Abstract
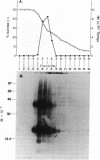
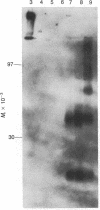
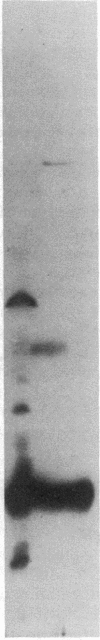
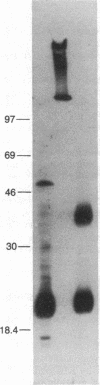
Hepatitis B surface antigen (HBsAg) has been extracted from yeast cells that produce HBsAg. These cells contain the gene for surface antigen carried on a plasmid that replicates in the cells. Analysis of the yeast-derived HBsAg by sucrose gradient centrifugation and by polyacrylamide gel electrophoresis shows that the antigen that is initially released from yeast cells is a high molecular weight aggregate of the fundamental Mr 25,000 subunit. Unlike HBsAg derived from human plasma, the yeast antigen is held together by noncovalent interactions and can be dissociated in 2% NaDodSO4 without the use of reducing agents. During in vitro purification of the yeast antigen, some disulfide bonds form spontaneously between the antigen subunits, resulting in a particle composed of a mixture of monomers and disulfide-bonded dimers. Treatment with 3 M thiocyanate converts the 20-nm particles into a fully disulfide-bonded form that is not disrupted in NaDodSO4 unless a reducing agent is added. This disulfide-bonded particle resembles the naturally occurring, plasma-derived surface antigen particle, and the in vitro formed particle has been used to prepare a vaccine for humans against hepatitis B virus infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aune T. M., Thomas E. L. Oxidation of protein sulfhydryls by products of peroxidase-catalyzed oxidation of thiocyanate ion. Biochemistry. 1978 Mar 21;17(6):1005–1010. doi: 10.1021/bi00599a010. [DOI] [PubMed] [Google Scholar]
- Boss M. A., Kenten J. H., Wood C. R., Emtage J. S. Assembly of functional antibodies from immunoglobulin heavy and light chains synthesised in E. coli. Nucleic Acids Res. 1984 May 11;12(9):3791–3806. doi: 10.1093/nar/12.9.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Burrell C. J., Mackay P., Greenaway P. J., Hofschneider P. H., Murray K. Expression in Escherichia coli of hepatitis B virus DNA sequences cloned in plasmid pBR322. Nature. 1979 May 3;279(5708):43–47. doi: 10.1038/279043a0. [DOI] [PubMed] [Google Scholar]
- Cabilly S., Riggs A. D., Pande H., Shively J. E., Holmes W. E., Rey M., Perry L. J., Wetzel R., Heyneker H. L. Generation of antibody activity from immunoglobulin polypeptide chains produced in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3273–3277. doi: 10.1073/pnas.81.11.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay P., Pourcel C., Louise A., Fritsch A., Tiollais P. Cloning in Escherichia coli and physical structure of hepatitis B virion DNA. Proc Natl Acad Sci U S A. 1979 May;76(5):2222–2226. doi: 10.1073/pnas.76.5.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham P. S. Removal of triton X-100 from aqueous solution using amberlite XAD-2. Anal Biochem. 1979 Jan 15;92(2):447–452. doi: 10.1016/0003-2697(79)90683-3. [DOI] [PubMed] [Google Scholar]
- GIVOL D., DELORENZO F., GOLDBERGER R. F., ANFINSEN C. B. DISULFIDE INTERCHANGE AND THE THREE-DIMENSIONAL STRUCTURE OF PROTEINS. Proc Natl Acad Sci U S A. 1965 Mar;53:676–684. doi: 10.1073/pnas.53.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Gerin J. L., Holland P. V., Purcell R. H. Australia antigen: large-scale purification from human serum and biochemical studies of its proteins. J Virol. 1971 May;7(5):569–576. doi: 10.1128/jvi.7.5.569-576.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleman M. R., McAleer W. J., Buynak E. B., McLean A. A. Quality and safety of human hepatitis B vaccine. Dev Biol Stand. 1983;54:3–12. [PubMed] [Google Scholar]
- Hitzeman R. A., Chen C. Y., Hagie F. E., Patzer E. J., Liu C. C., Estell D. A., Miller J. V., Yaffe A., Kleid D. G., Levinson A. D. Expression of hepatitis B virus surface antigen in yeast. Nucleic Acids Res. 1983 May 11;11(9):2745–2763. doi: 10.1093/nar/11.9.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janolino V. G., Swaisgood H. E. Isolation and characterization of sulfhydryl oxidase from bovine milk. J Biol Chem. 1975 Apr 10;250(7):2532–2538. [PubMed] [Google Scholar]
- Jilg W., Lorbeer B., Schmidt M., Wilske B., Zoulek G., Deinhardt F. Clinical evaluation of a recombinant hepatitis B vaccine. Lancet. 1984 Nov 24;2(8413):1174–1175. doi: 10.1016/s0140-6736(84)92740-5. [DOI] [PubMed] [Google Scholar]
- Katsoyannis P. G., Trakatellis A. C., Zalut C., Johnson S., Tometsko A., Schwartz G., Ginos J. Studies on the synthesis of insulin from natural and synthetic A and B chains. 3. Synthetic insulins. Biochemistry. 1967 Sep;6(9):2656–2668. doi: 10.1021/bi00861a003. [DOI] [PubMed] [Google Scholar]
- Kim C. Y., Bissell D. M. Stability of the lipid and protein of hepatitis-associated (Australia) antigen. J Infect Dis. 1971 May;123(5):470–476. doi: 10.1093/infdis/123.5.470. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McAleer W. J., Buynak E. B., Maigetter R. Z., Wampler D. E., Miller W. J., Hilleman M. R. Human hepatitis B vaccine from recombinant yeast. Nature. 1984 Jan 12;307(5947):178–180. doi: 10.1038/307178a0. [DOI] [PubMed] [Google Scholar]
- Mishiro S., Imai M., Takahashi K., Machida A., Gotanda T., Miyakawa Y., Mayumi M. A 49,000-dalton polypeptide bearing all antigenic determinants and full immunogenicity of 22-nm hepatitis B surface antigen particles. J Immunol. 1980 Apr;124(4):1589–1593. [PubMed] [Google Scholar]
- Peterson D. L., Roberts I. M., Vyas G. N. Partial amino acid sequence of two major component polypeptides of hepatitis B surface antigen. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1530–1534. doi: 10.1073/pnas.74.4.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillot J., Goueffon S., Keros R. G. Optimal conditions for elution of hepatitis B antigen after absorption onto colloidal silica. J Clin Microbiol. 1976 Sep;4(3):205–207. doi: 10.1128/jcm.4.3.205-207.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. A., Koshland M. E. Identification of a lymphocyte enzyme that catalyzes pentamer immunoglobulin M assembly. J Biol Chem. 1981 May 10;256(9):4633–4639. [PubMed] [Google Scholar]
- Roth R. A., Koshland M. E. Role of disulfide interchange enzyme in immunoglobulin synthesis. Biochemistry. 1981 Nov 10;20(23):6594–6599. doi: 10.1021/bi00526a012. [DOI] [PubMed] [Google Scholar]
- Sanchez Y., Ionescu-Matiu I., Melnick J. L., Dreesman G. R. Comparative studies of the immunogenic activity of hepatitis B surface antigen (HBsAg) and HBsAg polypeptides. J Med Virol. 1983;11(2):115–124. doi: 10.1002/jmv.1890110205. [DOI] [PubMed] [Google Scholar]
- Saxena V. P., Wetlaufer D. B. Formation of three-dimensional structure in proteins. I. Rapid nonenzymic reactivation of reduced lysozyme. Biochemistry. 1970 Dec 8;9(25):5015–5023. doi: 10.1021/bi00827a028. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., McLean A. A., West D. J., McAleer W. J., Miller W. J., Buynak E. B. Clinical evaluation in healthy adults of a hepatitis B vaccine made by recombinant DNA. JAMA. 1984 Jun 1;251(21):2812–2815. [PubMed] [Google Scholar]
- Steiner S., Huebner M. T., Dreesman G. R. Major polar lipids of hepatitis B antigen preparations: evidence for the presence of a glycosphingolipid. J Virol. 1974 Sep;14(3):572–577. doi: 10.1128/jvi.14.3.572-577.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukeno N., Shirachi R., Yamaguchi J., Ishida N. Reduction and reoxidation of Australia antigen: loss and reconstitution of particle structure and antigenicity. J Virol. 1972 Jan;9(1):182–183. doi: 10.1128/jvi.9.1.182-183.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmuness W., Stevens C. E., Harley E. J., Zang E. A., Oleszko W. R., William D. C., Sadovsky R., Morrison J. M., Kellner A. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980 Oct 9;303(15):833–841. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- Thornton J. M. Disulphide bridges in globular proteins. J Mol Biol. 1981 Sep 15;151(2):261–287. doi: 10.1016/0022-2836(81)90515-5. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Gray P., Quiroga M., Zaldivar J., Goodman H. M., Rutter W. J. Nucleotide sequence of the gene coding for the major protein of hepatitis B virus surface antigen. Nature. 1979 Aug 30;280(5725):815–819. doi: 10.1038/280815a0. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Medina A., Rutter W. J., Ammerer G., Hall B. D. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature. 1982 Jul 22;298(5872):347–350. doi: 10.1038/298347a0. [DOI] [PubMed] [Google Scholar]
- Vyas G. N., Williams E. W., Klaus G. G., Bond H. E. Hepatitis-associated Australia antigen. Protein, peptides and amine acid composition of purified antigen with its use in determining sensitivity of the hemagglutination test. J Immunol. 1972 Apr;108(4):1114–1118. [PubMed] [Google Scholar]
- Wetlaufer D. B., Saxena V. P., Ahmed A. K., Schaffer S. W., Pick P. W., Oh K. J., Peterson J. D. Protein thiol-disulfide interchange and interfacing with biological systems. Adv Exp Med Biol. 1977;86A:43–50. doi: 10.1007/978-1-4684-3282-4_3. [DOI] [PubMed] [Google Scholar]
- Wetzel R., Kleid D. G., Crea R., Heyneker H. L., Yansura D. G., Hirose T., Kraszewski A., Riggs A. D., Itakura K., Goeddel D. V. Expression in Escherichia coli of a chemically synthesized gene for a "mini-C" analog of human proinsulin. Gene. 1981 Dec;16(1-3):63–71. doi: 10.1016/0378-1119(81)90061-5. [DOI] [PubMed] [Google Scholar]
- Zurawski V. R., Jr, Foster J. F. The neutral transition and the environment of the sulfhydryl side chain of bovine plasma albumin. Biochemistry. 1974 Aug 13;13(17):3465–3471. doi: 10.1021/bi00714a007. [DOI] [PubMed] [Google Scholar]