Abstract
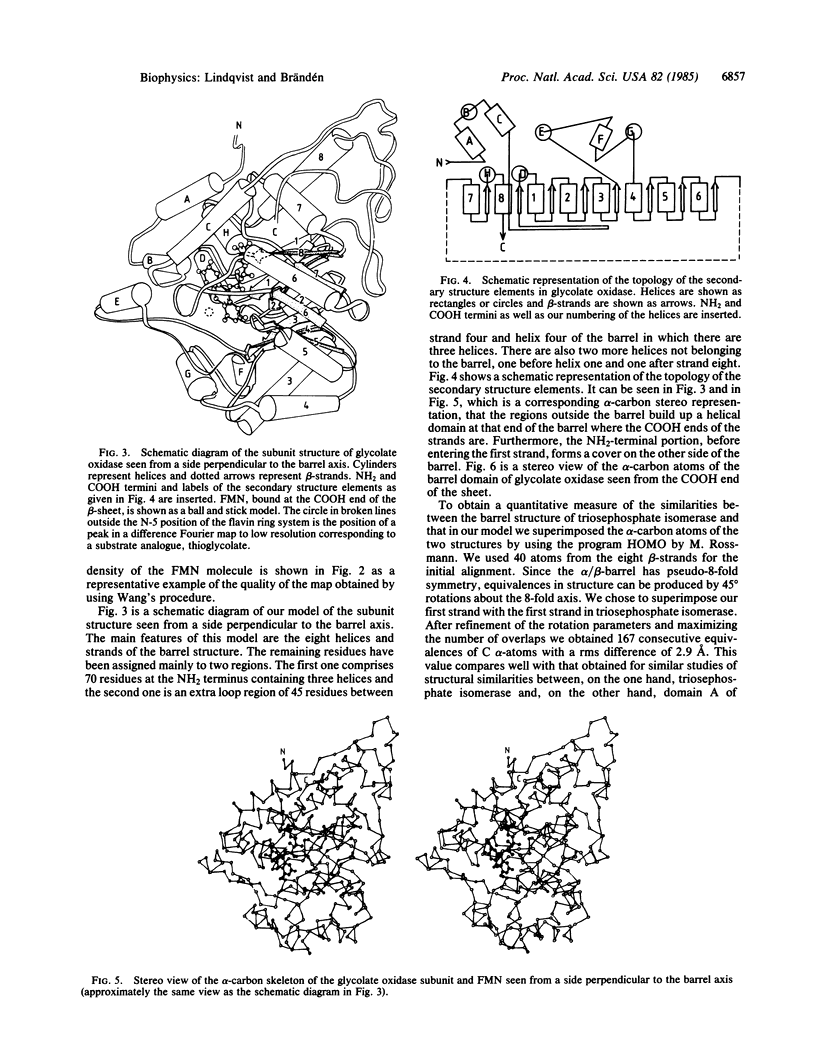
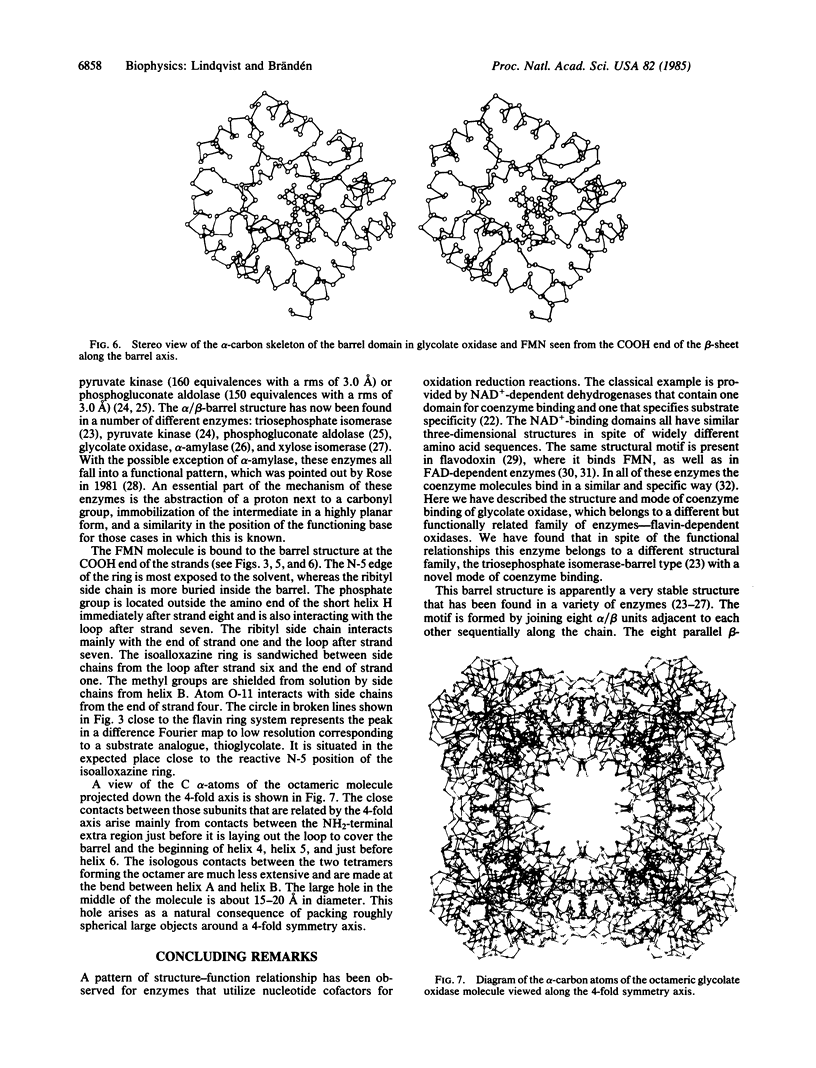
A high-resolution structure determination of glycolate oxidase from spinach is reported. X-ray data were collected on films at the synchrotron radiation source in Daresbury, England. The structure was solved by using two heavy-atom derivatives and a solvent-flattening procedure developed by B.-C. Wang. The subunit structure is essentially a structure of the eight-stranded α/β-barrel type first described for triosephosphate isomerase. In addition, there are 70 residues at the NH2 terminus and 45 residues between strand four and helix four of the barrel, which are arranged in a helical domain outside the COOH end of the parallel strands of the barrel. The active site is in a cleft between these domains with the coenzyme FMN essentially bound to the barrel and a substrate analogue, thioglycolate, bound to the helical domain. The molecule is octameric with 422 symmetry and has a 15- to 20-Å-wide hole in the middle.
Keywords: x-ray crystallography, flavoprotein, photorespiration
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson I., Brändén C. I. Large single crystals of spinach 1,5-bisphosphate carboxylase/oxygenase suitable for X-ray studies. J Mol Biol. 1984 Jan 25;172(3):363–366. doi: 10.1016/s0022-2836(84)80033-9. [DOI] [PubMed] [Google Scholar]
- Brändeén C. I. Relation between structure and function of alpha/beta-proteins. Q Rev Biophys. 1980 Aug;13(3):317–338. doi: 10.1017/s0033583500001712. [DOI] [PubMed] [Google Scholar]
- Burnett R. M., Darling G. D., Kendall D. S., LeQuesne M. E., Mayhew S. G., Smith W. W., Ludwig M. L. The structure of the oxidized form of clostridial flavodoxin at 1.9-A resolution. J Biol Chem. 1974 Jul 25;249(14):4383–4392. [PubMed] [Google Scholar]
- Carrell H. L., Rubin B. H., Hurley T. J., Glusker J. P. X-ray crystal structure of D-xylose isomerase at 4-A resolution. J Biol Chem. 1984 Mar 10;259(5):3230–3236. [PubMed] [Google Scholar]
- Lebioda L., Hatada M. H., Tulinsky A., Mavridis I. M. Comparison of the folding of 2-keto-3-deoxy-6-phosphogluconate aldolase, triosephosphate isomerase and pyruvate kinase. Implications in molecular evolution. J Mol Biol. 1982 Dec 5;162(2):445–458. doi: 10.1016/0022-2836(82)90537-x. [DOI] [PubMed] [Google Scholar]
- Liao L. L., Richardson K. E. The inhibition of oxalate biosynthesis in isolated perfused rat liver by DL-phenyllactate and n-heptanoate. Arch Biochem Biophys. 1973 Jan;154(1):68–75. doi: 10.1016/0003-9861(73)90035-0. [DOI] [PubMed] [Google Scholar]
- Lindqvist Y., Brändén C. I. Preliminary crystallographic data for glycolate oxidase from spinach. J Biol Chem. 1979 Aug 10;254(15):7403–7404. [PubMed] [Google Scholar]
- Lindqvist Y., Brändén C. I. Structure of glycolate oxidase from spinach at a resolution of 5.5 A. J Mol Biol. 1980 Oct 25;143(2):201–211. doi: 10.1016/0022-2836(80)90198-9. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Kusunoki M., Harada W., Tanaka N., Iga Y., Yasuoka N., Toda H., Narita K., Kakudo M. Molecular structure of taka-amylase A. I. Backbone chain folding at 3 A resolution. J Biochem. 1980 May;87(5):1555–1558. doi: 10.1093/oxfordjournals.jbchem.a132896. [DOI] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Phillips D. C., Sternberg M. J., Thornton J. M., Wilson I. A. An analysis of the structure of triose phosphate isomerase and its comparison with lactate dehydrogenase. J Mol Biol. 1978 Feb 25;119(2):329–351. doi: 10.1016/0022-2836(78)90440-0. [DOI] [PubMed] [Google Scholar]
- Randall W. C., Streeter K. B., Cresson E. L., Schwam H., Michelson S. R., Anderson P. S., Cragoe E. J., Jr, Williams H. W., Eichler E., Rooney C. S. Quantitative structure-activity relationships involving the inhibition of glycolic acid oxidase by derivatives of glycolic and glyoxylic acids. J Med Chem. 1979 Jun;22(6):608–614. doi: 10.1021/jm00192a002. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Rose I. A. Chemistry of proton abstraction by glycolytic enzymes (aldolase, isomerases and pyruvate kinase). Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):131–143. doi: 10.1098/rstb.1981.0067. [DOI] [PubMed] [Google Scholar]
- Schneider G., Brändén C. I., Lorimer G. Preliminary X-ray diffraction study of ribulose-1,5-bisphosphate carboxylase from Rhodospirillum rubrum. J Mol Biol. 1984 May 5;175(1):99–102. doi: 10.1016/0022-2836(84)90450-9. [DOI] [PubMed] [Google Scholar]
- Schulz G. E., Schirmer R. H., Sachsenheimer W., Pai E. F. The structure of the flavoenzyme glutathione reductase. Nature. 1978 May 11;273(5658):120–124. doi: 10.1038/273120a0. [DOI] [PubMed] [Google Scholar]
- Schuman M., Massey V. Effect of anions on the catalytic activity of pig liver glycolic acid oxidase. Biochim Biophys Acta. 1971 Mar 10;227(3):521–537. doi: 10.1016/0005-2744(71)90004-0. [DOI] [PubMed] [Google Scholar]
- Schwam H., Michelson S., Randall W. C., Sondey J. M., Hirschmann R. Purification and characterization of human liver glycolate oxidase. Molecular weight, subunit, and kinetic properties. Biochemistry. 1979 Jun 26;18(13):2828–2833. doi: 10.1021/bi00580a023. [DOI] [PubMed] [Google Scholar]
- Stuart D. I., Levine M., Muirhead H., Stammers D. K. Crystal structure of cat muscle pyruvate kinase at a resolution of 2.6 A. J Mol Biol. 1979 Oct 15;134(1):109–142. doi: 10.1016/0022-2836(79)90416-9. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., de Jong R. J., Kalk K. H., Hol W. G., Drenth J. Crystal structure of p-hydroxybenzoate hydroxylase. J Mol Biol. 1979 Jun 15;131(1):55–73. doi: 10.1016/0022-2836(79)90301-2. [DOI] [PubMed] [Google Scholar]



