B. breve, B. longum and B. infantis); four strains of Lactobacillus (L. plantarum, L. bulgaricus, L. casei and L. acidophilus) and one strain of Streptococcus (S. salivarius subsp. thermophilus). Each 2.5 g VSL#3 sachet contained 450 billion freeze-dried bacteria. Heat-killed VSL#3 was prepared by incubating VSL#3 at 121 °C for 20 min.
4.3. Induction of Acute DSS Colitis
Dextran sodium sulfate (DSS, Wako Pure Chemical Industries, Ltd, Osaka, Japan; molecular weight 5000 d), was dissolved in drinking water to a concentration of 5% DSS for 7 days and shifted to normal drinking water.
4.4. Experimental Protocol
Fifty-six SD rats were randomized into 7 groups (n = 8). Eight rats without pretreatment served as controls. In the DSS group, acute experimental colitis was induced as described above. In the placebo group, 200 μL normal saline was applied via gastric tube. In the live and heat-killed VSL#3 group, acute experimental colitis was induced as described above, eight rats were administrated with VSL#3 or heat-killed VSL#3 (0.6 g/kg/day) via gastric tube after induction of colitis. The mesalazine group was treated with 0.4 g/kg/day of mesalazine via gastric tube after induction of colitis. The VSL#3 + mesalazine group was treated with VSL#3 (0.6 g/kg/day) and mesalazine (0.4 g/kg/day) in the same manner. The dose of drug was according to body weight of rats at that time.
4.5. Sample Collection
All rats were injected with hydrated chloral and sacrificed after anesthesia. Their colons were washed with precooled saline, and a 0.5 cm portion of the terminal ileum was clipped, soaked in 4% formaldehyde, embedded in paraffin sections, stained with hematoxylin and eosin, and examined histologically. The remainder of each specimen was stored at −80 °C.
4.6. Assessment of Colitis
Colitis was evaluated by disease activity index (Table 2), with a modification by fecal property, fecal occult blood and body weight loss (the integral of each project sum up to a total DAI score). The length of the colon were measured and histological injury score of each colon was evaluated independently by two experienced pathologists (Table 3), with any differences settled by consultation with a third pathologist (the integral of each project sum up to a total histological injury score).
Table 2.
DAI score chart.
| Fecal Property | Fecal Occult Blood | Body Weight Decrease (%) | Integral |
|---|---|---|---|
| Normal | Normal | 0 | 0 |
| 1–5 | 1 | ||
| Relaxed | Positive Fecal Occult Blood | >5–10 | 2 |
| >10–15 | 3 | ||
| Loose stools | Naked Eye Fecal Occult Blood | >15 | 4 |
Normal stool, shaped stool; relaxed stool, pasty, unformed stools not attached to the anus; loose stools, unshaped stools attached to the anus.
Table 3.
Histology injury score chart.
| Integral | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Inflammation | None | Mild | Moderate | Severe | - |
| Mucosal damage | None | Mucous layer | Submucosa | Muscularis and serosa | - |
| Crypt damage | None | 1/3 | 2/3 | 100% | 100% with epithelium loss |
| Pathological change range | None | 0%–25% | 26%–50% | 51%–75% | 76%–100% |
4.7. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (PCR)
Total RNA was isolated from colonic tissue samples and subjected to the synthesis of cDNA. The individual cDNA species were amplified and PCR was performed by a real-time PCR system (ABI 7500, Foster, CA, USA). Primer sequences included those for mouse β-actin (5′-ACGTTGACAT CCGTAAAGAC-3′ and 5′-GAAGGTGGACAGTGAGGC-3′), IL-6 (5′-CGCAAAGCCAGAGT CATTC-3′ and 5′-TGGTCTTGGTCCTTAGCC-3′), IL-23 (5′-ACCTGCTGGACTCGGACAT-3′ and 5′-GGCGAGGCATCTGTTGAT-3′), STAT3 (5′-CAAGGGCTTCTCGTTCTG-3′ and 5′-GGGCTT TGTGCTTAGGAT-3′), and TGFβ (5′-CAATTCCTGGCGTTACCT-3′ and 5′-AAAGCCCTGTA TTCCGTC-3′). The amplification protocol consisted of an initial denaturation at 95 °C for 5 min, followed by 30 to 32 cycles of denaturation at 95 °C for 10 s, annealing at 56 °C for 20 s, and extension at 68 °C for 40 s, followed by a final extension at 68 °C for 5 min.
4.8. Western Blotting Analysis
In order to determine the levels of IL-6, IL-23, TGFβ, STAT3 and P-STAT3. Colorectal tissue were homogenized at 4 °C and supernatants after centrifugation at 14,000 g for 15 min, protein were subjected to SDS-PAGE before blotting onto a polyvinylidene difluoride membrane. The membrane was blocked with in a block buffer (5% skim milk) for 2 h at room temperature.
4.9. Statistical Analysis
All statistical analyses were performed using SPSS 16.0 software (SPSS Inc, Chicago, IL, USA). Data were expressed as mean ± standard deviation and compared by one-way ANOVA. A p-value < 0.05 was considered statistically significant.
5. Conclusions
In summary, our study showed that heat-killed VSL#3 ameliorate DSS-induced colitis in SD rats through its immunomodulatory activities. Heat-killed VSL#3 improved DAI, colon length and histological tissue injury in the DSS-induced acute experimental colitis. Moreover, heat-killed VSL#3 improved inflammation by suppressing MPO activation and decreasing IL-6 concentrations. Therefore, further studies are needed to elaborate the mechanisms of suppression of the IL-6/STAT3 pathway. Heat-killed VSL#3 may be a safer method than using live VSL#3 in some special conditions such as immunosuppressed individuals in future.
Acknowledgments
This study was funded by Science and Technology Program of Shenyang (No. f12-277-1-54).
Conflicts of Interest
The authors declared no conflict of interest.
References
- 1.Hans W., Scholmerich J., Gross V., Falk W. The role of the resident intestinal flora in acute and chronic dextran sulfate sodium-induced colitis in mice. Eur. J. Gastroenterol. Hepatol. 2000;12:267–273. doi: 10.1097/00042737-200012030-00002. [DOI] [PubMed] [Google Scholar]
- 2.Sellon R.K., Tonkonogy S., Schultz M., Dieleman L.A., Grenther W., Balish E., Rennick D.M., Sartor R.B. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taurog J.D., Richardson J.A., Croft J.T., Simmons W.A., Zhou M., Fernández-Sueiro J.L., Balish E., Hammer R.E. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shanahan F. Probiotics in inflammatory bowel disease-therapeutic rationale and role. Adv. Drug Deliv. Rev. 2004;56:809–818. doi: 10.1016/j.addr.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Fichera A., McCormack R., Rubin M.A., Hurst R.D., Michelassi F. Long-term outcome of surgically treated Crohn’s colitis: A prospective study. Dis. Colon Rectum. 2005;48:963–969. doi: 10.1007/s10350-004-0906-3. [DOI] [PubMed] [Google Scholar]
- 6.Ohkusa T., Nomura T., Terai T., Miwa H., Kobayashi O., Hojo M., Takei Y., Ogihara T., Hirai S., Okayasu I., et al. Effectiveness of antibiotic combination therapy in patients with active ulcerative colitis: A randomize, controlled Pilot trial with long-term follow-up. Scand. J. Gastroenterol. 2005;40:1334–1342. doi: 10.1080/00365520510023648. [DOI] [PubMed] [Google Scholar]
- 7.Gionchetti P., Rizzello F., Lammers K.M., Morselli C., Sollazzi L., Davies S., Tambasco R., Calabrese C., Campieri M. Antibiotics and probiotics in treatment of inflammatory bowel disease. World J. Gastroenterol. 2006;12:3306–3313. doi: 10.3748/wjg.v12.i21.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rachmilewitz D., Katakura K., Karmeli F., Hayashi T., Reinus C., Rudensky B., Akira S., Takeda K., Lee J., Takabayashi K., et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Cromer W.E., Ganta C.V., Patel M., Traylor J., Kevil C.G., Alexander J.S., Mathis J.M. VEGF-A isoform modulation in an preclinical TNBS model of ulcerative colitis: Protective effects of a VEGF164b therapy. J. Transl. Med. 2013;11:207. doi: 10.1186/1479-5876-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Rejaie S.S., Abuohashish H.M., Al-Enazi M.M., Al-Assaf A.H., Parmar M.Y., Ahmed M.M. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World. J. Gastroenterol. 2013;19:5633–5644. doi: 10.3748/wjg.v19.i34.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Z., Jiang Y., Yang Y., Shao J., Sun X., Chen J., Dong L., Zhang J. 3,3′-Diindolylmethane alleviates oxazolone-induced colitis through Th2/Th17 suppression and Treg induction. Mol. Immunol. 2013;53:335–344. doi: 10.1016/j.molimm.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Bishop J.L., Roberts M.E., Beer J.L., Huang M., Chehal M.K., Fan X., Fouser L.A., Ma H.L., Bacani J.T., Harder K.W. Lyn activity protects mice from DSS colitis and regulates the production of IL-22 from innate lymphoid cells. Mucosal. Immunol. 2013;18 doi: 10.1038/mi.2013.60. [DOI] [PubMed] [Google Scholar]
- 13.Gaudio E., Taddei G., Vetuschi A., Sferra R., Frieri G., Ricciardi G., Caprilli R. Dextran sulfate sodium (DSS) colitis in rats: Clinical, structural, and ultrastructural aspects. Dig. Dis. Sci. 1999;44:1458–1475. doi: 10.1023/a:1026620322859. [DOI] [PubMed] [Google Scholar]
- 14.Wirtz S., Neufert C., Weigmann B., Neurath M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 15.Madsen K., Cornish A., Soper P., McKaigney C., Jijon H., Yachimec C., Doyle J., Jewell L., de Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 16.Krawisz J.E., Sharon P., Stenson W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastro-Enterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- 17.Strober W., Fuss I.J., Blumberg R.S. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 18.Ramakers J.D., Verstege M.I., Thuijls G., Te Velde A.A., Mensink R.P., Plat J. The PPARgamma agonist rosiglitazone impairs colonic inflammation in mice with experimental colitis. J. Clin. Immunol. 2007;27:275–283. doi: 10.1007/s10875-007-9074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishimoto T. Interleukin-6: From basic science to medicine—40 years in immunology. Annu. Rev. Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 20.Atreya R., Neurath M.F. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin. Rev. Allergy Immunol. 2005;28:187–196. doi: 10.1385/CRIAI:28:3:187. [DOI] [PubMed] [Google Scholar]
- 21.Mitsuyama K., Sata M., Tanikawa K. Significance of interleukin-6 in patients with inflammatory bowel disease. Gastroenterol. Jpn. 1991;26:20–28. doi: 10.1007/BF02779504. [DOI] [PubMed] [Google Scholar]
- 22.Stevens C., Walz G., Singaram C., Lipman M.L., Zanker B., Muggia A., Antonioli D., Peppercorn M.A., Strom T.B. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig. Dis. Sci. 1992;37:818–826. doi: 10.1007/BF01300378. [DOI] [PubMed] [Google Scholar]
- 23.Waldner M.J., Neurath M.F. Novel cytokine-targeted therapies and intestinal inflammation. Curr. Opin. Pharmacol. 2009;9:702–707. doi: 10.1016/j.coph.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Kamada N., Hisamatsu T., Okamoto S., Sato T., Matsuoka K., Arai K., Nakai T., Hasegawa A., Inoue N., Watanabe N., et al. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J. Immunol. 2005;175:6900–6908. doi: 10.4049/jimmunol.175.10.6900. [DOI] [PubMed] [Google Scholar]
- 25.Wiekowski M.T., Leach M.W., Evans E.W., Sullivan L., Chen S.C., Vassileva G., Bazan J.F., Gorman D.M., Kastelein R.A., Narula S., et al. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J. Immunol. 2001;166:7563–7570. doi: 10.4049/jimmunol.166.12.7563. [DOI] [PubMed] [Google Scholar]
- 26.Elson C.O., Cong Y., Weaver C.T., Schoeb T.R., McClanahan T.K., Fick R.B., Kastelein R.A. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 27.Yen D., Cheung J., Scheerens H., Poulet F., McClanahan T., McKenzie B., Kleinschek M.A., Owyang A., Mattson J., Blumenschein W., et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt C., Giese T., Ludwig B., Mueller-Molaian I., Marth T., Zeuzem S., Meuer S.C., Stallmach A. Expression of interleukin-12- related cytokine transcripts in inflammatory bowel disease: Elevated interleukin-23p19 and interleukin-27p28 in Crohn’s disease but not in ulcerative colitis. Inflamm. Bowel. Dis. 2005;11:16–23. doi: 10.1097/00054725-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Duerr R.H., Taylor K.D., Brant S.R., Rioux J.D., Silverberg M.S., Daly M.J., Steinhart A.H., Abraham C., Regueiro M., Griffiths A., et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kehrl J.H., Wakefield L.M., Roberts A.B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M.B., Fauci A.S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J. Exp. Med. 1986;163:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marie J.C., Letterio J.J., Gavin M., Rudensky A.Y. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+ CD25+ regulatory T cells. J. Exp. Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piccirillo C.A., Letterio J.J., Thornton A.M., McHugh R.S., Mamura M., Mizuhara H., Shevach E.M. CD4(+) CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J. Exp. Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hata H., Sakaguchi N., Yoshitomi H., Iwakura Y., Sekikawa K., Azuma Y., Kanai C., Moriizumi E., Nomura T., Nakamura T., et al. Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J. Clin. Invest. 2004;114:582–588. doi: 10.1172/JCI21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alonzi T., Newton I.P., Bryce P.J., di Carlo E., Lattanzio G., Tripodi M., Musiani P., Poli V. Induced somatic inactivation of STAT3 in mice triggers the development of a fulminant form of enterocolitis. Cytokine. 2004;26:45–56. doi: 10.1016/j.cyto.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Musso A., Dentelli P., Carlino A., Chiusa L., Repici A., Sturm A., Fiocchi C., Rizzetto M., Pegoraro L., Sategna-Guidetti C., et al. Signal transducers and activators of transcription 3 signaling pathway: An essential mediator of inflammatorybowel disease and other forms of intestinal inflammation. Inflamm. Bowel Dis. 2005;11:91–98. doi: 10.1097/00054725-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki A., Hanada T., Mitsuyama K., Yoshida T., Kamizono S., Hoshino T., Kubo M., Yamashita A., Okabe M., Takeda K., et al. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J. Exp. Med. 2001;193:471–481. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mudter J., Weigmann B., Bartsch B., Kiesslich R., Strand D., Galle P.R., Lehr H.A., Schmidt J., Neurath M.F. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am. J. Gastroenterol. 2005;100:64–72. doi: 10.1111/j.1572-0241.2005.40615.x. [DOI] [PubMed] [Google Scholar]
- 38.Ukena S.N., Singh A., Dringenberg U., Engelhardt R., Seidler U., Hansen W., Bleich A., Bruder D., Franzke A., Rogler G., et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ueno N., Fujiya M., Segawa S., Nata T., Moriichi K., Tanabe H., Mizukami Y., Kobayashi N., Ito K., Kohgo Y. Heat-killed body of lactobacillus brevis SBC8803 ameliorates intestinal injury in a murine model of colitis by enhancing the intestinal barrier function. Inflamm. Bowel Dis. 2011;17:2235–2250. doi: 10.1002/ibd.21597. [DOI] [PubMed] [Google Scholar]
- 40.Malin M., Suomalainen H., Saxelin M., Isolauri E. Promotion of IgA immune response in patients with Crohn’s disease by oral bacteriotherapy with Lactobacillus GG. Ann. Nutr. Metab. 1996;40:137–145. doi: 10.1159/000177907. [DOI] [PubMed] [Google Scholar]
- 41.Cleveland J., Montville T.J., Nes I.F., Chikindas M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001;71:1–20. doi: 10.1016/s0168-1605(01)00560-8. [DOI] [PubMed] [Google Scholar]