Abstract
Humans and other primates shift their attention to follow the gaze of others [gaze following (GF)]. This behavior is a foundational component of joint attention, which is severely disrupted in neurodevelopmental disorders such as autism and schizophrenia. Both cortical and subcortical pathways have been implicated in GF, but their contributions remain largely untested. While the proposed subcortical pathway hinges crucially on the amygdala, the cortical pathway is thought to require perceptual processing by a region in the posterior superior temporal sulcus (pSTS). To determine whether pSTS is necessary for typical GF behavior, we engaged rhesus macaques in a reward discrimination task confounded by leftward- and rightward-facing social distractors following saline or muscimol injections into left pSTS. We found that reversible inactivation of left pSTS with muscimol strongly suppressed GF, as assessed by reduced influence of observed gaze on target choices and saccadic reaction times. These findings demonstrate that activity in pSTS is required for normal GF by primates.
Keywords: gaze following, joint attention, superior temporal sulcus, face-selective neurons, autism
INTRODUCTION
Orienting attention in the same direction that another looks is a social competence, known as gaze following (GF), thought to be foundational to joint attention and theory of mind (Posner and Cohen, 1984; Emery et al., 1997; Deaner and Platt, 2003; Shepherd, 2010). GF is widely found in diverse animals, including primates (Tomasello et al., 1998), birds (Bugynar et al., 2004; Jaime et al., 2009) and even tortoises (Wilkinson et al., 2010). In humans, GF is an early-emerging joint attention behavior that predicts subsequent social and language skills (Brooks and Meltzoff, 2005, 2008); dysfunctional joint attention is a defining feature of neurodevelopmental disorders such as autism (American Psychiatric Association, 1994; Charman et al., 1997; Senju et al., 2004; Zilbovicius et al., 2006).
Despite its importance to normal social behavior, the neural mechanism mediating GF remains unclear. Current models suggest GF either relies primarily upon evolutionarily primitive subcortical circuitry, as suggested by its speed, reflexivity, early development and phylogenetic ubiquity (Sewards and Sewards, 2002; Johnson, 2005), or depends upon cortical areas optimized for detecting the gaze of others and sharing attention (Shepherd, 2010; Shepherd and Cappuccio, 2012). A critical finding regarding this argument is Sato and colleague’s discovery (Sato et al., 2007) that gaze cues influence orienting even when presented subliminally, and that certain subliminal gaze cues are as effective as supraliminal cues at influencing attention. A follow-up study by the same group suggested that Asperger’s patients are primarily impaired in GF to subliminal gaze cues, relative to neurotypicals (Sato et al., 2010), consistent with the Johnson model (Johnson, 2005; Senju and Johnson, 2009a,b) in which subcortically mediated social orienting acts to bias cortical development toward processing socially relevant signals. The relevance of this hypothesized subcortical pathway, previously implicated in fear processing (Morris et al., 1999), was bolstered by reports (Akiyama et al., 2007; Okada et al., 2008) that amygdala lesions disrupt GF in human adults.
Even if a subcortical pathway for GF exists, however, it seems likely that cortical pathways are also important. While gaze perception is not logically necessary for GF (Sato et al., 2007; Shepherd, 2010; Shepherd and Cappuccio, 2012), several results suggest that cortical perceptual processing is critical. In two split-brain patients, GF was found to be restricted to a single hemisphere (typically right), thereby emphasizing the importance of lateralized cortical areas in the behavior (Kingstone et al., 2000). Additionally, one patient with a large right superior temporal lesion failed to both perceive and to follow gaze (Akiyama et al., 2006a,b). In macaques, large bilateral lesions in superior temporal gyrus lead to deficiencies in gaze perception (Campbell et al., 1990), but effects on GF were not measured.
Given that various cortical and subcortical pathways have been implicated in gaze processing (Perrett et al., 1982, 1985; Kawashima et al., 1999; Allison et al., 2000; Akiyama et al., 2007; Gothard et al., 2007; Senju and Johnson, 2009b), how might we determine whether cortical processing is actually necessary for GF in healthy adults? We focus our attention on the putative bottleneck, posterior superior temporal sulcus (pSTS). In humans, strong activation of pSTS during GF tasks indicate that this area is capable of extracting socially-relevant directional cues, including averted gaze (Hoffman and Haxby, 2000; Materna et al., 2008; Sato et al., 2008; Kamphuis et al., 2009). While pSTS in neurotypicals responds differentially to congruent vs incongruent gaze cues, this difference is absent in autistic individuals (Pelphrey et al., 2005), consistent with a role for this area in the pathophysiology of autism. In macaques engaged in GF tasks, pSTS, along with more anterior areas of STS, responds differentially to observing faces gazing in different directions (Kamphuis et al., 2009). Classic single-unit recordings from anterior STS in macaques found neurons selective for the orientation of observed faces and eyes (Perrett et al., 1982, 1985; Tsao et al., 2006). More recently, fMRI-guided recordings in macaques have identified distinct patches in the STS that contain high concentrations of face-selective neurons, including one such patch located in the pSTS (Tsao et al., 2006). Taken together, these results raise the possibility that face-orientation selective neurons in pSTS play a critical role in GF. However, the necessity of pSTS for normal social GF has never been experimentally demonstrated using reversible lesions or microstimulation techniques.
To directly probe the functional anatomy of GF, we engaged rhesus macaques in a novel reward discrimination task with task-irrelevant social gaze cues as distractors following injections of muscimol or saline into left pSTS. Intact macaques reliably shifted their own gaze in the direction of observed gaze, as indexed by target choices and reaction times (RTs). We also found that unilateral muscimol injections to pSTS strongly suppressed GF, whereas saline injections did not, supporting the hypothesis that this area is necessary for normal GF.
MATERIALS AND METHODS
Subjects and general procedures
All experiments were carried out in two male rhesus macaques (Macaca mulatta; denoted as M1 and M2) pair-housed at the Duke University Medical Center. Monkeys’ water access was controlled outside of experimental sessions to motivate performance. All procedures were conducted in accordance with the Public Health Service’s Guide for the Care and Use of Animals and approved by Duke University Institutional Animal Care and Use Committee.
Experiments were run on a Dell Precision T3400 computer, using custom software written in MATLAB Psychtoolbox-2. Monkeys viewed stimuli on a dark background on 20″ Sony Trinitron CRT monitor (1280 × 1024 resolution and 60 Hz refresh rate) 17″ away. Eye position was monitored at 500 Hz using an Eyelink II gaze-tracking system. Monkeys’ heads were held still by a surgically implanted titanium prosthesis (Crist Instruments). All surgeries were carried out using aseptic techniques and followed with analgesics and antibiotics.
Behavioral methods
Reward discrimination task
Monkeys were fixated for 500 ms on a central white rectangle 1° × 1°. This was followed by a face (10° × 10° in size) of a monkey—chosen randomly from a pool of 66 headshots of eight monkeys from our colony—with head and eyes facing either to the subject’s left (‘cue-left’) or right (‘cue-right’). Duration of face display followed a hazard function (µ = 0.5 s, σ = 1 s). If monkeys failed to fixate within the borders of the face the trial was terminated and an inter-trial interval (ITI) of 500 ms ensued. After face offset, two targets (1° × 1° white rectangles) appeared as radial pairs on the perimeter of an imaginary circle, reflected through the origin, with a radius of 15°. The angular location (θ) on the perimeter of the imaginary circle determined the exact positions of the target pair and these positions varied randomly for every trial. The vertically located pair was not used. In 1 out of 10 trials in a single block we used a gray square as cue image instead of facial images and these trials were labeled ‘neutral’ trials.
For every target pair, the one on the subject’s left was the ‘left target’ and the one on the right was the ‘right target’. Cue-congruent targets were in the same direction as gaze in the face image; cue-incongruent targets were in the opposite direction. Following target presentation, monkeys had 300 ms to shift gaze to a single target (±7.5°) and maintain gaze for at least 300 ms. The monkey was then rewarded with a squirt of fruit juice. Reward size was determined by solenoid open time in milliseconds.
For each block of trials, left and right targets were assigned fixed but unequal juice amounts, which varied across blocks. Differential reward value for the two targets, Δ reward, varied between −100 and 100 ms over seven blocks (pairs of juice values used: 100/200, 120/180, 140/160, 150/150, 160/140, 180/120 and 200/100 ms; Figure 1a). ITI of 500 ms followed reward delivery. To encourage sampling of both target sides, choice trials were sparsely interleaved with single-target trials in which only one target was presented and a gaze shift to it was rewarded according to the reward schedule of the current block. Only choice trials were analyzed for this study. On a single day, 1000–1200 successful trials were obtained from each monkey and data from a day were only included for analysis if the monkey met a criterion of ≥75% correct.
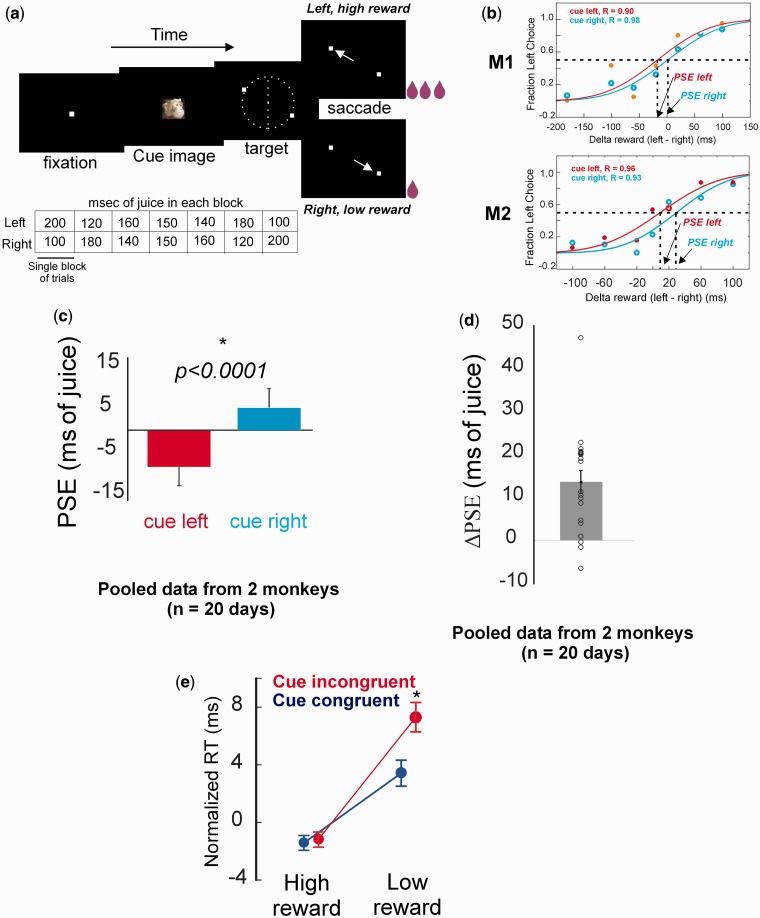
Fig. 1.
(a) Schematic of the reward discrimination task and block structure. For details about the task see ‘Materials and Methods’ section. (b) Choice curves for left target choice from a single session; reward for left target increases along x-axis; top: monkey M1, bottom: monkey M2. In both monkeys choice for left target reliably increased with increasing reward size, but the choice curves for cue-left (red) and cue-right (blue) trials were shifted from each other along the x-axis. (c) Mean ± SEM PSEs for cue-left and cue-right trials, pooled data from two monkeys. (d) Single-session and mean ± SEM ΔPSE values from two monkeys. (e) Normalized saccade RT showing interaction of reward congruency and cue congruency. Cue-congruent saccades were faster than cue-incongruent ones, but only when made toward the low-reward targets.
Passive face viewing task
Electrophysiological recording from face-selective neurons was used to identify pSTS for injections, using a passive-viewing task. An initial fixation (500 ms) phase was followed by presentation of an image for a fixed duration of 1 s. Images were randomly selected from a pool of 30 monkey faces (headshots with head and gaze turned either straight, to left or to right, 10 of each) and 30 objects (fruits and vegetables, flowers or insects, 10 of each). Following image presentation, a single target was presented at variable locations along the perimeter of an imaginary circle of radius 15°. After shifting gaze to the target the monkey was rewarded with a 150 ms squirt of juice, an ITI of 500 ms ensued and the next trial implemented.
Magnetic resonance imaging
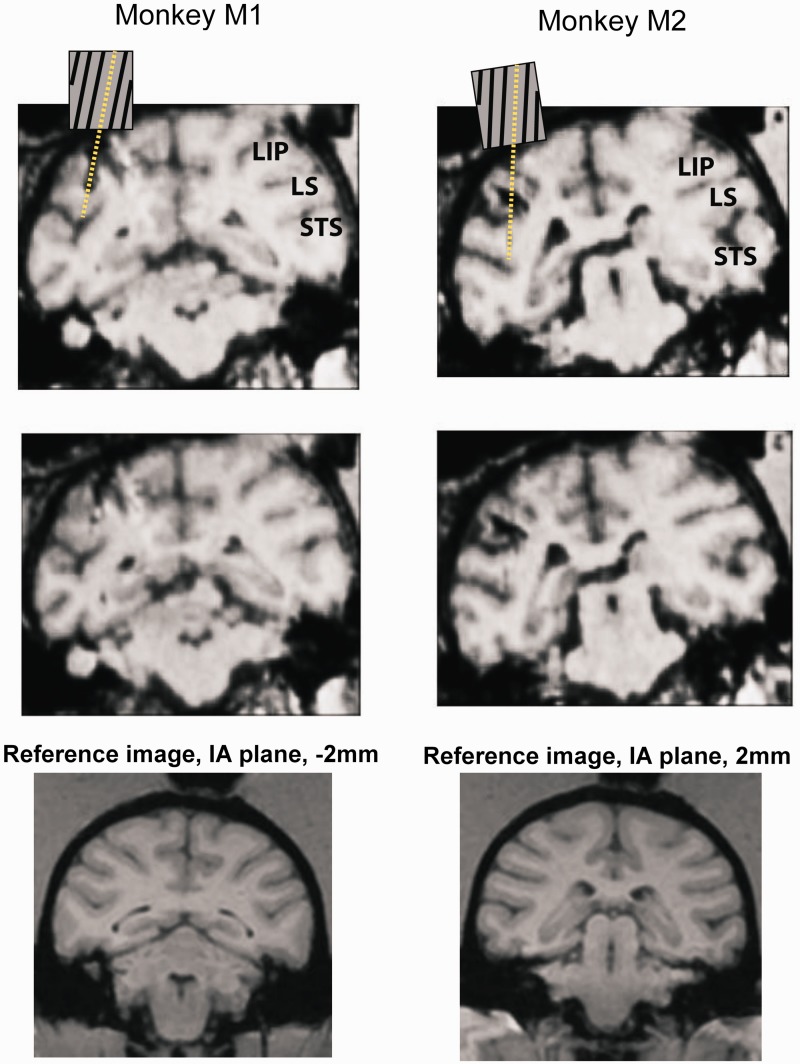
To localize the site of electrophysiological recordings and saline/muscimol injections, we carried out MRI scans using a Siemens 3T MR Imaging Instrument located at the Center for Advanced Magnetic Resonance Development at Duke University Medical Center. Coronal and sagittal scans were made with 0.5-mm slice thickness. Before starting experiments, an initial scan was done with 10 and 15° angled grids placed in the recording chamber and electrodes positioned along the grid channels. From these scans we determined which angled grid channels would target pSTS. Upon conclusion of all recording and injection experiments, a second set of scans was carried out to note any visible tracks left by the electrodes and the microsyringe (Figure 3). Scans were compared to reference images from an online macaque brain atlas (http://brainmaps.org/index.php?action=viewslides&datid=141&start=1) to ascertain the position of sections with respect to interaural plane.
Fig. 3.
Two successive coronal sections (0.5-mm thickness) from structural MRI scans in monkey M1 and M2 showing approach of electrode tracks toward left pSTS. In the top section, a schematic (not drawn to scale) of the angular grid shows the electrode path extending to pSTS. Matching reference images from an online macaque brain atlas (source: http://brainmaps.org/index.php?action=viewslides&datid=141&start=1) shown below the MRI scans identify the scans in M1 and M2 to lie at −2 and 2 mm from interaural plane, respectively. LIP: lateral intraparietal sulcus; LS: lateral sulcus.
Microelectrode recordings
Detailed description of single-unit recordings with tungsten electrodes and Plexon spike-sorting system has been published previously (Shepherd et al., 2009). Monkeys M1 and M2 were used in this previous study to record from lateral intraparietal area (LIP) through recording chambers implanted over the left parietal lobe. The same parietal chambers were used for extending electrodes toward the left pSTS in the current study. To target pSTS, a 23-gauge hypodermic guide tube containing a tungsten electrode (Frederick Haer) was inserted through a plastic grid (Crist Instruments) with angled (10°) channels spaced at 1-mm intervals. Only a single tungsten electrode was inserted through one channel during one recording session. The optimal grid orientation for pSTS was estimated from MRI scans. The center of the grid was located 3-mm posterior to inter-aural zero (IA0) and 12-mm lateral from midline of the brain. The grid was positioned such that the angled channels pointed lateral at an angle of 10° (Figure 2a, top). In this orientation, only two to three channels near the center of the grid provided reliable access to pSTS in each monkey (Figure 2a, bottom). Electrodes were lowered through these central channels 21–24 mm along the angled tract until neurons with face-responsive activity were encountered. Based on both visible electrode paths on MRI images (Figure 3) and neuronal activity matched to a macaque brain atlas, we estimate that we recorded and injected muscimol primarily in the upper banks and the fundus of the pSTS between −2 and 2 mm of IA0.
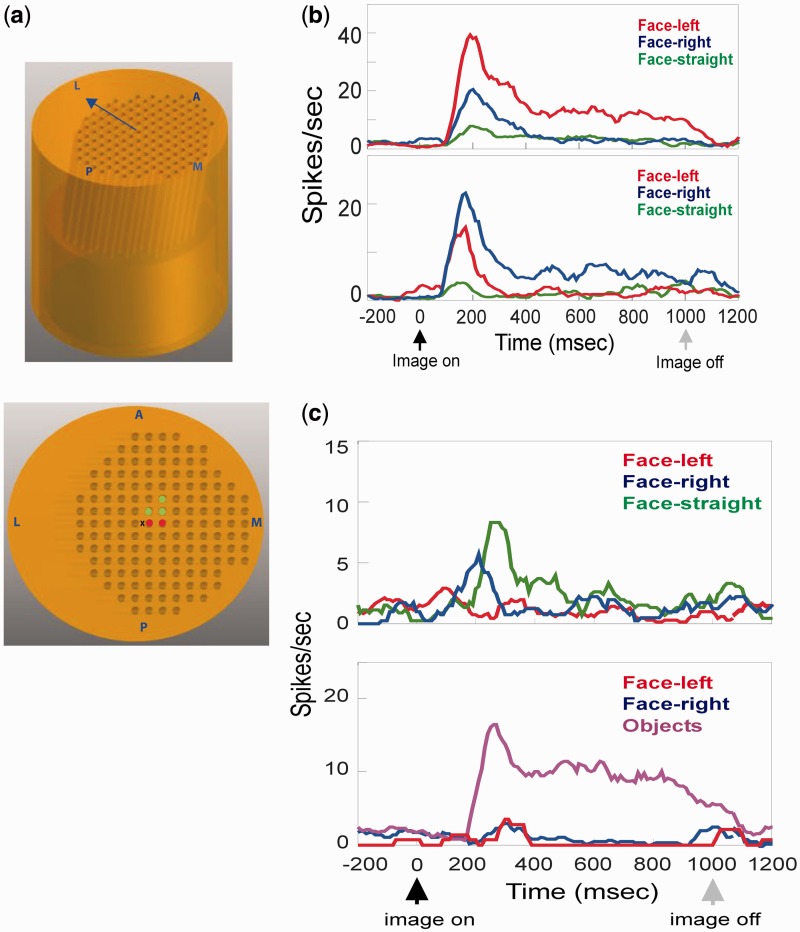
Fig. 2.
(a) The 10°-angled grid in sideview (top) and topview (bottom) showing the layout of channels. The grid was positioned such that the angled channels pointed in the lateral direction (arrow, top). Center of the grid (marked X, bottom) was located 3-mm posterior and 12-mm lateral to IA0. Two channels in M1 (red) and three channels in M2 (green) were used for electrophysiology and muscimol injections. Anterior (A), posterior (P), medial (M) and lateral (L) directions are shown. (b) Firing rates of two pSTS neurons selective for left (top) and right (bottom) face orientations. (c) Firing rates of two pSTS neurons selective for straight face (top) and objects (bottom).
Upon isolation of single waveform, 30 trials of passive face-viewing task with face and object images each were run. Images of monkey faces and objects were randomly chosen from the pool of images described above. If neuronal responses to facial images were overall greater than that to the images of objects, equal numbers of trials with face-left, face-right and face-straight images were further run to test face orientation selectivity. Cells with responses selective for object over faces were not further tested, except for a small subset (n = 3) of cells for which equal numbers of trials with object, face-left and face-right images were further run.
Muscimol injections in pSTS
To inject muscimol, a long-acting agonist of GABAA channels, a Hamilton microsyringe (Crist) was advanced to pSTS through the angled channels on the grid. A total of 3–5 μl of muscimol solution (10 μg/μl) was pressure injected through the microsyringe and spread over multiple spots. Along each of the two tracks through which the microsyringe was advanced, we injected muscimol at two different depths separated by 1 mm, resulting in a total of four different loci of injection per session. At each locus we pressure-injected 0.75–1.25 μl muscimol, in steps of 0.25 μl separated by intervals of 2 min to allow for slow diffusion. On saline control days, 3–5 μl of 0.9% sterile saline solution was injected into pSTS following the same procedure. Saline and muscimol were injected in the same monkey on alternate days. Following injection the microsyringe was slowly (10 μm/s) retracted from the brain before behavioral testing started. On average about 30 min elapsed between the first bolus of injection and the onset of behavioral testing, and all behavioral testing was completed within 2.5 h of the first injection.
Data analysis
Reward discrimination task analysis
For each block, the cue-left and cue-right trials were separated, and the fraction of times the monkey chose the left target (# times left target chosen/# total trials in block) calculated for each subset. A cumulative normal function was fit to the plot of the ‘fraction left choice’ values against the ‘Δ reward’ values (juice for left target minus juice for right target) for each block. Cumulative normal fits were calculated in Statistica 9 (StatSoft, Tulsa, OK, USA). ‘Point of subjective equivalence’ or PSE, defined as the Δ reward value at which the fraction left choice was 0.5, was estimated from the fitted curve following the Hooke–Jeeves and Quasi–Newton non-linear estimation method, with the following values: start values, parameter 1 = 0.5, parameter 2 = 100; initial steps, parameter 1 = 0.5, parameter 2 = 10. Only fitted curves with R > 0.75 were included in further analysis and the PSE estimates were constrained between −60 and 60 ms. PSEs obtained for cue-left and cue-right trials were designated PSE:cue-left and PSE:cue-right, respectively. GF effect was estimated by the difference between PSE:cue-right and PSE:cue-left (ΔPSE = PSE:cue-right minus PSE:cue-left).
RT analysis
To analyze saccade RTs, the monkeys’ left eye position was monitored using the Eyelink II system at a resolution of 2 ms throughout the experiment session. Saccade RTs were defined as the time elapsed between the onset of the targets and the onset of the saccade, defined by velocity (≥50°/s for ≥5 consecutive 2 ms samples). Saccade offset was defined as the time at which the eye was detected within a 7.5° radius around the target center. Normalized RTs were generated by subtracting the daily average RTs from individual trial RTs and these normalized RTs from all trials from all days in both monkeys were pooled for statistical analysis.
We classified each saccade made to a target along two dimensions: ‘reward congruency’ denoted whether the saccade was made to a high or low-reward target and ‘cue congruency’ denoted whether the saccade was made toward a face congruent or incongruent target. Normalized RTs of all saccades were then analyzed using a 2 × 2 × 2 ANOVA design, with RT as the dependent variable. Trials with neutral cue (gray square) were outnumbered 10:1 by facial cue trials and thus were not included in the same overall ANOVA for RT. For saline and muscimol conditions the RTs of neutral trials were analyzed separately to test for general visual deficiency caused by pSTS inactivation. For baseline experiments (no injections), the three categorical factors in the ANOVA were subject (M1 or M2), reward congruency (high or low) and cue congruency (congruent or incongruent). For pSTS inactivation experiments, the three categorical factors were treatment (saline or muscimol), reward congruency (high or low) and cue congruency (congruent or incongruent). Fisher’s post hoc LSD (FPLSD) test was used to compare RTs between sub-categories for those interaction terms that showed significance at P < 0.05 level (shown in red in Table 1).
Table 1.
ANOVA results for normalized saccade RT during GF choice task
| Factors | d.o.f a | F | P |
|---|---|---|---|
| GF choice task, baseline (no injections) | |||
| Subject (M1, M2) | 1 | 17.7 | 0.000026 |
| Juice congruency (high, low) | 1 | 74.04 | 0.000000 |
| Cue congruency (congruent, incongruent) | 1 | 7.1 | 0.007914 |
| Subject × juice congruency | 1 | 54.6 | 0.000000 |
| Subject × cue congruency | 1 | 0.3 | 0.579525 |
| Juice congruency × cue congruencyb | 1 | 5.6 | 0.018369 |
| Subject × juice congruency × cue congruency | 1 | 0.08 | 0.775165 |
| GF choice task, with saline or muscimol injections | |||
| Treatment (saline, muscimol) | 1 | 0.24 | 0.620895 |
| Juice congruency (high, low) | 1 | 135.8 | 0.000000 |
| Cue congruency (congruent, incongruent) | 1 | 3.2 | 0.072401 |
| Treatment × juice congruency | 1 | 1.7 | 0.187776 |
| Treatment × cue congruency | 1 | 1.4 | 0.233408 |
| Juice congruency × cue congruency | 1 | 0.27 | 0.601579 |
| Treatment × juice congruency × cue congruencyc | 1 | 2.51 | 0.113354 |
aDegrees of freedom.
bFisher LSD test:
high juice–cue congruent vs high juice–cue incongruent: P = 0.84.
low juice–cue congruent vs low juice–cue incongruent: P = 0.007.
cFisher LSD test:
Saline:
high juice–cue congruent vs high juice–cue incongruent: P = 0.5.
low juice–cue congruent vs low juice–cue incongruent: P = 0.03.
Muscimol:
high juice–cue congruent vs high juice–cue incongruent: P = 0.25.
low juice–cue congruent vs low juice–cue incongruent: P = 0.86.
Frequency distributions of the measured variables (PSE:cue-right, PSE:cue-left etc.) closely resembled normal distributions. Therefore, parametric tests were carried out for statistical comparisons throughout the study. Non-parametric comparisons closely paralleled the parametric results, and have been described in the Supplementary Data.
Analysis of STS neuronal responses
For each isolated and sorted single unit, we first compared the number of total spikes fired in response to object and face images during the 1-s image-viewing interval (t-test). Neurons that showed significantly greater responses to faces over objects (P < 0.05) were further tested with face-straight, face-left and face-right images for at least 30 trials each. Total spikes during the image-viewing interval for these trials were then compared using one-way ANOVA. Neurons were considered face-orientation selective if the overall ANOVA showed significance at P = 0.05 level, and subsequently each of the three face-orientation categories were further compared to each other using Fisher’s post hoc LSD test to ascertain the most effective face stimulus for the neuron. We also constructed peri-stimulus time histograms (PSTHs) by aligning the spike trains to the onset of the face/object image and calculating firing rates in 10 ms bins. Before plotting, the PSTHs were smoothed by 10-point moving average method using the ‘smooth’ function in Matlab.
RESULTS
Monkeys viewed an image of a familiar monkey (cue) with its head and eyes oriented to the subject’s left (‘cue-left’) or right (‘cue-right’) and then chose between a left and a right target, which were differentially rewarded (Figure 1a; see ‘Materials and Methods’ section). The face’s direction of gaze did not predict the differential reward associated with each target. Psychometric curves revealed that monkeys reliably chose the target offering larger reward (Figure 1b), but choice probabilities were also influenced by observed gaze direction. Choice curves for cue-left and cue-right trials were shifted to the left and right, respectively, indicating monkeys required less juice to choose a target congruent with observed gaze direction. Cumulative normal functions fit to preference curves yielded PSE, an index of the impact of the observed gaze direction on target choice (Deaner et al., 2005). Pooled data from two monkeys revealed that the mean PSE:cue-right was greater than mean PSE:cue-left (Figure 1c; mean PSE:cue-left = −8.56 ± 4.3 ms, PSE:cue-right = 5.1 ± 4.6 ms, P < 0.0001, n = 20, paired t-test). The effect of gaze cues on target choice in each session, as estimated from the difference between PSE:cue-right and PSE:cue-left (ΔPSE), was significantly different from zero (Figure 1d; mean ΔPSE = PSE:cue-right minus PSE:cue-left = 13.63 ± 2.7 ms juice, n = 20; t-test against mean = 0, P < 0.0001), indicating GF.
RTs for choice saccades were evaluated as a secondary measure of GF (Deaner and Platt, 2003; Shepherd et al., 2006) on each trial as a function of reward congruency (i.e. choice of the high or low-reward target) and cue congruency (i.e. in the same or opposite direction as the face cue). Normalized RTs pooled from both monkeys were faster for reward- and cue-congruent target choices (2 × 2 × 2 ANOVA, main effects of reward and cue congruency; Table 1). Interaction effects revealed that congruent gaze cues facilitated RTs only when monkeys shifted gaze to low-reward targets (Figure 1e, reward × cue interaction), suggesting unmasking of GF under low-reward conditions.
STS is known to harbor neurons selective for faces, even face orientations (Perrett et al., 1985; Tsao et al., 2006). Therefore, to functionally identify STS for subsequent muscimol injections, we carried out electrophysiological recordings in each monkey through electrodes advanced at an angle from a chamber over the left parietal lobe (for layout of grid see Figure 2a) while the monkeys viewed images of faces or objects (see ‘Materials and Methods’ section). Out of 79 neurons recorded in two monkeys, 10 were selective for faces over objects (13%), 28 were selective for objects over faces (35%) and 41 were not selectively responsive to either category (52%). A majority (7/10) of the face-selective neurons further responded selectively to faces of a particular orientation (Figure 2b), consistent with prior studies (Perrett et al., 1985). After termination of all experiments, electrode and microsyringe paths were further confirmed by MRI to target the very posterior portion of STS (within 2 mm of interaural plane), with all recordings and inactivations in the upper bank and fundus of pSTS (Figure 3; for further detail see ‘Materials and Methods’ section).
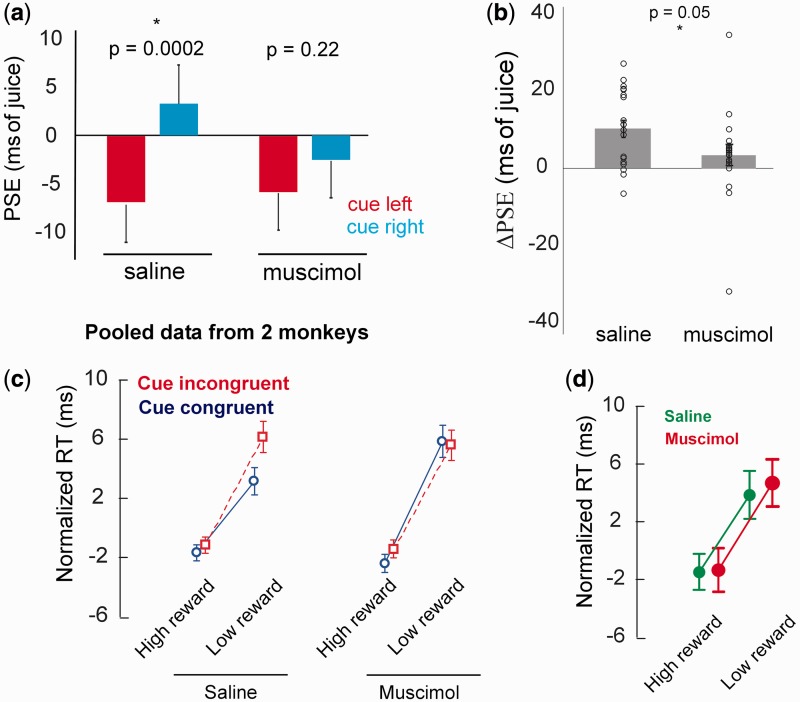
Next, both monkeys performed the reward discrimination task following injections of saline (control days) or muscimol (experiment days) in left pSTS. Muscimol injections did not decrease the monkeys’ efficiency in task performance as assessed from the percent failed trials (error rates; M1: saline: 3069/12 578, muscimol: 3125/12 692, P = 0.7, chi-square test; M2: saline: 2643/12 739, muscimol: 2488/12 559, P = 0.1, chi-square test). However, muscimol injections strongly reduced the magnitude of cue-induced PSE shifts, consistent with suppressed GF, whereas saline had little effect on GF. Injection in left pSTS appeared to impair processing of rightward much more than leftward oriented face cues (Figure 4a; saline: mean PSE:cue-left = −6.96 ± 4.18 ms, PSE:cue-right = 3.26 ± 4.04 ms, P = 0.0002, n = 20, paired t-tests; muscimol: mean PSE:cue-left = −5.93 ± 3.93 ms, PSE:cue-right = −2.58 ± 3.95 ms, P = 0.22, n = 20, paired t-tests). ΔPSE following saline was significantly greater than zero while ΔPSE following muscimol was not; and ΔPSE:saline was significantly greater than ΔPSE:muscimol (Figure 4b; mean ΔPSE: saline: 10.23 ± 2.2 ms, muscimol: 3.35 ± 2.6 ms, P = 0.05, n = 20, t-test; one sample t-test against mean = 0, saline: P = 0.0002, muscimol: P = 0.2).
Fig. 4.
(a) Mean ± SEM PSEs for cue-left and cue-right trials following saline and muscimol injections in pSTS, pooled data from two monkeys. (b) Single-session and mean ± SEM ΔPSE values from saline and muscimol sessions, pooled from two monkeys. (c) Normalized saccade RTs showing reward × cue congruency interactions for saline and muscimol sessions. (d) Normalized saccade RTs for trials with neutral cue (gray square), under saline and muscimol conditions. Note the bigger error bars compared to facial cue trials in (c), due to the sample size for neutral cues being ∼1/10 that of facial cues.
ANOVA revealed overall RTs were unaffected by muscimol, mitigating concern about generalized effects on saccades (Table 1). The interaction term treatment × juice congruency × cue congruency did not reach significance (P = 0.11), but as in our preliminary behavioral data, RTs were faster for cue-congruent saccades primarily when reward was low and when saline rather than muscimol had been injected into pSTS (Figure 4c). For comparison with our earlier data, we confirmed this trend through a direct FPLSD test (FPLSD of RT by cue congruency for low-reward trials:treatment: saline, P = 0.032; treatment: muscimol, P = 0.86), suggesting muscimol reduced the facilitatory effect of observed gaze on target selection under low-reward conditions.
To test if the observed effect of muscimol was caused by general visual deficiency instead of deficiency in face-specific visual processing induced by pSTS inactivation, we separately analyzed the RTs of saccades in the smaller subset of trials where neutral images (a gray square), instead of faces, were used as cues. A 2 × 2 ANOVA of RTs for neutral trials using reward congruency (high or low) and treatment (saline or muscimol) as the two factors revealed that there was no main effect of treatment (P = 0.37; Figure 4d). This confirms that reversible inactivation of pSTS did not cause any deficiency in general visual processing or the alerting effects of any temporally-predictive cue on RT. Muscimol in left pSTS also did not cause neglect or extinction of targets in the contralateral hemifield as the RTs of saccades to left and right targets in the muscimol sessions were indistinguishable (P = 0.98, unpaired t-test, n = 2734 and 2830 for left and right saccades repectively).
DISCUSSION
These findings suggest that cortical activity in the area of pSTS is required for normal GF. The necessity of pSTS for typical primate GF indicated by these data is compatible with both domain-specific (selective for social attention) and domain-general (selective for reorienting or reflexive cuing, cf. the ‘ventral attention network’ of (Corbetta and Shulman, 2002; Corbetta et al., 2008) accounts of pSTS activity. Notably, human pSTS has been implicated in gaze perception, theory of mind and language tasks—all diagnostic dysfunctions in autism spectrum disorders—supporting the possibility that diverse social cognition deficits in autism and other social cognitive disorders may arise through dysfunction in this region (Boddaert et al., 2004; Zilbovicius et al., 2006). A detailed electrophysiological characterization of pSTS was beyond the scope of the current study; we carried out single unit recordings only to confirm that our inactivations were targeted to regions of pSTS containing face-selective neurons. Earlier studies of single unit recordings in STS found an overall low fraction (∼10%) of cells in this area to be face-selective (Perrett et al., 1982, 1985). However, recent studies using fMRI-assisted single unit recordings have revealed multiple patches along the antero-posterior axis of STS that contain almost exclusively face-selective neurones (Tsao et al., 2006). We anticipated that our electrodes, which approached STS from a chamber placed over parietal lobe near interaural zero, would intercept the most posterior of these face patches (Tsao et al., 2006, Figure 1A). Even though we did find face- and face-orientation-selective cells, we never found an area with face-selective cells as concentrated as in fMRI-identified face-patches. Because a limited number of recording grid channels gave access to the temporal lobe (Figure 2a), we cannot rule out having intercepted pSTS just outside the posterior face patch. Nevertheless, given that comparable volumes of injected muscimol can spread 1–3 mm from the injection site (Martin and Ghez, 1999), it is not beyond the bounds of speculation that muscimol activity at the nearby posterior face patch caused the observed effects on GF behavior.
Interestingly, muscimol in left pSTS barely reduced the impact of leftward gaze cues, but strongly reduced the effect of rightward cues (i.e. gaze directed toward the muscimol-inactivated visual field). This is consistent with deficits in detecting gaze directed toward the contralateral hemifield reported in a human patient with a lesion in STG (Akiyama et al., 2006b), and therefore raises the possibility that pSTS primarily processes gaze directed toward the contralateral visual hemifield. However, this is not corroborated by electrophysiological studies so far: recordings in STS to date have shown existence of neurons selective for gaze directed toward both hemifields (Perrett et al., 1982, 1985). But STS is a large, and potentially non-homogeneous, area that may contain pockets of clustered neurons highly selective for gaze directed to contralateral hemifield, and recordings from a much larger sample may be necessary to unravel such localized bias.
Some residual GF might have remained intact following unilateral pSTS inactivation, as evidenced by the similar fraction of sessions yielding PSE:cue-right greater than PSE:cue-left under saline and muscimol conditions. This was confirmed by non-parametric comparisons between PSE:cue-right and PSE:cue-left values from muscimol sessions (Supplementary Data). The intact hemisphere (right) and subcortical pathways may be responsible for this weak residual GF. Subcortical responses are thought to play a role in innate, reflexive orienting responses—e.g. to fearful faces (Morris et al., 1999), staring eyes (Sewards and Sewards, 2002) and perhaps social stimuli more generally (Johnson, 2005)—and may help explain the rapidity and phylogenetic breadth of GF behaviors (Shepherd, 2010). Unfortunately, since we inactivated pSTS unilaterally, we cannot resolve whether the residual GF is due to the intact pSTS or the intact subcortical pathways. The stronger effect of muscimol on GF to contralateral hemifield, however, argues against a role for the subcortical pathway.
Several caveats must be considered in interpreting these results. First, we must consider the possibility that unilateral muscimol injections to the left pSTS region may have resulted in some form of neglect or extinction in the contralateral hemifield. We believe we can discount this possibility: if extinction occurred, we would expect the preference curves for both cue-left and cue-right trials to shift strongly away from the neglected side. No such shift was evident: the curves for leftward and rightward cues were not shifted, on average, but were merely closer together than when measured after saline injections (Figure 4a). Also, there was no difference in RT of saccades to right and left targets following muscimol in left pSTS, thus ruling out the possibility of general neglect of the right hemifield caused by inactivation of left pSTS.
Second, we must consider the possibility that pSTS injections disrupted visual processing in the central visual field for all stimuli, and not merely gaze cues. We find this criticism unpersuasive for two reasons, one empirical and one conceptual: First, neither RT nor error rates were significantly increased after muscimol relative to saline, suggesting that visual processing was not globally disrupted. Second, let us momentarily assume that cortical processing of foveal vision was disrupted for non-social as well as social cues: our data still support the necessity of cortical processing for typical primate GF.
It is an interesting question whether pSTS is also necessary for non-social cuing, such as flickered lights (‘bottom–up’/exogenous attention) or acculturated predictive symbols (e.g. arrows, ‘top–down’/endogenous attention). Cortex near pSTS is known to be part of a ventral attention system implicated both in reflexive attention/reorienting and in social processing (Corbetta et al., 2008). The exact role of this region in social and symbolic cuing has remained unclear despite several studies (Engell et al., 2010), in part due to the limited temporal precision of imaging techniques. Electrophysiological investigation will no doubt help address these issues, but it must be noted that no direct parallel to arrow cues exists in animals; most modern-day humans learn to respond automatically to arrows through a lifetime of acculturation.
In sum, our findings are most consistent with a model in which neurons in pSTS (and downstream STS face patches, cf. Freiwald and Tsao, 2010) decode the direction of observed gaze and influence attention through projections to ipsilateral orienting areas. For example, the LIP contribues to visual attention and saccade preparation, and neuronal firing rates in this area reflect both target selection and oculomotor response time (Goldberg et al., 2006) and are modulated by behaviorally-relevant variables such as reward expectation (Platt and Glimcher, 1999) and social relevance (Klein et al., 2008). Therefore, the muscimol injected in pSTS may have interrupted the flow of social information through STS to LIP. This model is consistent with Shepherd and colleagues’ report that neurons in LIP are sensitive to observed gaze direction, and that the dynamics of gaze-congruent neuronal responses match the dynamics of GF behavior (Shepherd et al., 2009). Note that because LIP itself was otherwise uncompromised, pSTS inactivation influenced GF effects without significantly increasing overall RT. Our findings show this pathway is crucial to normal GF, and imply that pathologies of joint attention deficits (including autism) may involve disrupted processing of gaze cues within pSTS or disrupted flow of information from pSTS and its downstream targets to ispilateral orienting areas such as LIP.
In conclusion, we find that pSTS is required for typical GF in the macaque. We take this as strong evidence for cortical involvement in fast GF responses among anthropoid primates, and adds to the evidence that dysfunction in pSTS may contribute to joint attention deficits in human pathology.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We thank all the members of the Platt Lab for constructive comments on design and analysis of the experiments.
This work was supported by the National Institute of Mental Health [grants 1R01-MH-086712-01 and 5-R03 MH066259-02 to M.L.P]; and two grants from the Autism Speaks Foundation.
REFERENCES
- Akiyama T, Kato M, Muramatsu T, Saito F, Umeda S, Kashima H. Gaze but not arrows: a dissociative impairment after right superior temporal gyrus damage. Neuropsychologia. 2006a;44:1804–10. doi: 10.1016/j.neuropsychologia.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Kato M, Muramatsu T, Saito F, Nakachi R, Kashima H. A deficit in discriminating gaze direction in a case with right superior temporal gyrus lesion. Neuropsychologia. 2006b;44:161–70. doi: 10.1016/j.neuropsychologia.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Kato M, Muramatsu T, Umeda S, Saito F, Kashima H. Unilateral amygdala lesions hamper attentional orienting triggered by gaze direction. Cerebral Cortex. 2007;17:2593–600. doi: 10.1093/cercor/bhl166. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Science. 2000;4:267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1994) In: Diagnostic and Statistical Manual. 4th edn. Washington, DC: American Psychiatric Association; 1994. Autistic disorder. [Google Scholar]
- Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23:364–9. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Brooks R, Meltzoff AN. The development of gaze following and its relation to language. Developmental Science. 2005;8:535–43. doi: 10.1111/j.1467-7687.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R, Meltzoff AN. Infant gaze following and pointing predict accelerated vocabulary growth through two years of age: a longitudinal, growth curve modeling study. Journal of Child Language. 2008;35:207–20. doi: 10.1017/s030500090700829x. [DOI] [PubMed] [Google Scholar]
- Bugynar T, Stowe M, Heinrich B. Ravens, Corvus corax, follow gaze direction of humans around obstacles; 2004. Proceedings of the Royal Society B, 271, 1331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R, Heywood CA, Cowey A, Regard M, Landis T. Sensitivity to eye gaze in prosopagnosic patients and monkeys with superior temporal sulcus ablation. Neuropsychologia. 1990;28:1123–42. doi: 10.1016/0028-3932(90)90050-x. [DOI] [PubMed] [Google Scholar]
- Charman T, Swettenham J, Baron-Cohen S, Cox A, Baird G, Drew A. Infants with autism: an investigation of empathy, pretend play, joint attention, and imitation. Developmental Psychology. 1997;33:781–9. doi: 10.1037//0012-1649.33.5.781. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaner RO, Platt ML. Reflexive social attention in monkeys and humans. Current Biology. 2003;13:1609–13. doi: 10.1016/j.cub.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Deaner RO, Khera AV, Platt ML. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Current Biology. 2005;15:543–8. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Lorincz EN, Perrett DI, Oram MW, Baker CI. Gaze following and joint attention in rhesus monkeys (Macaca mulatta) Journal of Comparative Psychology. 1997;111:286–93. doi: 10.1037/0735-7036.111.3.286. [DOI] [PubMed] [Google Scholar]
- Engell AD, Nummenmaa L, Oosterhof NN, Henson RN, Haxby JV, Calder AJ. Differential activation of frontoparietal attention networks by social and symbolic spatial cues. Social Cognitive & Affective Neuroscience. 2010;5:432–40. doi: 10.1093/scan/nsq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiwald WA, Tsao DY. Functional compartmentalization and viewpoint generalization within the macaque face-processing system. Science. 2010;330:845–51. doi: 10.1126/science.1194908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg ME, Bisley JW, Powell KD, Gottlieb J. Saccades, salience and attention: the role of the lateral intraparietal area in visual behavior. Progress in Brain Research. 2006;155:157–75. doi: 10.1016/S0079-6123(06)55010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. Journal of Neurophysiology. 2007;97:1671–83. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nature Neuroscience. 2000;3:80–4. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Jaime M, Lopez JP, Lickliter R. Bobwhite quail (Colinus virginianus) hatchlings track the direction of human gaze. Animal Cognition. 2009;12:559–65. doi: 10.1007/s10071-009-0214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. Subcortical face processing. Nature Reviews Neuroscience. 2005;6:766–74. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Kamphuis S, Dicke PW, Thier P. Neuronal substrates of gaze following in monkeys. European Journal of Neuroscience. 2009;29:1732–8. doi: 10.1111/j.1460-9568.2009.06730.x. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Sugiura M, Kato T, et al. The human amygdala plays an important role in gaze monitoring. A PET study. Brain. 1999;122(Pt 4):779–83. doi: 10.1093/brain/122.4.779. [DOI] [PubMed] [Google Scholar]
- Kingstone A, Friesen CK, Gazzaniga MS. Reflexive joint attention depends on lateralized cortical connections. Psychological Science. 2000;11:159–66. doi: 10.1111/1467-9280.00232. [DOI] [PubMed] [Google Scholar]
- Klein JT, Deaner RO, Platt ML. Neural correlates of social target value in macaque parietal cortex. Current Biology. 2008;18:419–24. doi: 10.1016/j.cub.2008.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. Journal of Neuroscience Methods. 1999;86:145–59. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Materna S, Dicke PW, Thier P. The posterior superior temporal sulcus is involved in social communication not specific for the eyes. Neuropsychologia. 2008;46:2759–65. doi: 10.1016/j.neuropsychologia.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear; 1999. Proceedings of the National Academy of Sciences of the United States of America, 96, 1680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Sato W, Kubota Y, et al. Involvement of medial temporal structures in reflexive attentional shift by gaze. Social Cognitive & Affective Neuroscience. 2008;3:80–8. doi: 10.1093/scan/nsm027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–48. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Rolls ET, Caan W. Visual neurones responsive to faces in the monkey temporal cortex. Experimental Brain Research. 1982;47:329–42. doi: 10.1007/BF00239352. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Smith PA, Potter DD, et al. Visual cells in the temporal cortex sensitive to face view and gaze direction; 1985. Proceedings of the Royal Society London B: Biological Sciences, 223, 293–317. [DOI] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–8. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma HaB D, editor. Attention and Performance. Vol. X. Erlbaum: 1984. pp. 531–56. [Google Scholar]
- Sato W, Okada T, Toichi M. Attentional shift by gaze is triggered without awareness. Experimental Brain Research. 2007;183:87–94. doi: 10.1007/s00221-007-1025-x. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Yoshikawa S. Time course of superior temporal sulcus activity in response to eye gaze: a combined fMRI and MEG study. Social Cognitive & Affective Neuroscience. 2008;3:224–32. doi: 10.1093/scan/nsn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W, Uono S, Okada T, Toichi M. Impairment of unconscious, but not conscious, gaze-triggered attention orienting in Asperger's disorder. Research in Autism Spectrum Disorders. 2010;4:782–786. [Google Scholar]
- Senju A, Johnson MH. The eye contact effect: mechanisms and development. Trends in Cognitive Sciences. 2009a;13:127–34. doi: 10.1016/j.tics.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Senju A, Johnson MH. Atypical eye contact in autism: models, mechanisms and development. Neuroscience & Biobehavioral Reviews. 2009b;33:1204–14. doi: 10.1016/j.neubiorev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Dairoku H, Hasegawa T. Reflexive orienting in response to eye gaze and an arrow in children with and without autism. Journal of Child Psychology and Psychiatry. 2004;45:445–58. doi: 10.1111/j.1469-7610.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. Innate visual object recognition in vertebrates: some proposed pathways and mechanisms. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology. 2002;132:861–91. doi: 10.1016/s1095-6433(02)00119-8. [DOI] [PubMed] [Google Scholar]
- Shepherd SV. Following gaze: gaze-following behavior as a window into social cognition. Frontiers in Neuroscience. 2010;4:5. doi: 10.3389/fnint.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd SV, Deaner RO, Platt ML. Social status gates social attention in monkeys. Current Biology. 2006;16:R119–20. doi: 10.1016/j.cub.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Shepherd SV, Klein JT, Deaner RO, Platt ML. Mirroring of attention by neurons in macaque parietal cortex; 2009. Proceedings of the National Academy of Sciences of the United States of America, 106, 9489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd SV, Cappuccio M. Sociality, attention and the mind's eyes. In: Seemann A, editor. Joint Attention: New Developments. Cambridge, MA, USA: MIT Press; 2012. [Google Scholar]
- Tomasello M, Call J, Hare B. Five primate species follow the visual gaze of conspecifics. Animal Behaviour. 1998;55:1063–9. doi: 10.1006/anbe.1997.0636. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–4. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson A, Mandl I, Bugnyar T, Huber L. Gaze following in the red-footed tortoise (Geochelone carbonaria) Animal Cognition. 2010;13:765–9. doi: 10.1007/s10071-010-0320-2. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N. Autism, the superior temporal sulcus and social perception. Trends in Neurosciences. 2006;29:359–66. doi: 10.1016/j.tins.2006.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.