Abstract
In this study, a functional magnetic resonance imaging paradigm originally employed by Takahashi et al. was adapted to look for emotion-specific differences in functional brain activity within a healthy German sample (N = 14), using shame- and guilt-related stimuli and neutral stimuli. Activations were found for both of these emotions in the temporal lobe (shame condition: anterior cingulate cortex, parahippocampal gyrus; guilt condition: fusiform gyrus, middle temporal gyrus). Specific activations were found for shame in the frontal lobe (medial and inferior frontal gyrus), and for guilt in the amygdala and insula. This is consistent with Takahashi et al.’s results obtained for a Japanese sample (using Japanese stimuli), which showed activations in the fusiform gyrus, hippocampus, middle occipital gyrus and parahippocampal gyrus. During the imagination of shame, frontal and temporal areas (e.g. middle frontal gyrus and parahippocampal gyrus) were responsive regardless of gender. In the guilt condition, women only activate temporal regions, whereas men showed additional frontal and occipital activation as well as a responsive amygdala. The results suggest that shame and guilt share some neural networks, as well as having individual areas of activation. It can be concluded that frontal, temporal and limbic areas play a prominent role in the generation of moral feelings.
Keywords: shame, guilt, fMRI, moral emotion, gender, culture
INTRODUCTION
Brain imaging studies have recently been employed to investigate the concept of morality and its related self-conscious emotions (e.g. Greene et al., 2001; Moll et al., 2002; Berthoz et al., 2006). The primary self-conscious emotions are shame, guilt, embarrassment and pride (Lewis, 2010). This article deals with two self-conscious emotions: shame and guilt. A further self-conscious emotion, embarrassment, is described as a less intensive form of shame (Lewis, 2010). In accord with this, functional magnetic resonance imaging (fMRI) studies thus far have found no differences between the two emotions. Following this, our description of studies of shame will encompass both shame and embarrassment. Shame and guilt can be differentiated theoretically: While the feeling of shame implicates the presence of other people; guilt can arise and persist without others (Takahashi et al., 2004). Compared with shame, the feeling of guilt is a super ordinate entity and generally interculturally important for living together (Ausubel, 1955). Guilt in particular is grounded in social relationships, and its prime function is to adjust interpersonal relationships (De Rivera, 1984; Caplovitz Barrett, 1995). For example, Baumeister et al. (1994) have argued that guilt serves relationship-enhancing functions by motivating people to treat partners well and to avoid interpersonal transgressions. Shame has also been perceived as one of the moral emotions that motivate prosaically behaviour (e.g. Emde and Oppenheim, 1995), and as moral emotion it is linked to the interests of other people (Haidt, 2003). However, in contrast to guilt, the case for shame as a moral emotion is less clear. In a series of recent studies, prosocial effects were found for guilt but not for shame (De Hooge et al., 2007). Guilt experiences increased prosaically behaviour in everyday situations and in a social dilemma, but these effects were not found when participants recalled experiences of shame (De Hooge et al., 2007).
Generally, from the neurobiological view, it is believed that a fronto-temporo-limbic network is involved in the generation of emotions. Research in the neurobiology of social and moral emotions suggests that the medial orbitofrontal gyrus plays an important role (Moll et al., 2002). Other important structures thought to be involved in these emotions include the medial frontal gyrus, the posterior cingular gyrus (Greene et al., 2001), the anterior temporal lobe, the superior temporal sulcus (STS) and subcortical structures, such as the amygdala and hypothalamus (Moll et al., 2005).
Additional findings in the areas of theory of mind, empathy, social cognition and self-referential cognition provide important insights into neural networks also crucial for the emotions of shame and guilt. The anterior paracingulate cortex, superior temporal sulci and the temporal poles are thought to house networks enabling people to take on perspectives of others (Gallagher and Frith, 2003). Networks for emotional empathy are thought to be located within the superior temporal and inferior frontal areas, as well as the limbic system (Carr et al., 2003). Finally, areas related to self-referential thoughts are postulated to be controlled by cortical midline structures, e.g. the orbital and medial prefrontal cortex and the posterior cingulate cortex (Northoff and Bermpohl, 2004).
So far, activity of the neural system for self-conscious emotions and their underlying appraisals has been localized to frontal and temporal areas, as well as to the amygdala and cingulate gyrus. Frontal areas are associated with the generation of emotions, such as embarrassment and guilt, temporal areas are linked with the capacity to make inferences about the mind of others and knowledge of social norms. The amygdala plays an important role in marking one’s own emotions and the emotions of others, taking into account knowledge about social norms (Adolphs, 2003; Beer, 2007). In processing the emotion of embarrassment, convergent findings of frontal, temporal and limbic brain participation have been provided by lesion and imaging studies (Devinsky et al., 1982; Blair and Cipolotti, 2000; Berthoz et al., 2002; Beer et al., 2003, 2006; Ruby and Decety, 2004; Takahashi et al., 2004). Functional brain imaging studies on processing the moral emotion of guilt support the idea of an associated diffuse activation in frontal and temporal regions (Shin et al., 2000; Takahashi et al., 2004), but to our knowledge, only one study has investigated the differences in embarrassment and guilt with fMRI (Takahashi et al., 2004).
Takahashi et al. (2004) directly compared imagination of embarrassment and guilt, measuring associated functional brain activity with fMRI and using sentences evoking shame and guilt. Their results indicate overlapping activity for both conditions in the medial prefrontal cortex, the left posterior STS and the visual cortex. For embarrassment, they found a distinctly greater activation in the right temporal cortex and hippocampus relative to guilt. Thus, the processing of embarrassment seemed to be more complex, more self-conscious and more memory-related than the processing of guilt in the Japanese sample.
We predict that German and Japanese people differ in their experience of shame and guilt, owing to certain critical cultural differences between the two cultures: First, the expression of self-conscious, moral feelings of shame or guilt will inter alia vary, depending not only on social contexts (Tangney, 1992) but also in behaviour repertoires or in regulation processes (Mesquita and Frijda, 1992). In addition, there are findings, specifically contrasting American and Japanese people, suggesting that culture plays a role in determining the kind of situations in which mixed emotions, that is, emotions with both positive and negative feelings, are experienced. For example, Japanese feel more mixed emotions in positive situations than Americans (Miyamoto et al., 2010). Japanese have a stronger self-critical focus arising from an enhanced need for positive self-regard (Heine et al., 1999). Overall, it seems to make a difference if these emotions are measured in individualistic or collectivistic cultures, because of the different stages of development and socialization (Wallbott and Scherer, 1998).
To assess shame and guilt in a German sample, this study adopted Takahashi et al.’s (2004) experimental fMRI paradigm.
Referring to the empirically verified differences at the psychological level of experience of self-conscious emotions between Japanese people and those of a Western country people (e.g. the USA: Heine et al., 1999; Miyamoto et al., 2010), we predict in parallel observable differences at the level of functional neurobiology.
Since there were as many men as women included in this study, the authors took the opportunity to carry out an exploratory analysis of gender-related activation patterns, because there are very few gender studies on emotions (e.g. Schneider et al., 2000) and no single study investigating gender differences in shame and guilt.
In addition to brain activation data, behavioural parameters on shame and guilt are assessed through self-report questionnaires.
METHODS
Sample
Fourteen healthy right-handed participants voluntarily took part in this study (overall mean age = 29 years, s.d. = 2.95, range = 22–33 years, df = 12; gender specific: seven men, mean age = 29 years, s.d. = 2.27, range = 25–32 years; seven women, mean age = 28 years, s.d. = 3.58, range = 22–33 years). All participants were medication free and reported to be in good physical and mental condition. The following classification was used for education level: 0 = no graduation, 1 = 9 years of school, 2 = 10 years of school, 3 = 13 years of school without graduation and 4 = general qualification for university entrance. The mean level of education on this scale was 2.6 (s.d. = 1.44, df = 12; gender specific: male mean level of education = 2.28, s.d. = 1.60; female mean level of education = 3.00, s.d. = 1.29; t = −0.918, P = 0.377). To screen the level of language-based intelligence, we used a German Vocabulary Test (WST; Schmidt and Metzler, 1992). This test requires participants to identify a single real word out of a number of pseudowords. The test is sensitive for crystallized verbal intelligence (Schmidt and Metzler, 1992). The mean level of verbal intelligence was 113.64 (s.d. = 14.81, df = 12; gender specific: male mean level of verbal intelligence = 111.00, s.d. = 16.01; female mean level of verbal intelligence = 116.28, s.d. = 14.2; t = −0.653, P = 0.526) (Table 1).
Table 1.
Sample description with age, level of education and verbal intelligence
| Women |
Men |
Altogether |
t | P | d | df | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | |||||
| Age (range = 22–33) | 28 | 3.58 | 29 | 2.27 | 29 | 2.95 | 0.803 | 0.438 | −0.334 | 12 |
| Level of education | 3.00 | 1.29 | 2.28 | 1.60 | 2.6 | 1.44 | −0.918 | 0.377 | 0.495 | 12 |
| Level of language- based intelligence | 116.28 | 14.2 | 111.00 | 16.01 | 113.64 | 14.81 | −0.653 | 0.526 | 0.348 | 12 |
Mean (T-values), s.d. = standard deviation, t = t-values, P = level of significance and d = Cohen's d.
All participants reported being free of current or previous neurological disorders, psychiatric illnesses, and reported no history of drug or alcohol dependence. To verify this, we used the structured clinical interview for DSM-IV (SCID) I and II (Wittchen et al., 1997).
All participants received detailed information before deciding whether to participate, and gave written consent for participating in the study. Participants were not paid for their attendance. The study has been approved by the Ethics Committee of the Medical Faculty of the LMU Munich and has been performed in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Experimental stimuli
Following the study of Takahashi et al. (2004), our sentences were classified into three categories: neutral, guilt or shame. Short German sentences in the past tense were used to make them comparable with the sentences from Takahashi et al. (2004).
A total of 90 sentences (30 neutral, 30 guilt-laden and 30 shame-laden) were selected from a pool of 107 sentences. We validated each stimulus sentence using a group of healthy participants. By calculating a non-parametric Friedman’s two-way analysis, the lengths of sentences do not differ between all three conditions: shame, guilt and neutral (range of length in guilt condition = 3–13, range of length in shame condition = 5–12, range of length in neutral condition = 3–10; P = 0.695).
The pre-selection of the sentences was anonymous and rated on a six-point scale (0 = not ashamed/innocent; 5 = very ashamed/guilty). Forty-five participants (23 men; mean age = 25, s.d. = 4.04, range = 20–33) evaluated the sentences according to self-referring shame and guilt and the sentences with the strongest scores in the pre-evaluation were selected. Examples of the sentences are presented in the Supplementary Table S1. The sentences, with the highest score for the category, were selected for the experimental stimuli. Only sentences that scored higher than three on one scale were included, sentences scoring high on both scales were excluded.
Visual presentation and timing of these stimuli were programmed using Presentation® software (Neurobehavioral Systems, 2005). Inside the MR scanner, the participants viewed the stimuli over a head-coil compatible mirror system (300 cm screen to mirror, 15 cm mirror to participant’s eyes). Stimuli were projected on a translucent screen in white capitals (font: Arial, font size: 16) on black background by a commercially available video beamer (INTouch, resolution of 1024 × 768 pixel) protected by a faraday cage.
Each stimulus was presented for 4000 ms, the next started after an inter-stimulus interval of 400 ms. Stimuli were presented in a block design and were pseudo-randomized within blocks; each block consisted of five sentences of the same category, and it began with a fixation cross. There was a rest period of 20 s between each block. The instructions were as follows: ‘As you read this sentence, please imagine the situation described’ (Figure 1).
Fig. 1.
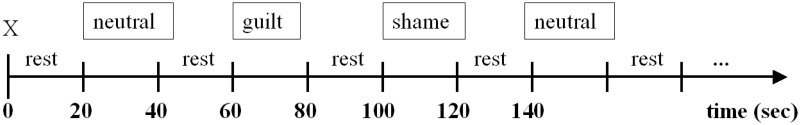
Block design paradigm.
Magnetic resonance
Functional MRI images were collected using a Siemens 1.5-T scanner (Siemens Magnetom Vision, Erlangen, Germany) with T2*-weighted echo planar imaging sequences based on a blood oxygen level-dependent (BOLD) protocol [time of repetition (TR): 4000 ms, time of echo (TE): 100 ms, flip angle (FA): 90°, matrix: 64 × 64, field of view (FoV): 240 mm× 240 mm, pixel size: 3.75 mm × 3.75 mm] parallel to the anterior commissure–posterior commissure. Twenty-eight transversal slices (slice thickness: 4 mm) from the cerebellum to the cortex were acquired sequentially. For visualization of the functional data, magnetization-prepared rapid gradient-echo images (MPRAGE) were recorded following the functional measurements within a period of ∼10 min (TR: 11.4 ms, TE: 4.4 ms, FA: 8°, matrix: 256 × 256, FoV: 250 mm× 250 mm). In total, 144 sagittal slices (slice thickness: 1.25 mm, pixel size: 1.05 mm× 1.05 mm) were acquired sequentially. The sentences were presented using the software Presentation® (Version 0.80, Neurobehavioral Systems).
Statistical analysis
Statistical analyses were carried out using SPSS 17 (SPSS Inc., 2008). For the neuropsychological data and experimental paradigm, independent t-tests were performed to compare the two groups (men and women). All analyses were two-tailed and the significance level was defined as P < 0.05.
Image pre-processing (motion correction, slice scan time correction, temporal smoothing, mean intensity correction and functional-to-anatomical co-registration, transformation to Talairach standard space) and statistical calculations were performed by means of BrainVoyager QX (Version 2.2; Goebel and Jansma, 2006). After data pre-processing, a general linear model was built with the following conditions: shame, guilt and neutral. The conditions were convolved with a haemodynamic response function (Friston et al., 1995; Boynton et al., 1996) and were counted among a fixed effect analysis. To correct for multiple comparisons, the false discovery rate (FDR) controlling procedure was applied on the resulting P values for all voxels. The value of q specifying the maximum FDR tolerated on average was set to 0.05 (Benjamini and Hochberg, 1995). In a voxel-based approach, contrast maps were computed for the predictions shame, guilt and neutral within each of the participant samples of men and women.
Standard stereotactic coordinates for the voxel displaying local maximum activation were determined within the areas where significant relative changes in neural activity associated with the demands of the different memory conditions were found. These local maxima were anatomically localized by reference to a standard stereotactic atlas (Talairach and Tournoux, 1988) using TalairachClient (Version 2.4.2).
RESULTS
Subjective ratings of experimental stimuli
After the fMRI scan, the participants were asked to rate the sentences on a five-point scale (0 = not ashamed/innocent; 5 = very ashamed/guilty) in the category of shame and guilt. We assumed that those items scaled around zero can be considered as neutral. The ratings of the sentences used in our paradigm lead to the following results: The items that had been depicted as shame items received higher values in the category ‘shame’ (range = 1.90–4.07, mean = 2.87, s.d. = 0.68) than in the category ‘guilt’ (range = 0.93–3.10, mean = 1.86, s.d. = 0.59; P = 0.001, computed with Wilcoxon Signed Rank Test). The same applies for the guilt items. The guilt sentences received higher values in the category ‘guilt’ (range = 1.27–4.67, mean = 3.67, s.d. = 0.87) than in the category ‘shame’ (range = 0.13–4.27, mean = 2.98, s.d. = 1.16; P = 0.002). The neutral sentences received values around zero and differ significantly from the two affective categories (range = 0.00–0.40, mean = 0.04, s.d. = 0.11; for both comparisons neutral vs guilt and neutral–shame; P = 0.000).
fMRI results
First, to calculate the emotion-specific activation, we used the contrasts of ‘guilt minus neutral’ and ‘shame minus neutral’ as performed by Takahashi et al. (2004). Second, we added the contrast of ‘shame minus guilt’ and vice versa. Finally, we report pilot data on gender differences. The neutral condition did not show any significantly increased activation compared with guilt or shame for any of the comparisons.
‘Shame minus neutral’ condition
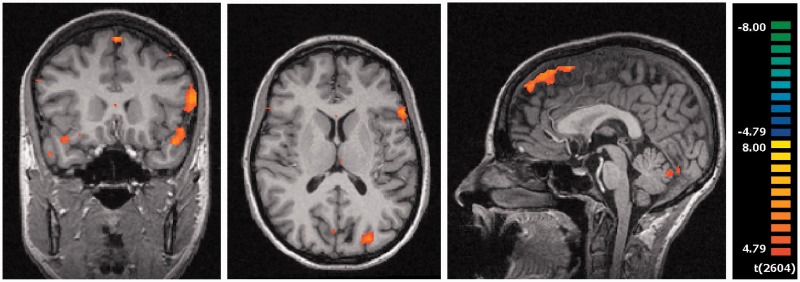
First, in the shame condition (relative to the neutral condition), the participants produced an activation in the visual areas [right cuneus (BA 17), right lingual gyrus (BA 18) and left middle occipital gyrus (BA 19)], in the temporal lobe [right fusiform gyrus (BA 19), bilateral parahippocampal gyrus (BA 27, 30), left superior (BA 38) and left middle temporal gyrus (BA 21)] and in a frontal network [left superior (BA 6, 8, 9), bilateral inferior (BA 45, 47), left middle (BA 9) and right medial frontal gyrus (BA 8)] (Figure 2; Table 2).
Fig. 2.
Brain activation in the condition shame minus neutral, red = shame activation: (A) superior temporal gyrus (−42, 17, 14); (B) middle occipital gyrus (x = −27, y = −85, z = 13); (C) superior frontal gyrus (x = −0, y = 63, z = −34); P < 0.0002.
Table 2.
Activation during shame minus neutral and guilt minus neutral condition
| Conditions | Brain region | BA | Hemisphere | Coordinate |
Z-score | Voxel | ||
|---|---|---|---|---|---|---|---|---|
| Shame > neutral; P < 0.0002 | Right/left | x | y | z | ||||
| Middle occipital gyrus/occipital gyrus/lingual gyrus | 19/18 | l | −27 | −85 | 13 | 6,25 | 1431 | |
| Cuneus/lingual gyrus | 17/18 | r | 3 | −82 | 10 | 5,4 | 203 | |
| Lingual gyrus/fusiform gyrus | 18/19 | r | 6 | −70 | −23 | 6,08 | 706 | |
| Parahippocampal gyrus/parahippocampal gyrus | 27/30 | l/r | −6 | −34 | 1 | 5,65 | 329 | |
| Superior temporal gyrus/middle temporal gyrus | 38/21 | l | −42 | 17 | −14 | 6,37 | 2181 | |
| Superior frontal gyrus | 6 | l | −9 | 2 | 68 | 6,39 | 506 | |
| Inferior frontal gyrus | 47 | r | 36 | 20 | −14 | 6,16 | 371 | |
| Inferior frontal gyrus/middle frontal gyrus | 45/9 | l | −54 | 20 | 13 | 6,77 | 1164 | |
| Superior frontal gyrus/medial frontal gyrus/superior frontal gyrus | 8/8/6 | r | 3 | 41 | 52 | 6,49 | 4287 | |
| Superior frontal gyrus | 9 | 0 | 63 | 34 | 6,05 | 389 | ||
| Guilt > neutral; P < 0.0002 | ||||||||
| Cuneus | 18 | l | −3 | −90 | 10 | 5,51 | 57 | |
| Lingual gyrus | 17 | l | −12 | −95 | −14 | 6,02 | 150 | |
| Lingual gyrus | 18 | l | −15 | −88 | −8 | 7,10 | 350 | |
| Lingual gyrus | 18 | r | 3 | −78 | 4 | 6,39 | 464 | |
| Lingual gyrus | 18 | r | 3 | −76 | 1 | 7,02 | 1024 | |
| Fusiform gyrus | 18 | l | −24 | −92 | −11 | 6,41 | 645 | |
| Fusiform gyrus | 19 | r | 24 | −76 | −35 | 6,84 | 1949 | |
| Fusiform gyrus | 19 | r | −31 | −76 | −29 | 5,77 | 57 | |
| Superior temporal gyrus | 39 | l | −54 | −55 | 13 | 6,10 | 234 | |
| Superior temporal gyrus | 22 | l | −51 | −19 | 1 | 6,42 | 177 | |
| Middle temporal gyrus | 21 | l | −60 | −34 | 1 | 7,49 | 401 | |
| Middle temporal gyrus | 21 | l | −60 | −10 | −8 | 6,67 | 716 | |
| Middle temporal gyrus | 21 | r | 54 | 5 | −11 | 6,52 | 120 | |
| Insula | 13 | l | −45 | 10 | 14 | 5,83 | 161 | |
| Insula/middle frontal gyrus | 13/46 | l | −54 | 17 | 22 | 7,47 | 1247 | |
| Precentral gyrus | 6 | l | −45 | −4 | 53 | 5,21 | 118 | |
| Precentral gyrus | 44 | l | −51 | 5 | 11 | 6,62 | 446 | |
| Orbitofrontal gyrus | 11 | l | −6 | 54 | 43 | 6,81 | 2607 | |
| Superior frontal gyrus | 8 | r | 18 | 47 | 43 | 6,23 | 511 | |
BA = Broadmann area, l = left hemisphere, r = right hemisphere.
‘Guilt minus neutral’ condition
Table 2 also shows the results from the comparison of the ‘guilt minus neutral’ condition. In the guilt condition, outspreading activation was found in the left hemisphere, which approached significance in comparison with the ‘shame minus neutral’ condition.
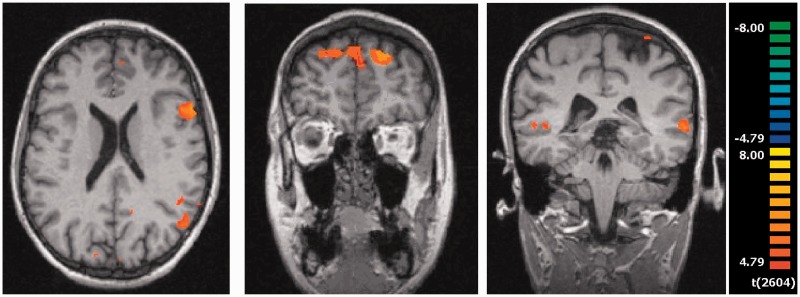
The guilt condition is some similarities of brain activation to the shame condition, specifically in the visual cortex [left cuneus (BA 18), bilateral lingual gyrus (BA 17, 18)], in the temporal lobe [bilateral fusiform gyrus (BA 18, 19), left superior (BA 22, 39) and bilateral middle temporal gyrus (BA 21)] and in the frontal area [right superior (BA 8) and left middle frontal gyrus (BA 13, 46)], which were not seen in the neutral condition.
Unlike the shame condition, there was additional activation in the left insula (BA 13), the left orbitofrontal gyrus (BA 11) and the left precentral gyrus (BA 6, 44) (Figure 3).
Fig. 3.
Brain activation in the condition guilt minus neutral, red = guilt activation: (A) insula (x = −54, y = 17, z = 22); (B) superior frontal gyrus (x = 18, y = 47, z = 43); (C) middle temporal gyrus (x = −60, y = −34, z = 1); P < 0.0002.
Table 2 contains the results of the activations in the conditions ‘shame minus neutral’ and ‘guilt minus neutral’ with the associated Brodmann areas, hemispheres, coordinates, z-score and the number of voxel.
‘Shame minus guilt’ condition (and vice versa)
The shame vs guilt condition were both compared (shame > guilt and vice versa). Because of the subtraction method used for the contrast, only the areas that occur in one of the two conditions are presented (red = shame, blue = guilt). Thus, regions that are activated in both conditions are not indicated.
There was activation of different regions within the temporal lobe in both conditions [shame: cingulate gyrus (BA 32), bilateral parahippocampal gyrus (BA 27, 20, 34, 36); guilt: right fusiform gyrus (BA 37) and left middle temporal gyrus (BA 21)]. Additional frontal activation was found in the shame condition [anterior cingulate cortex (BA 24), right rectal gyrus (BA 11), right medial (BA 10) and right inferior frontal gyrus (BA 47)]. In the guilt condition, an activation of the right amygdala and the insula (BA 13) could be detected, which was not found in the shame condition (Table 3).
Table 3.
Overview of the neuronal activation from shame–guilt and guilt–shame
| Conditions | Brain region | BA | Hemisphere | Coordinate |
Z-score | Voxel | ||
|---|---|---|---|---|---|---|---|---|
| Shame–guilt; P < 0.002 | Right/left | x | y | z | ||||
| Anterior cingulum/cingulate gyrus | 24/32 | 0 | 29 | 25 | 4,72 | 128 | ||
| Cingulate gyrus/cingulate gyrus | 23/31 | r | 3 | −37 | 28 | 4,11 | 135 | |
| Parahippocampal gyrus | 36 | l | −21 | −37 | −11 | 3,65 | 40 | |
| Parahippocampal gyrus/parahippocampal gyrus | 30/27 | r | 9 | −34 | 7 | 4,05 | 209 | |
| Parahippocampal gyrus/rectal gyrus | 34/11 | r | 12 | −7 | −17 | 4,32 | 101 | |
| Medial frontal gyrus | 10 | r | 21 | 53 | 13 | 3,89 | 51 | |
| Extra-nuclear/inferior frontal gyrus | 13/47 | r | 30 | 17 | −8 | 4,27 | 92 | |
| Guilt–shame; P < 0.002 | ||||||||
| Fusiform gyrus | 37 | r | 33 | −55 | −17 | 4,27 | 62 | |
| Middle temporal gyrus | 21 | l | −61 | −31 | −2 | 4,65 | 325 | |
| Amygdala/insula | 13 | r | 16 | −7 | 7 | 4,37 | 82 | |
BA = Broadmann area, l = left hemisphere, r = right hemisphere.
Gender effects
Within the female sample there was an activation in the shame and guilt condition (shame > guilt) in the bilateral middle temporal gyrus (BA 19, 21, 39). In addition, the shame condition also activates the parietal lobe [right precuneus (BA 7, 31), right supramarginal gyrus (BA 40)], the temporal lobe [cingulate gyrus (BA 31), parahippocampal gyrus (BA 27)] and the frontal lobe [right precentral gyrus (BA 6), bilateral middle frontal gyrus (BA 10)] (Supplementary Table S2).
The male participants showed activations in shame and guilt (shame > guilt) in the right fusiform gyrus (BA 37). In contrast to the guilt condition, men showed more activation in the left parahippocampal gyrus (BA 35, 36) during the shame condition. The bilateral anterior cingulate cortex (BA 32) and the right orbital frontal gyrus (BA 47) also showed relatively increased activation. The guilt condition was associated with activation in the uncus (BA 34), the right amygdala. Additionally, there was also activation seen in the left inferior occipital and lingual gyrus (BA 18), and bilateral middle frontal gyrus (BA 10) (Supplementary Table S3).
DISCUSSION
The results will be discussed in light of current research, first, examining the effects of gender in the German sample, then, the differences between the Japanese and the German sample and finally, addressing the limitations of the study. The pre-experimental subjective ratings and post-experimental control rating suggest that it is legitimate to assume that the sentences presented during the experiment evoked the intended emotional responses of shame and guilt during scanning. In our German sample, both emotions were associated with the activity in the temporal lobe (shame: anterior cingulate cortex, parahippocampal gyrus; guilt: fusiform gyrus, middle temporal gyrus). For the contrast of both emotions, the ‘shame minus guilt’ comparisons revealed greater activity in the medial and inferior frontal lobe related to shame. The ‘guilt minus shame’ condition revealed greater activity in the amygdala and the insula related to guilt.
As there were specific activation patterns in the shame and guilt conditions, which differed from the neutral condition, it can be reasonably assumed that the imagined affect-laden situations produced the measured changes in BOLD response.
The results show a pronounced activation in the right hemisphere in the shame condition.
More precisely, activation was seen in the bilateral parahippocampal gyrus, the right rectal and right cingular gyrus, the anterior cingulate cortex and the right inferior and medial frontal gyrus.
In the guilt condition, however, both hemispheres showed less neural activity than in the shame condition. These differences were specifically seen for activity in the right fusiform gyrus, the left middle temporal gyrus, the right insula and the amygdala. The activation of the right amygdala in the guilt condition may indicate the involvement of a reactivity to aversive stimuli (Ochsner and Gross, 2005) and social judgment (Adolphs et al., 1998). It could be caused by guilt-associated emotional responses and social consequences, which could have a higher impact during imagination of guilt-related situations than shame-related situations. This is because guilt is typically associated with punishment meted out by a group or society. There are several possible interpretations of the observed insula activation. The use of emotional recall imagery for affective induction could explain the recruitment of the insula in its regulative function (Phan et al., 2002). Alternately, the increased activation of the amygdala and insula could also reflect an attempt at suppression of negative emotions (Goldin et al., 2008). Finally, the amygdala activation could stand for the identification of an emotional stimulus and thus reflect an affective response (Phillips et al., 2003).
The amygdala was active in the guilt condition, but not in the shame condition. This could either be attributable to the previously mentioned affective responses related to guilt, or to factors relating to the imagination of shame-related situations. It has been shown that the audience is an essential aspect in the development of shame coupled with an activation of the amygdala (Finger et al., 2006). This socially relevant aspect could be weakened in our task, where the participant only imagined a shame reaction. An alternative explanation for our findings could relate to the nature of the emotion shame, as the feeling of shame, when developed from an actual infringement, is an intensive, long-lasting emotion (Miller, 2007). The results of our study support the idea that frontal and temporal areas play a prominent role during moral and self-conscious emotional states. This is in line with another study of the self-conscious emotions of empathy and forgiveness, which also represent emotions pre-supposing complex social interaction (Farrow et al., 2001). They found these emotions to be associated with activation in the prefrontal and temporal cortex (including amygdala and cingulate). All together, the frontal lobes seem to be associated with the application of social norms and the generation of embarrassment and guilt, the temporal lobes to be associated with making inferences about the others’ minds and experiencing self-conscious emotions (Beer, 2007).
The preliminary results of the gender-related comparisons suggest that both women and men activate frontal and temporal areas during the imagination of shame. In the guilt condition, women only activate temporal regions, in contrast, men showed additional frontal and occipital activation as well as responsive amygdalae. This could mean that women associate more memory contents and more social associations in the shame condition, while attempting to control the associated recall at the same time. Women probably modulate shameful memories more than guilty ones and are possibly better at visualizing situations or responses of others with a more fully elaborated recall of associations for a given situation. The activation of the amygdala in male participants in the guilt condition seems to be rooted in its role for interpersonal outcome and for perceived self-discrepancy in men. This perceived significance of guilt in men is related to guilt-specific emotional consequences or emotion regulation strategies, which go along with an increase of amygdala activity in comparison to women (McRae et al., 2008). The finding of gender differences here is only preliminary, and there are many possible explanations, ranging from gender-based upbringing, phylogenetic differences evolved over the course of human history or differences in socialization (see also Buss, 1999; Brody and Hall, 2008). Our results offer an interesting potential starting point for research investigating the origins of differences in self-conscious emotions.
The comparison of our findings to the original results of Takahashi et al. (2004) revealed that both cultural samples, Japanese and German, display more frontal and temporal activation in the shame condition than in the guilt condition (see also Berthoz et al., 2002).
In the ‘shame minus neutral’ condition, both samples showed activity in the visual cortex, the temporal lobe/temporo-parietal junction and the frontal lobe during the imagination of shameful scenarios. While activation patterns specific to the Japanese sample were found in the shame condition in the left hippocampus (Takahashi et al., 2004), in our German sample we found activations in the left middle occipital gyrus, the parahippocampal gyri bilaterally and the middle frontal gyrus. Although a technical effect cannot be ruled out here, the results of both studies would indicate a mobilization for memory relevant areas during the shame condition in both cultures.
In the ‘guilt minus neutral’ condition, there was activation in the bilateral visual cortex, in the temporo-parietal junction and activation in the frontal lobe in both cultural samples. These are regions critical for imagination and social cognition. In contrast to the German sample, the Japanese sample showed additional activity in the medial frontal gyrus, a region relevant for perspective taking, while we found additional activation in the Germans in the bilateral fusiform gyrus, left superior temporal gyrus, insula, middle and orbitofrontal gyrus and left precentral gyrus, a network that is involved in affective and mnestic processing.
Although it is possible that the differences in brain activation between the two cultures could be the product of differences in fMRI techniques, we interpret the findings as indicating that shame manifests itself similarly across cultures, whereas guilt is based more on specific social standards. In a prior study from Matsumoto et al. (1988) on different emotions in American and Japanese people, it was found that emotions, such as joy, fear, anger, sadness, disgust, shame and guilt are equal across individual and collective cultures, even though their intensity may differ cross-culturally. This is in accordance with the assumption that emotions are universal and genetically pre-determined (see also Bedford, 2004).
This study provides evidence that the activation reflecting the processing of shame is more complex than feeling guilt. One reason for this could be that shame is not so much based on general social standards but rather on culturally independent social settings. Nevertheless, shame may indeed fluctuate according to individual standards, and the specificity of particular social situations. For example, it can be difficult to imagine the reactions of other people to a specific shame situation, thus people may have problems in visualizing the possible thoughts and behavioural reactions of others. The imagination of guilt, on the other hand, may be based less on predicting others’ reactions, and more on the culture-specific normative moral knowledge of right and wrong. However, the task of imagining guilt likely includes an a priori decision on whether someone is guilty or innocent before vivid visualization and feeling of guilt. This could be the reason why there is generally less activation in the brain in regions associated with situational moral decision making.
Limitations of the study
One limitation of the study might be in the comparison of our data with that of Takahashi et al. (2004), because we used a different experimental setting (only German participants, different laboratory and German sentences) and did not use the primary data from his study for an integrative analysis, only the results given in the article for theoretical comparison.
We could not control the success of imagination and generation of moral feelings, but only indirectly gauge it by rating it after the scanning. The participants were instructed to imagine the described situations, but they themselves were free to regulate how strongly they felt the emotions. It is possible that some participants had really experienced some of the situations, and not others, which could bring about corresponding activation differences (Fink et al., 1996).
Another potential objection to our methods is that the feelings of shame and guilt could arise at the same time in the same situations (Eisenberg, 2000) and therefore might be entangled with each other. However, for this study, we chose pre-experimentally situations that differentiated well between shame and guilt as supported by a priori evaluations.
Finally, the small sample size may constrict the generalizability and validity of the comparisons. To exclude these effects and to provide further support for the gender difference, future research needs to be conducted with larger samples.
CONCLUSION
Reasons for similarity between Takahashi et al.’s (2004) findings and our own could be attributed to the possibility that shame is processed similarly across cultures as it is linked more directly to biophysiological processes (Kemeny et al., 2004), whereas guilt is based more on culture dependent and learned social standards (Ausubel, 1955). For example, further cross-cultural comparisons in other individualistic and collective populations or in samples with various faiths, e.g. such as members of Christian or Muslim faiths could bring more insight into the processing of shame and guilt (Bierbrauer, 1992).
Further analysis of gender differences indicate gender-specific processing of these stimuli reflected by inconsistent differences (Brody and Hall, 2008) so far. Future studies in larger gender samples could investigate the true nature of these differences, that is, whether they are more based upon upbringing or have deeper phylogenetic roots.
Additionally, analysing pathological deviations in self-conscious emotions in clinical groups, such as antisocial participants could yield further insights into this area. So far it has been demonstrated that rule-breaking behaviour is in part due to impairments in some of the neurobiological areas also crucial for moral cognition and behavior (Rain and Yang, 2006).
It would also be of interest to extend this research to cover the neuronal basis of other self-conscious emotions like revenge or honour in comparison to shame and guilt, which could be important in subjects with dissocial disorder. Similar to obsessive-compulsive disorder (OCD) patients changes in the serotonin household, as well as a dysfunction of specific brain regions (prefrontal cortex and limbic regions, e.g. amygdala) were shown so far in this subjects (Kiehl, 2006). In summary, there are numerous research questions, which would enhance our understanding of neuronal processing of emotion.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
We thank James Moran and Sanni Norweg for proofreading the manuscript and Ute Coates for technical assistance during scanning.
REFERENCES
- Adolphs R. Is the human amygdala specialized for processing social information? Annals of the New York Academy of Sciences. 2003;985:326–40. doi: 10.1111/j.1749-6632.2003.tb07091.x. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–4. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Ausubel DP. Relationships between shame and guilt in the socializing process. Psychological Review. 1955;62(5):378–90. doi: 10.1037/h0042534. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Stillwell AM, Heatherton TF. Guilt: an interpersonal approach. Psychological Bulletin. 1994;115(2):243–67. doi: 10.1037/0033-2909.115.2.243. [DOI] [PubMed] [Google Scholar]
- Bedford OA. The individual experience of guilt and shame in Chinese culture. Culture & Psychology. 2004;10:29–52. [Google Scholar]
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology. 2003;85(4):594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Beer JS. Neural system for self-conscious emotions and their underlying appraisals. In: Tracy JL, Robins RW, Tangney JP, editors. The Self-Conscious Emotions. Theory and Research. New York: The Guilford Press; 2007. pp. 53–62. [Google Scholar]
- Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. Journal of Cognitive Neuroscience. 2006;18(6):871–9. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B Statistical Methodology. 1995;57:289–300. [Google Scholar]
- Berthoz S, Armony JL, Blair RJR, Dolan RJ. An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain. 2002;125(8):1696–1708. doi: 10.1093/brain/awf190. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Grèzes J, Armony JL, Passingham RE, Dolan RJ. Affective response to one's own moral violations. NeuroImage. 2006;31(2):945–50. doi: 10.1016/j.neuroimage.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Bierbrauer G. Reactions to violation of normative standards: a cross-cultural analysis of shame and guilt. International Journal of Psychology. 1992;27(2):181–93. [Google Scholar]
- Blair RJR, Cipolotti L. Impaired social response reversal. A case of “acquired sociopathy. Brain. 2000;123(6):1122–41. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. Journal of Neuroscience. 1996;16:4207–21. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody LR, Hall JA. Gender and emotion in context. In: Lewis M, Haviland-Jones JM, Feldmann Barett L, editors. Handbook of Emotions. New York: The Guilford Press; 2008. pp. 395–408. [Google Scholar]
- Buss DM. Evolutionary Psychology. A New Science of the Mind. Boston, MA: Allyn and Bacon; 1999. [Google Scholar]
- Caplovitz Barrett K. A functionalist approach to shame and guilt. In: Tangney JP, Fischer KW, editors. Self-conscious Emotions: The Psychology of Shame, Guilt, Embarrassment, and Pride. New York, NY: The Guilford Press; 1995. pp. 25–63. [Google Scholar]
- Carr L, Iacoboni M, Dubeau M-C, Mazziotta JC, Lenzi GL. Mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(9):5497–502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hooge IE, Zeelenberg M, Breugelmans SM. Moral sentiments and cooperation: differential influences of shame and guilt. Cognition and Emotion. 2007;21(5):1025–42. [Google Scholar]
- De Rivera J. Emotional experience and qualitative methodology. American Behavioral Scientist. 1984;27(6):677–88. [Google Scholar]
- Devinsky O, Hafler DA, Victor J. Embarrassment as the aura of a complex partial seizure. Neurology. 1982;32(11):1284–5. doi: 10.1212/wnl.32.11.1284. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. Emotion, regulation, and moral development. Annual Review of Psychology. 2000;51:665–97. doi: 10.1146/annurev.psych.51.1.665. [DOI] [PubMed] [Google Scholar]
- Emde RN, Oppenheim D. Shame, guilt and the oedipal drama: developmental considerations concerning morality and the referencing of critical others. In: Tangney JP, Fischer KW, editors. Self-conscious Emotions: The Psychology of Shame, Guilt, Embarrassment, and Pride. New York, NY: The Guilford Press; 1995. pp. 413–36. [Google Scholar]
- Farrow TFD, Zhen Y, Wilkinson ID, et al. Investigating the functional anatomy of empathy and forgiveness. Neuroreport. 2001;12(11):2433–8. doi: 10.1097/00001756-200108080-00029. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Kamel N, Mitchell DGV, Blair JR. Caught in the act: the impact of audience on the neural response to morally and socially inappropriate behavior. NeuroImage. 2006;33(1):414–21. doi: 10.1016/j.neuroimage.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss W-D. Cerebral representation of one’s own past: neural networks involved in autobiographical memory. Journal of Neuroscience. 1996;16(13):4275–82. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;3:165–89. [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Science. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Goebel R, Jansma H. Brain Voyager QX (Version 2.2). [Computer Software and Manual] Maastricht, The Netherlands: Brain Innovation B.V.; 2006. [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. Biological Psychiatry. 63: 2008. The neural bases of emotion regulation: reappraisal and suppression of negative emotion; pp. 577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J, Sommerville R, Nystrom L, Darley J, Cohen J. Science. 293: 2001. An fMRI investigation of emotional engagement in moral judgment; p. 2105. [DOI] [PubMed] [Google Scholar]
- Haidt J. The moral emotions. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of Affective Sciences. Oxford: Oxford University Press; 2003. pp. 852–70. [Google Scholar]
- Heine SJ, Lehman DR, Markus HR, Kitayama S. Psychological Review. 106: 1999. Is there a universal need for positive self-regard? pp. 766–94. [DOI] [PubMed] [Google Scholar]
- Kemeny ME, Gruenewlad TL, Dickerson SS. Psychological Inquiry. 15: 2004. Shame as the emotional response to threat to the social self: implications for behaviour; pp. 153–60. [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142(2–3):107–28. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. Self-conscious emotions: embarrassment, pride, shame, and guilt. In: Lewis M, Haviland-Jones JM, Feldmann Barett L, editors. Handbook of Emotions. New York: The Guilford Press; 2010. pp. 742–56. [Google Scholar]
- Matsumoto D, Kudoh T, Scherer K, Wallbott H. Antecedents of and reactions to emotions in the United States and Japan. Journal of Cross Cultural Psychology. 1988;19(3):267–86. [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JJD, Gross JJ. Gender differences in emotion regulation: an fMRI study of cognitive reappraisal. Group Processes & Intergroup Relations. 2008;11:143–62. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita B, Frijda NH. Cultural variations in emotions: a review. Psychological Bulletin. 1992;112(2):179–204. doi: 10.1037/0033-2909.112.2.179. [DOI] [PubMed] [Google Scholar]
- Miller RS. Neural system for self-conscious emotions and their underlying appraisals. In: Tracy JL, Robins RW, Tangney JP, editors. The Self-conscious Emotions. Theory and Research. New York: The Guilford Press; 2007. pp. 53–62. [Google Scholar]
- Miyamoto Y, Uchida Y, Ellsworth PC. Culture and mixed emotions: co-occurrence of positive and negative emotions in Japan and the United States. Emotion. 2010;10(3):404–15. doi: 10.1037/a0018430. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Bramati IE, Grafman J. Functional networks in emotional moral and nonmoral social judgments. NeuroImage. 2002;16:696–703. doi: 10.1006/nimg.2002.1118. [DOI] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. The neural basis of human moral cognition. Neuroscience. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Science. 2004;8(3):102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wagner T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry. 2003;54(5):504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Presentation®. Neurobehavioral Systems (Version 0.80) [Computer software] 2005. http://www.neurobs.com/ [Google Scholar]
- Rain A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Social Cognitive and Affective Neuroscience. 2006;1(3):203–13. doi: 10.1093/scan/nsl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. Journal of Cognitive Neuroscience. 2004;16(6):988–99. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Schmidt K-H, Metzler P. Wortschatztest (WST) Weinheim: Beltz Test GmbH; 1992. [Google Scholar]
- Shin LM, Dougherty DD, Orr SP, et al. Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biological Psychiatry. 2000;48:43–50. doi: 10.1016/s0006-3223(00)00251-1. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Kessler C, Salloum JB, Posse S. Gender differences in regional cerebral activity during sadness. Human Brain Mapping. 2000;9(4):226–38. doi: 10.1002/(SICI)1097-0193(200004)9:4<226::AID-HBM4>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS Inc. (Version 17.0) [Computer Software] Chicago, IL: SPSS; 2008. [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y. Brain activation associated with evaluative processes of guilt and embarrassment: an fMRI study. NeuroImage. 2004;23:967–74. doi: 10.1016/j.neuroimage.2004.07.054. [DOI] [PubMed] [Google Scholar]
- TalairachClient (2010) (Version 2.4.2.) [Computer Software] http://www.talairach.org./client.html. [Google Scholar]
- Talairach J, Tournoux P. Stuttgart: Thieme; 1988. Co-planar stereotaxic atlas of the human brain. [Google Scholar]
- Tangney JP. Situational determinants of shame and guilt in young adulthood. Personality and Social Psychology Bulletin. 1992;18:199–206. [Google Scholar]
- Wittchen H-U, Zaudig M, Fydrich T. SKID-I und SKID-II. Struktuiertes Klinisches Interview für DSM-IV. Göttingen: Hogrefe Verlag; 1997. [Google Scholar]
- Wallbott HG, Scherer KR. Cultural determinants in experiencing shame and guilt. In: Tangney JP, Fischer KW, editors. Self-conscious Emotions. The Psychology of Shame, Guilt, Embarrassment, and Pride. New York: The Guilford Press; 1998. pp. 465–87. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.