Abstract
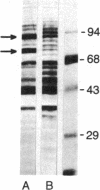
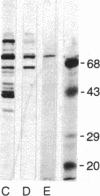
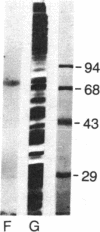
When HeLa cells are shifted from 37 degrees C to 45 degrees C, the synthesis of two proteins increases. Their approximate molecular masses are 73 kDa [heat shock protein 73 (hsp73)] and 87 kDa (hsp87), respectively. One of them, the hsp73, shows a specific affinity for poly(A). This protein is identical with a protein regularly found associated with translatable mRNAs in all vertebrate cells. It is well characterized by its high affinity to the poly(A) sequence of polyribosomal mRNA, and it occurs free in cytoplasm. hsp73 and the poly(A)-binding protein have the same isoelectric point and molecular size. The peptide analysis indicates that they are identical.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhayat O., Vincent A., Goldenberg S., Person A., Scherrer K. The translation of the messenger for the poly(A)-binding protein-associated with translated mRNA is suppressed. A case of cytoplasmic repression in duck erythroblasts. FEBS Lett. 1983 Oct 3;162(1):25–32. doi: 10.1016/0014-5793(83)81042-4. [DOI] [PubMed] [Google Scholar]
- Arrigo A. P., Fakan S., Tissières A. Localization of the heat shock-induced proteins in Drosophila melanogaster tissue culture cells. Dev Biol. 1980 Jul;78(1):86–103. doi: 10.1016/0012-1606(80)90320-6. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Bienz M., Gurdon J. B. The heat-shock response in Xenopus oocytes is controlled at the translational level. Cell. 1982 Jul;29(3):811–819. doi: 10.1016/0092-8674(82)90443-3. [DOI] [PubMed] [Google Scholar]
- Blobel G. A protein of molecular weight 78,000 bound to the polyadenylate region of eukaryotic messenger RNAs. Proc Natl Acad Sci U S A. 1973 Mar;70(3):924–928. doi: 10.1073/pnas.70.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Civelli O., Vincent A., Maundrell K., Buri J. F., Scherrer K. The translational repression of globin mRNA in free cytoplasmic ribonucleoprotein complexes. Eur J Biochem. 1980 Jun;107(2):577–585. doi: 10.1111/j.1432-1033.1980.tb06066.x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Courgeon A. M., Maisonhaute C., Best-Belpomme M. Heat shock proteins are induced by cadmium in Drosophila cells. Exp Cell Res. 1984 Aug;153(2):515–521. doi: 10.1016/0014-4827(84)90618-9. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R., Carroll E., 3rd Reconstitution of functional mRNA-protein complexes in a rabbit reticulocyte cell-free translation system. Mol Cell Biol. 1985 Feb;5(2):342–351. doi: 10.1128/mcb.5.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi-Scherrer M. T., Maundrell K., Civelli O., Scherrer K. Transcriptional and post-transcriptional regulation in duck erythroblasts. Dev Biol. 1982 Sep;93(1):126–138. doi: 10.1016/0012-1606(82)90246-9. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Nemer M. Heterogeneous ribonucleoprotein particles in the cytoplasm of sea urchin embryos. J Mol Biol. 1968 Mar 28;32(3):543–565. doi: 10.1016/0022-2836(68)90342-2. [DOI] [PubMed] [Google Scholar]
- Jeffery W. R. Characterization of polypeptides associated with messenger RNA and its polyadenylate segment in Ehrlich ascites messenger ribonucleoprotein. J Biol Chem. 1977 May 25;252(10):3525–3532. [PubMed] [Google Scholar]
- Kish V. M., Pederson T. Poly (A)-rich ribonucleoprotein complexes from HeLa cell messenger RNA. J Biol Chem. 1976 Oct 10;251(19):5888–5894. [PubMed] [Google Scholar]
- Kloetzel P. M., Bautz E. K. Heat-shock proteins are associated with hnRNA in Drosophila melanogaster tissue culture cells. EMBO J. 1983;2(5):705–710. doi: 10.1002/j.1460-2075.1983.tb01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaThangue N. B. A major heat-shock protein defined by a monoclonal antibody. EMBO J. 1984 Aug;3(8):1871–1879. doi: 10.1002/j.1460-2075.1984.tb02061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee G. T., Engelhardt D. L. Peptide coding capacity of polysomal and non-polysomal messenger RNA during growth of animal cells. J Mol Biol. 1979 Apr 5;129(2):221–233. doi: 10.1016/0022-2836(79)90278-x. [DOI] [PubMed] [Google Scholar]
- Lenk R., Ransom L., Kaufmann Y., Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977 Jan;10(1):67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Mazur G., Schweiger A. Identical properties of an mRNA-bound protein and a cytosol protein with high affinity for polyadenylate. Biochem Biophys Res Commun. 1978 Jan 13;80(1):39–45. doi: 10.1016/0006-291x(78)91101-4. [DOI] [PubMed] [Google Scholar]
- McKenzie S. L., Henikoff S., Meselson M. Localization of RNA from heat-induced polysomes at puff sites in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1117–1121. doi: 10.1073/pnas.72.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto R., Fodor E. Cell-specific expression of heat shock proteins in chicken reticulocytes and lymphocytes. J Cell Biol. 1984 Oct;99(4 Pt 1):1316–1323. doi: 10.1083/jcb.99.4.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter T., Penman S. "Prompt" heat shock proteins: translationally regulated synthesis of new proteins associated with the nuclear matrix-intermediate filaments as an early response to heat shock. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4737–4741. doi: 10.1073/pnas.80.15.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid H. P., Köhler K., Setyono B. Interaction of cytoplasmic messenger-RNA with proteins: their possible function in the regulation of translation. Mol Biol Rep. 1983 May;9(1-2):87–90. doi: 10.1007/BF00777478. [DOI] [PubMed] [Google Scholar]
- Schmid H. P., Köhler K., Setyono B. Possible involvement of messenger RNA-associated proteins in protein synthesis. J Cell Biol. 1982 Jun;93(3):893–898. doi: 10.1083/jcb.93.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid H. P., Schönfelder M., Setyono B., Köhler K. 76-kDa poly(A)-protein is involved in the formation of 48 S initiation complexes. FEBS Lett. 1983 Jun 27;157(1):105–110. doi: 10.1016/0014-5793(83)81125-9. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Perry R. P. Characterization of the messenger RNA released from L cell polyribosomes as a result of temperature shock. J Mol Biol. 1972 Feb 14;63(3):577–590. doi: 10.1016/0022-2836(72)90449-4. [DOI] [PubMed] [Google Scholar]
- Setyono B., Greenberg J. R. Proteins associated with poly(A) and other regions of mRNA and hnRNA molecules as investigated by crosslinking. Cell. 1981 Jun;24(3):775–783. doi: 10.1016/0092-8674(81)90103-3. [DOI] [PubMed] [Google Scholar]
- Setyono B., Schmid H. P., Köhler K. The role of acidic proteins from cytoplasmic fractions of Krebs II ascites cells for efficient translation. Z Naturforsch C. 1979 Jan-Feb;34(1-2):64–75. doi: 10.1515/znc-1979-1-215. [DOI] [PubMed] [Google Scholar]
- Slater A., Cato A. C., Sillar G. M., Kioussis J., Burdon R. H. The pattern of protein synthesis induced by heat shock of HeLa cells. Eur J Biochem. 1981 Jul;117(2):341–346. doi: 10.1111/j.1432-1033.1981.tb06343.x. [DOI] [PubMed] [Google Scholar]
- Spirin A. S. The second Sir Hans Krebs Lecture. Informosomes. Eur J Biochem. 1969 Aug;10(1):20–35. doi: 10.1111/j.1432-1033.1969.tb00651.x. [DOI] [PubMed] [Google Scholar]
- Standart N., Vincent A., Scherrer K. The polyribosomal poly(A)-binding protein is highly conserved in vertebrate species. Comparison in duck, mouse and rabbit. FEBS Lett. 1981 Nov 30;135(1):56–60. doi: 10.1016/0014-5793(81)80942-8. [DOI] [PubMed] [Google Scholar]
- Tissières A., Mitchell H. K., Tracy U. M. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974 Apr 15;84(3):389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Goldenberg S., Scherrer K. Comparisons of proteins associated with duck-globin mRNA and its polyadenylated segment in polyribosomal and repressed free messenger ribonucleoprotein complexes. Eur J Biochem. 1981 Feb;114(2):179–193. doi: 10.1111/j.1432-1033.1981.tb05135.x. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Purification of the major mammalian heat shock proteins. J Biol Chem. 1982 Dec 25;257(24):14949–14959. [PubMed] [Google Scholar]
- van Venrooij W. J., van Eekelen C. A., Jansen R. T., Princen J. M. Specific poly-A-binding protein of 76,000 molecular weight in polyribosomes is not present on poly A of free cytoplasmic mRNP. Nature. 1977 Nov 10;270(5633):189–191. doi: 10.1038/270189a0. [DOI] [PubMed] [Google Scholar]