Abstract
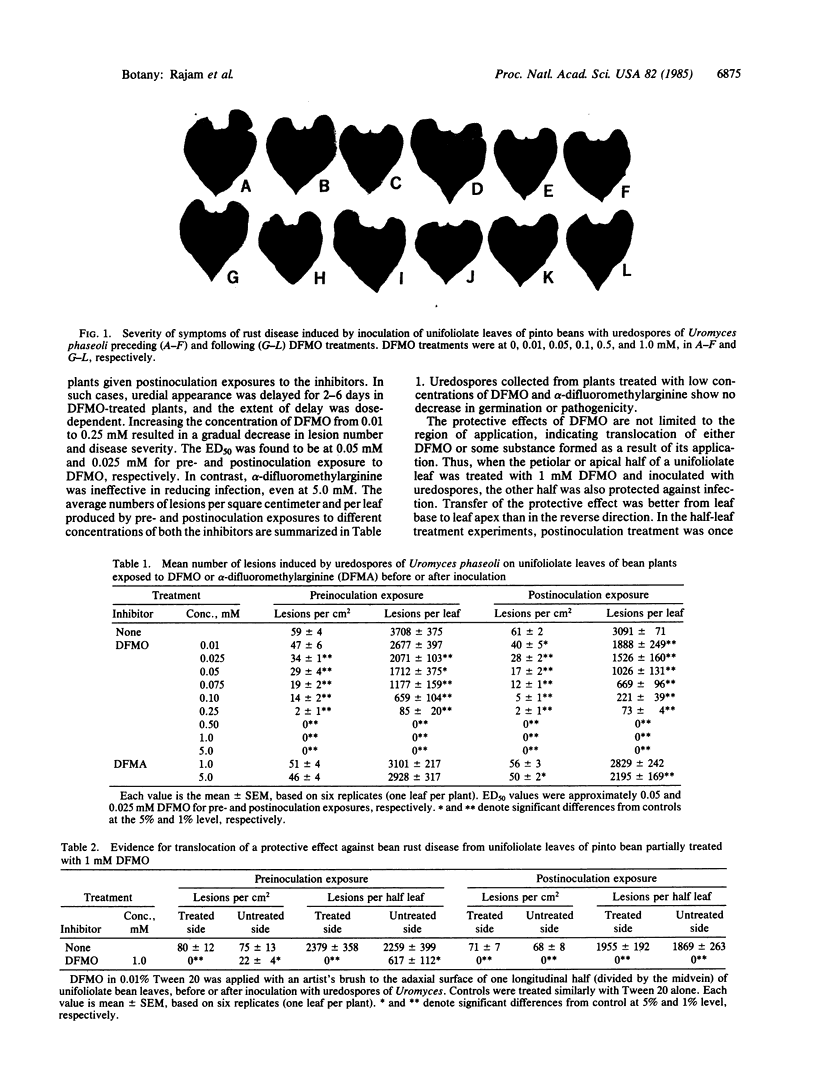
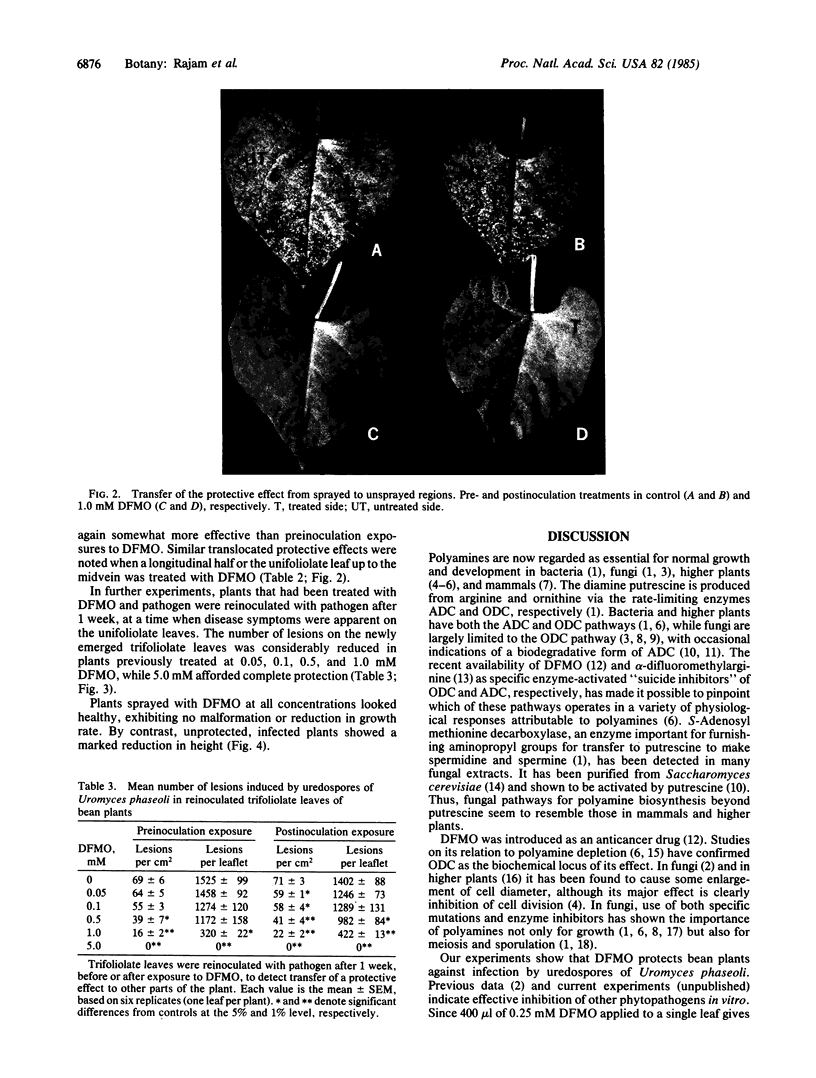
DL-alpha-Difluoromethylornithine (DFMO), an inhibitor of the polyamine biosynthetic enzyme ornithine decarboxylase (EC 4.1.1.17), strongly retards the growth of several species of phytopathogenic fungi in vitro. Such inhibition can be completely reversed by putrescine or spermidine, confirming the essentiality of polyamines for growth of fungal hyphae. We now show that DFMO can protect bean plants (Phaseolus vulgaris Linnaeus cv. Pinto) against infection by uredospores of the bean rust fungus, Uromyces phaseoli Linnaeus, race O. Unifoliolate leaves of 10-day-old greenhouse-grown seedlings were sprayed with 400 microliter per leaf of DFMO at various concentrations in 0.01% Tween 20 at pH 7.0 before or after inoculation with uredospores of Uromyces. After 16 hr in darkness in dew chambers to facilitate spore germination, plants were transferred to the greenhouse, arranged randomly, and examined for local lesions 7 days later. All concentrations of DFMO 0.50 mM or higher gave complete protection against the pathogen; at lower concentrations, postinoculation treatments with DFMO were generally more effective than preinoculation. The appearance of lesions on plants treated with lower concentrations of DFMO was retarded 2-6 days. DFMO also confers protection on unsprayed parts of treated plants, indicating the translocation of some protective effect from sprayed areas. DL-alpha-Difluoromethylarginine, an analogous inhibitor of arginine decarboxylase (EC 4.1.1.19), which is the rate-limiting enzyme in an alternative pathway for polyamine biosynthesis in higher plants, confers no protection even at 5 mM. This emphasizes ornithine decarboxylase as the biochemical locus of choice for the prevention of plant diseases by inhibiting polyamine metabolism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brawley J. V., Ferro A. J. Polyamine biosynthesis during germination of yeast ascospores. J Bacteriol. 1979 Nov;140(2):649–654. doi: 10.1128/jb.140.2.649-654.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E., Arad S. M., Heimer Y. M., Mizrahi Y. Participation of ornithine decarboxylase in early stages of tomato fruit development. Plant Physiol. 1982 Aug;70(2):540–543. doi: 10.1104/pp.70.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Isolation and characterization of Saccharomyces cerevisiae mutants deficient in S-adenosylmethionine decarboxylase, spermidine, and spermine. J Bacteriol. 1978 Apr;134(1):208–213. doi: 10.1128/jb.134.1.208-213.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio A., McCann P. P., Bey P. DL-alpha-(Difluoromethyl)arginine: a potent enzyme-activated irreversible inhibitor of bacterial decarboxylases. Biochemistry. 1981 May 26;20(11):3163–3168. doi: 10.1021/bi00514a027. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- Paulus T. J., Davis R. H. Regulation of polyamine synthesis in relation to putrescine and spermidine pools in Neurospora crassa. J Bacteriol. 1981 Jan;145(1):14–20. doi: 10.1128/jb.145.1.14-20.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pösö H., Sinervirta R., Jänne J. S-adenosylmethionine decarboxylase from baker's yeast. Biochem J. 1975 Oct;151(1):67–73. doi: 10.1042/bj1510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajam M. V., Galston A. W. The effects of some polyamine biosynthetic inhibitors on growth and morphology of phytopathogenic fungi. Plant Cell Physiol. 1985;26(4):683–692. doi: 10.1093/oxfordjournals.pcp.a076958. [DOI] [PubMed] [Google Scholar]
- Slocum R. D., Kaur-Sawhney R., Galston A. W. The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys. 1984 Dec;235(2):283–303. doi: 10.1016/0003-9861(84)90201-7. [DOI] [PubMed] [Google Scholar]
- Stevens L., Winther M. D. Spermine, spermidine and putrescine in fungal development. Adv Microb Physiol. 1979;19:63–148. doi: 10.1016/s0065-2911(08)60198-8. [DOI] [PubMed] [Google Scholar]
- Tabor C. W. Mutants of Saccharomyces cerevisiae deficient in polyamine biosynthesis: studies on the regulation of ornithine decarboxylase. Med Biol. 1981 Dec;59(5-6):272–278. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P. A., Morris D. R. Polyamine auxotrophs of Saccharomyces cerevisiae. J Bacteriol. 1978 Apr;134(1):214–220. doi: 10.1128/jb.134.1.214-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]