Abstract
There are marked individual differences in the pattern of cortical (sulcogyral) folding in the orbitofrontal cortex (OFC), and there is a growing literature suggesting that these individual differences are associated with risk for psychotic disorders. To date, however, no study has investigated whether OFC folding patterns are associated with broader risk factors relevant to a range of psychopathology. This study helps address this knowledge gap by examining whether OFC sulcogyral folding patterns are associated with putative risk factors, specifically affective temperament and psychiatric symptoms, in a large community sample (N = 152) of adolescents. Results showed that the most common pattern of folding (‘Type I’, marked by discontinuity of the medial orbital sulcus and continuity of the lateral orbital sulcus) was associated with low levels of Surgency, high levels of Negative Affectivity (in girls) and higher depressive symptoms. This pattern was also associated with reduced thickness of OFC gray matter. Overall, the findings, combined with previous work, suggest some specificity of neurodevelopmental risk for different types of psychopathology. Thus, these results have the potential to inform the early identification of at-risk individuals.
Keywords: gyrification, temperament, orbitofrontal cortex, risk, adolescence
INTRODUCTION
The orbitofrontal cortex (OFC) is a functionally heterogeneous brain region that has been particularly implicated in contributing to aspects of social and emotional behavior (Kringelbach and Rolls, 2004). Both lesion and neuroimaging studies provide evidence that structural aspects of the OFC are important for mediating these functions. Indeed, both lesions and individual differences in volume of the OFC have been shown to be associated with dysfunction in social and emotional behavior. For example, patients with OFC lesions have difficulty with reinforcement learning and often present with disinhibited or socially inappropriate behavior and emotional changes (Rolls et al., 1994). We have found that individuals with reduced OFC volume are more likely to display sad affective behaviors (Whittle et al., 2008a) and have reduced temperamental effortful control (Whittle et al., 2008b). Furthermore, volumetric changes have been noted in individuals suffering from a number of forms of psychopathology, including depression, anxiety, personality disorders and schizophrenia (Bremner et al., 2002; Tebartz van Elst et al., 2003; Kang et al., 2004; Riffkin et al., 2005). However, the location of volumetric change within the OFC and the direction of change have been inconsistent across studies and the specificity of volumetric deficits to one disorder compared with another has not been established. These inconsistencies in volumetric studies may be partially due to the limitations inherent to the interpretation of volumetric measurements. For example, volumes may be affected by factors such as illness duration and medications (Lorenzetti et al., 2009), and it is difficult to establish whether volumetric differences reflect a risk factor or biomarker for the disorder vs a consequence of illness-related factors. Furthermore, a brain region’s volume is determined by other structural properties including cortical thickness and surface area (Fornito et al., 2008), making it difficult to know the mechanistic processes associated with volumetric differences or changes.
One alternative approach to investigating the relationship between OFC structure and psychopathology is via the characterization of sulcogyral patterns. Sulcogyral patterns are largely established by birth, are partly genetically influenced and are thought to result from differential growth of the cortex (i.e. differences in the growth rate between cortical layers cause the surface to buckle or fold) (Xu et al., 2010). Hence, sulcogyral patterns provide a means of investigating possible neurodevelopmental markers of a disorder that are less likely to be influenced by confounds such as medication or illness duration.
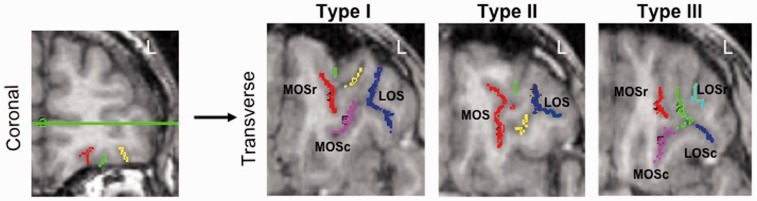
Based on the configuration of the four major orbitofrontal gyri (the medial, lateral, anterior and posterior gyri), three basic OFC sulcogyral configurations have been identified in healthy adults (Chiavaras and Petrides, 2000). These are characterized by the continuity of rostral and caudal sections of the medial and lateral orbitofrontal sulci (Figure 1). ‘Type I’ (the most common type) is characterized by discontinuity of the (rostral and caudal) medial orbitofrontal sulcus (MOS) and continuity of the (rostral and caudal) lateral orbitofrontal sulcus (LOS); ‘Type II’ is characterized by continuity of both medial and lateral orbitofrontal sulci and ‘Type III’ (least common) is characterized by disconnections in both medial and lateral orbitofrontal sulci. To date, six studies have investigated the association between OFC sulcogyral patterns and psychopathology—mostly in the context of psychotic disorders. In patients with both first episode and chronic schizophrenia, an increased prevalence of the Type III OFC sulcogyral pattern, and a decreased prevalence of the Type I pattern, was found compared with controls (Nakamura et al., 2007, 2008; Takayanagi et al., 2010; Uehara-Aoyama et al., 2011). A fifth study has shown that an increased Type III pattern, and decreased Type I pattern, characterized genetically at-risk individuals who went on to develop schizophrenia vs controls and high-risk individuals who did not go on to develop schizophrenia (Chakirova et al., 2010). Although not based on OFC sulcogyral pattern types, Roppongi et al. (2010) found no differences between panic disorder patients and controls in the number of sulci apparent in the posterior OFC [posterior orbitofrontal sulci (POS)].
Fig. 1.
Example of each OFC sulcogyral type. To determine Type, sulci are first traced on coronal images (left panel) and then viewed on transverse images (right panel). Type I: rostral and caudal segments of the lateral orbital sulcus are continuous (dark blue); rostral (red) and caudal (magenta) medial orbital sulci are not connected. Type II: both medial (red) and lateral (dark blue) orbital sulci are continuous. Type III: for the medial orbital sulci, rostral (red) and caudal (magenta) segments are not connected; for the lateral orbital sulci, rostral (cyan) and caudal (dark blue) segments are also not connected. r = rostral, c = caudal, LOS = lateral orbital sulcus, MOS = medial orbital sulcus.
Based on the results of these studies, it has been suggested that Type III OFC sulcogyral pattern may represent a biomarker or risk factor for schizophrenia (Chakirova et al., 2010). However, it is currently not known whether OFC sulogyral patterns are associated with risk for other specific psychiatric disorders or risk for psychopathology in general. Two of the aforementioned studies provide some preliminary evidence that OFC sulcogyral patterns might be associated with personality dimensions that have been linked with risk for various forms of psychopathology (Clark et al., 1994). First, Nakamura et al. (2007) found that in 50 healthy controls, while Type I was not associated with any personality dimension, Type II pattern was associated with higher Positive Emotionality (sensitivity to signals of incentive reward), as measured by the Multidimensional Personality Questionnaire (Tellegen and Waller, 1994), whereas Type III was associated with higher Constraint/Effortful Control (tendency to inhibit and restrain impulse expression, unconventional behavior and risk-taking). Second, Roppongi et al. (2010) found that 28 healthy controls with no POS had lower Extraversion (similar to Positive Emotionality) and higher Openness (active imagination, aesthetic sensitivity, attentiveness to inner feelings, preference for variety and intellectual curiosity, as measured by the Neuroticism-Extraversion-Openness Personality Inventory, Costa and McCrae, 1992), while those with one POS had lower Openness, and those with two POS had higher Conscientiousness (tendency for carefulness, thoroughness, self-organization, deliberation and need for achievement). Interestingly, some of these associations were reversed for patients with psychopathology. In Nakamura et al.’s study, Type III pattern was associated with lower Constraint in patients (n = 50) with schizophrenia. Furthermore, in Roppongi et al.’s study, panic disorder patients (n = 28) with no POS had lower Openness. The reason for these differences in findings for patients vs controls is unknown, but there are two likely possibilities. First, adult personality (the dependent variable in each study) may be shaped by experience (Strelau, 1987), including illness-related factors, and thus may not be a good candidate for a risk or vulnerability factor. Second, the sample sizes in each of these studies were relatively small. For example, there were less than five participants in some POS groups in Roppongi et al.’s (2010) study, reducing power and generalizability. Thus, while there is some initial evidence that OFC sulcogyral patterns might be associated with personality, which may represent risk for a number of psychiatric disorders, further research is needed with larger samples, investigating vulnerability factors that are more stable and less influenced by experience. To this end, investigating affective temperament might be fruitful, as it is thought to be established at birth, heritable and relatively stable (Rothbart and Bates, 2006). Furthermore, conducting such an investigation in a younger sample would be advantageous as individual differences in temperament would be less likely to be influenced by experiential factors than in adulthood (Strelau, 1987).
Additionally, one potentially key variable that has not yet been comprehensively investigated in regard to sulcogyral OFC patterns is sex. There is consistent evidence that males and females differ in brain structure, including gyrification patterns in other parts of the brain (e.g. anterior cingulate cortex; Yücel et al., 2001; Whittle et al., 2009). Furthermore, there is evidence that gyrification patterns can have different effects on behavior for males and females. For example, in our work, the presence of a paracingulate sulcus in the medial surface of left hemisphere was found to be more important in predicting temperamental Negative Emotionality for males than females (Whittle et al., 2009). Till date, only two studies have investigated sex differences in OFC sulcogyral pattern types (Chiavaras and Petrides, 2000; Uehara-Aoyama et al., 2011), with neither study finding any significant differences between healthy males and females. However, Uehara-Aoyama et al. (2001) found that Type III pattern characterized schizophrenia in males but not females, suggesting that further investigation of the interaction between sex and OFC sulcogyral pattern in predicting psychopathology is warranted.
The aim of this study was to investigate OFC sulcogyral patterns in a large community sample of adolescents and determine whether certain patterns are associated with affective temperament, which is a core vulnerability factor for psychopathology (Whittle et al., 2006). We also aimed to investigate whether sex moderated the relationship between sulcogyral pattern and temperament. Based on the existing literature, we hypothesized that Type III sulcogyral folding pattern would be associated with general risk for psychopathology, as indicated by at risk affective temperament (i.e. high Negative Emotionality/Neuroticism and low Effortful Control/Constraint). We also hypothesized that there would be sex differences in these relationships. As secondary aims, we investigated whether sulcogyral patterns were associated with (i) sub-clinical psychiatric symptoms (i.e. internalizing and externalizing symptoms), given that such symptoms are also a risk factor for later onset of mental disorder (e.g. Pine et al., 1999) and (ii) measures of cortical thickness, so as to broaden our understanding of the functional relevance of OFC gyrification.
METHODS
Participants
Participants were 152 community-based early adolescent individuals (72 female, 80 male; mean age 12.6 years, s.d. 0.4 years; range 11.4–13.7 years), from a larger sample of 2479 grade 6 students (from 97 separate schools, representative of Victorian school sector type and socioeconomic classification) as part of a broader adolescent development study conducted at Orygen Youth Health, The University of Melbourne, Melbourne, Australia, the aim of which was to investigate risk factors for psychopathology during adolescence. One hundred thirty-nine participants were right handed as established using the Edinburgh Handedness Inventory (Oldfield, 1971). Nineteen participants met criteria for past or current mental illness, established using The Schedule for Affective Disorders and Schizophrenia for School-Aged Children: Epidemiologic Version (K-SADS-E, Orvaschel and Puig-Antich, 1994). The diagnoses were as follows: depressive disorder, n = 1; social phobia, n = 1; separation anxiety disorder, n = 1; obsessive compulsive disorder, n = 2; simple phobia, n = 2; oppositional defiant disorder, n = 8; attention deficit hyperactivity disorder, n = 3; conduct disorder, n = 1.
Selection procedure
Participants were selected on the bases of an in-school screening (see Yap et al., 2008, for further details). The aim of the selection procedure was to ascertain a sample of adolescents with maximal variability in temperamental risk for psychopathology. To this end, we selected adolescents who were representative of the full range of scores across two higher order temperament dimensions (Negative Affectivity and Effortful Control) measured by a revised version the Early Adolescent Temperament Questionnaire (EATQ-R). See below for information about this instrument. Thus, equal numbers of adolescents were recruited across the following ranges of scores on the aforementioned two higher order factors of the EATQ-R: 0–1 s.d. ± mean, 1–2 s.d. ± mean, 2–2.5 s.d. ± mean and >2.5 s.d. ± mean. Of the 425 selected adolescents, 152 agreed to participate in the MRI study. No differences between participants who agreed to the MRI and those who were selected but declined were observed on temperament {Negative Affectivity [t(412) = 0.58, P = 0.56]; Effortful Control [t(412) = 0.32, P = 0.75]; Surgency [t(412) = 0.56, P = 0.58]; Affiliation [t(412) = −0.71, P = 0.48] or sex [χ2(1) = 0.54, P = 0.46]}. Furthermore, the distribution of temperament scores did not differ between those participating and those selected (Kolmogorov–Smirnov tests: P’s > 0.183). Written informed consent was obtained from all adolescents and their parents/guardians prior to participation, in accordance with local ethics committee guidelines.
Measures
EATQ-R
All participants completed the EATQ-R (Ellis and Rothbart, 2001) at two time points (in-school screening and MRI/diagnostic assessment 6 months to 1 year after screening). The EATQ-R contains items loading onto 10 sub-scales and four higher order factors: Negative Affectivity (comprising items from the Frustration sub-scale), Effortful Control (comprising items from Activation Control, Attention and Inhibitory Control sub-scales), Surgency [comprising Fear (reversed-scored), Shyness (reversed-scored) and Surgency items] and Affiliation (comprising Affiliation, Pleasure Sensitivity and Perceptual Sensitivity items). Note that Surgency represents an internalizing–externalizing dimension with high scores indicating high extraversion and low scores indicating high introversion (i.e. high fear and shyness). Confirmatory factor analysis was employed with data from the large school screening sample to verify the factor structure of the EATQ-R suggested by initial work with the instrument (Putnam et al., 2001). See Whittle et al. (2009) for further details. Factor scores from each administration were highly correlated (Effortful Control: r = 0.657, P < 0.001; Negative Affectivity: r = 0.652, P < 0.001; Surgency: r = 0.679, P < 0.001; Affiliation: r = 0.579, P < 0.001) and were averaged, with the resulting values used for all subsequent analyses.
Internalizing and externalizing symptoms
As part of a comprehensive diagnostic assessment, participants completed the Centre for Epidemiological Studies-Depression Scale (CES-D; Radloff, 1977), the Beck Anxiety Inventory (BAI; Beck et al., 1988) and the externalizing component of the Child Behavior Checklist (CBCL; Achenbach, 1991).
Image acquisition
MRI scans were performed on a 3 Tesla scanner at the Brain Research Institute, Austin and Repatriation Medical Centre, Melbourne, Australia, using a gradient echo volumetric acquisition sequence (repetition time = 36 ms; echo time = 9 ms; flip angle = 35°, field of view = 20 cm2, pixel matrix = 410 × 410) to obtain 124 T1-weighted contiguous 2 mm thick slices (voxel dimensions = 0.4883 × 0.4883 × 1.5 mm).
Image pre-processing
Images were transferred to an SGI/Linux workstation for morphometric analysis. Image pre-processing was carried out using tools from the FMRIB software library (http://www.frmib.ox.ac.uk/fsl). Each 3D scan was stripped of all non-brain tissue (Smith, 2002) and aligned to the MNI 152 average template (six-parameter rigid body transform with trilinear interpolation) using FLIRT (Jenkinson and Smith, 2001). This registration served to align each image axially along the AC–PC plane and sagittally along the interhemispheric fissure without any deformation. Images were resampled to 1 mm3.
OFC sulcogyral pattern classification
OFC sulcogyral patterns were classified according to the criteria described by Chiavaras and Petrides (2000). Images were classified on a LINUX workstation using the biomedical imaging software package Analyze 10.0 (Mayo Clinic). We identified all sulci appearing on the orbital surface. Sulci were highlighted in coronal section slice by slice using the manual tracing tool, and then viewed in transverse and sagittal planes to aid in the visual classification of OFC pattern type. Sulcogyral patterns were classified into three types based on the continuity of the medial and lateral orbitofrontal sulci (Figure 1). Type I pattern is characterized by a LOS that is continuous from the rostral to the caudal aspect of the OFC and a MOS that is disconnected such that the rostral segment is separate from the caudal segment. Type II pattern is characterized by continuity of both MOS and LOS and Type III pattern is characterized by discontinuity of both the MOS and LOS.
Regarding identification of the rostral part of the MOS, care was taken not to mistake this sulcus for a fragment or dimple in the medial orbital gyrus. To this end, an eligible sulcus was considered the rostral MOS if it was at least 4 mm long and 4 mm deep. Furthermore, it was often difficult to establish sulcus continuity, particularly in cases where the depth of a sulcus varied along its rostro-caudal extent. In ambiguous cases, we considered that a sulcus was continuous when it was clearly continuous on three adjacent axial slices.
We identified whether zero, one or two POS were present in each hemisphere. A POS was considered present if it was at least 4 mm long and 4 mm deep. POS were not measured in three participants due to poor image quality.
Inter-rater reliability was performed by C.B. and S.L.W. on 56 randomly selected brains from the Melbourne Neuropsychiatry Centre database. Intra-rater reliability was performed on a subset (N = 18) of these brains. Inter-class correlation coefficients (Cronbach’s α) were 0.82 and 0.89 for inter- and intra-rater reliability, respectively.
Cortical thickness
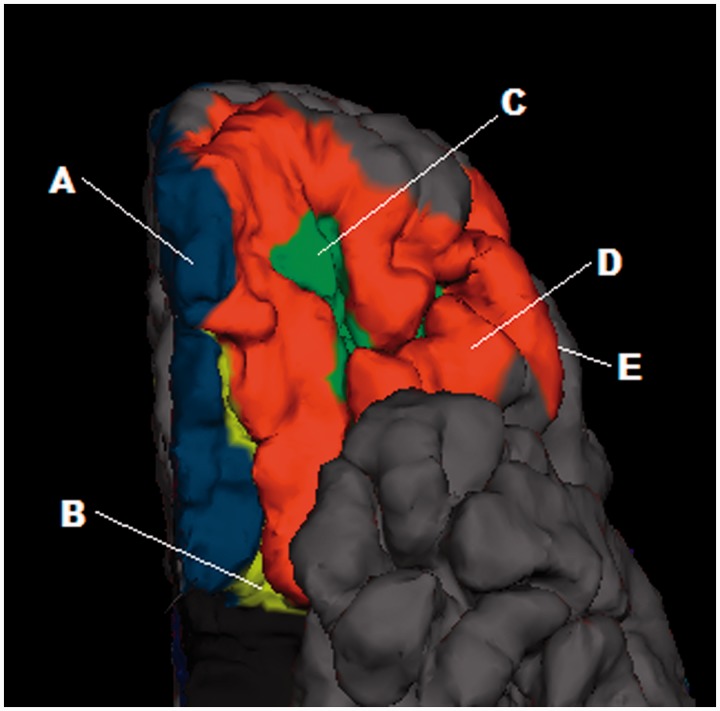
FreeSurfer (version 4.5) software (http://surfer.nmr.mgh.harvard.edu/fswiki/) was used to obtain cortical thickness estimates for the OFC. Prior to FreeSurfer processing, all images were corrected for tissue signal inhomogeneity using a nonparametric non-uniformity intensity normalization method optimized for 3 Tesla images (Sled et al., 1998; Zheng et al., 2009). Cortical thickness in five orbitofrontal regions (gyrus rectus, orbital gyrus, medial orbital sulcus, lateral orbital sulcus and orbital-H shaped sulcus; see Figure 2) was estimated using FreeSurfer by means of an automated segmentation procedure, which has been described previously (Fischl et al., 2002). This procedure automatically assigns a neuroanatomical label to each voxel in a MRI volume based on probabilistic information estimated automatically from a manually labeled training set. Cortical thickness measures were not available for 38 participants due to poor image quality in the OFC region (caused by artifact as a result of orthodontic braces or signal dropout).
Fig. 2.
Rendered T1-weighted MR image of the ventral anterior aspect of the left hemisphere of an example brain. Orbitofrontal regions for which gray matter thickness was extracted are shown. A = gyrus rectus; B = medial orbital sulcus; C = orbital-H shaped sulcus; D = orbital gyrus; E = lateral orbital sulcus (hidden behind orbital gyrus).
Statistical analysis
Pearson’s bivariate correlations were performed to describe the pattern of association between temperament and symptom measures. Chi-squared analyses were performed to assess OFC sulcogyral pattern type and POS frequency differences in the sample. The effects of sex and OFC sulcogyral patterns (and their interaction) on each continuous temperament score were tested using univariate analysis of covariance (ANCOVA). Age and intracranial volume (ICV) were used as covariates. Note that ICV did not differ by sulcogyral pattern type or POS frequency (P’s > 0.05), but both age and ICV were included as covariates due to theoretical effects on sulcogyral folding (White et al., 2010). Furthermore, it is a common misconception that the independent variable and covariate need to be significantly correlated in order to warrant use of ANCOVA (Miller and Chapman, 2001). For sulcogyral pattern variables showing significant association with temperament, post hoc ANCOVAs were employed to test for their effects on cortical thickness, and internalizing and externalizing symptoms. Multivariate normality was indicated via Shapiro-Wilks W test and plots of residuals vs predicted values. Levene’s tests indicated that the homogeneity of variance assumption was met for all analyses.
RESULTS
Table 1 shows descriptive information for the sample. There was a significant sex difference in Effortful Control (with females showing higher scores), but no sex differences in any other variable. Table 2 shows the pattern of association between temperament and symptom variables. While Effortful Control and Negative Affectivity were strongly associated with all symptom measures, Surgency and Affiliation were only weakly associated with symptoms (associations were significant for the former but not the latter).
Table 1.
Descriptive information for the sample
| Mean | s.d. | Range | |
|---|---|---|---|
| CESD | 31.96 | 9.79 | 20–75 |
| BAI | 8.80 | 8.50 | 0–43 |
| CBCL | 16.69 | 12.10 | 0–62 |
| Surgency | 3.36 | 0.67 | 1.89–4.71 |
| Affiliation | 3.39 | 0.57 | 1.66–4.81 |
| Effortful control | 3.42** | 0.63 | 1.77–4.83 |
| Negative Affectivity | 3.20 | 0.79 | 1.21–5.00 |
**Sex difference, P < 0.01.
Table 2.
Associations (Pearson’s r) between temperament dimensions and symptoms
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Effortful control | – | −0.62*** | 0.31*** | 0.12 | −0.56*** | −0.33*** | −0.53*** |
| 2. Negative Affectivity | – | −0.45*** | −0.06 | 0.51*** | 0.44*** | 0.53*** | |
| 3. Surgency | – | −0.07 | −0.23** | −0.20* | −0.17* | ||
| 4. Affiliation | – | 0.10 | 0.13 | −0.08 | |||
| 5. CES-D | – | 0.62*** | 0.70*** | ||||
| 6. BAI | – | 0.59*** | |||||
| 7. CBCL | – |
*P < 0.05, **P < 0.01, ***P < 0.001.
Table 3 shows the frequency of OFC sulcogyral pattern types and POS in the total sample and in males and females, separately. For the total sample, the frequency of sulcogyral pattern types was similar for both hemispheres. Type I was most common and Type III was least common [left: χ2 (2) = 38.90, P < 0.001; right: χ2 (2) = 41.90, P < 0.001]. There were no sex differences in pattern type distribution for the left [χ2 (2) = 2.87, P = 0.238] or right [χ2 (2) = 3.26, P = 0.196] hemisphere. The distributions of pattern types were not significantly different from those originally reported by Chiavaras and Petrides (2000) for the left [χ2 (2) = 1.31, P = 0.519] or right [χ2 (2) = 1.95, P = 0.377] hemisphere.
Table 3.
Frequency of (a) OFC sulcogyral pattern types and (b) POS, in males, females and the total sample
| Males |
Females |
Total |
|||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| (a) Type | Left | ||||||
| 1 | 44 | 54 | 42 | 60 | 86 | 57 | |
| 2 | 26 | 32 | 14 | 20 | 40 | 26 | |
| 3 | 12 | 15 | 14 | 20 | 26 | 17 | |
| Total | 82 | 100 | 70 | 100 | 152 | 100 | |
| Right | |||||||
| 1 | 43 | 52 | 35 | 64 | 88 | 58 | |
| 2 | 24 | 29 | 12 | 17 | 36 | 24 | |
| 3 | 15 | 18 | 13 | 19 | 28 | 18 | |
| Total | 82 | 100 | 70 | 100 | 152 | 100 | |
| (b) POS | Left | ||||||
| 0 | 41 | 51 | 42 | 62 | 83 | 56 | |
| 1 | 34 | 42 | 22 | 32 | 56 | 38 | |
| 2 | 6 | 7 | 4 | 6 | 10 | 7 | |
| Total | 81 | 100 | 68 | 100 | 149 | 100 | |
| Right | |||||||
| 0 | 37 | 46 | 41 | 60 | 78 | 52 | |
| 1 | 33 | 41 | 23 | 34 | 56 | 38 | |
| 2 | 11 | 14 | 4 | 6 | 15 | 10 | |
| Total | 81 | 100 | 68 | 100 | 149 | 100 | |
Percentages (rounded) are also given.
For POS, zero sulci was most common and two sulci was least common in both hemispheres [left: χ2 (2) = 55.63, P < 0.001; right: χ2 (2) = 41.17, P < 0.001]. There were no sex differences in number of POS for the left [χ2 (2) = 2.00, P = 0.369] or right [χ2 (2) = 4.16, P = 0.125] hemisphere. OFC sulcogyral pattern was not associated with POS frequency in the left [χ2 (4) = 0.496, P = 0.974] or right [χ2 (4) = 3.14, P = 0.535] hemisphere. The distribution of POS frequencies were significantly different from those originally reported by Chiavaras and Petrides (2000) for the left [χ2 (2) = 17.47, P < 0.001] and right [χ2 (2) = 15.33, P < 0.001] hemisphere. In both hemispheres, our participants had a greater number of absent POS, and a lesser number of single POS, as compared with the data reported by Chiavaras and Petrides (2000).
Temperament
OFC sulcogyral pattern type
See Table 4 for significance tests for all temperament analyses. There were no main effects of left sulcogyral pattern type for any temperament dimension; however, there was a significant sex by left sulcogyral pattern interaction in the prediction of Negative Affectivity. Analyses conducted for each sex separately revealed that left sulcogyral pattern predicted Negative Affectivity for females [F(2,65) = 4.48, P = 0.015] but not males [F(2,77) = 0.97, P = 0.383]. For females, those with a Type I pattern in the left hemisphere had higher Negative Affectivity than those with a Type II (P = 0.011) or III (P = 0.043) pattern.
Table 4.
Effects of OFC folding patterns (sulcogyral type and POS frequency) on temperament
| Folding pattern | Folding pattern X sex | Post hoc tests | |
|---|---|---|---|
| Left type | |||
| Negative affectivity | – | F(2,144) = 4.29, P = 0.016 | I > II & IIIb |
| Effortful control | – | – | |
| Surgency | – | – | |
| Affiliation | – | – | |
| Right type | |||
| Negative affectivity | – | – | |
| Effortful control | – | – | |
| Surgency | F(2,144) = 3.11, P = 0.048 | III > I | |
| Affiliation | – | – | |
| Right POS | |||
| Negative affectivity | – | – | |
| Effortful control | – | – | |
| Surgency | – | – | |
| Affiliation | F(2,141) = 4.05, P = 0.019 | – | 1 > 0 & 2a |
Note that results are not shown for Left POS as there were no significant effects. Non-significant (P > 0.05) statistics are not presented to improve clarity, but are available from the authors by request. I, II, and III indicate sulcogyral pattern types I, II and III, respectively. 0, 1 and 2 indicate zero, one and two POS, respectively.
Post hoc tests are shown at P < 0.05 (aP < 0.1).
b Significant finding only for females.
Right sulcogyral pattern type was significantly associated with Surgency. Those with a Type III pattern in the right hemisphere had higher Surgency than those with a Type I pattern (P = 0.021). There were no significant interaction effects with sex.
POS
There were no main effects of left POS for any temperament dimension. Right POS was significantly associated with Affiliation, whereby individuals with one POS had higher Affiliation than those with zero POS (P = 0.009) and tended to have higher Affiliation than those with two POS (P = 0.072). There were no significant interactions between sex and left or right POS in predicting temperament.
Symptoms
OFC sulcogyral pattern type
Given the significant results found for temperament, analyses for symptoms were restricted to left pattern type in females and right pattern type in the whole sample (see Table 5). For females, there were no effects of left sulcogyral pattern type for any symptom score. For the whole sample, there was a significant effect of right sulcogyral pattern type on depressive symptoms, whereby individuals with a Type I pattern in the right hemisphere had higher depressive symptoms than those with a Type II (P = 0.009) or Type III pattern (P = 0.049).
Table 5.
Effects of OFC folding patterns (sulcogyral type and POS frequency) on symptoms and cortical thickness (for variables where folding patterns were significant for temperament dimensions).
| Folding pattern | Post hoc tests | |
|---|---|---|
| Left type | ||
| Left orbital gyrus thickness | F(4,48) = 3.273, P = 0.047b | I < IIb |
| Left medial orbital sulcus thickness | F(4,48) = 3.452, P = 0.040b | I & III < IIb |
| Right type | ||
| Depressive symptoms | F(2,131) = 4.43, P = 0.014 | I > II & III |
| Right gyrus rectus thickness | F(4,108) = 6.08, P = 0.003 | I & II < III |
| Right lateral orbital sulcus thickness | F(4,108) = 3.618, P = 0.030 | I < II & III |
| Right POS | ||
| Depressive symptoms | F(2,128) = 3.51, P = 0.033 | 2 > 0 & 1a |
Note that only significant effects are shown for clarity; statistics for non-significant (P > 0.05) effects are available from the authors. I, II and III indicate sulcogyral pattern types I, II and III, respectively. 0, 1 and 2 indicate zero, one and two POS, respectively.
Post hoc tests are shown at P < 0.05 (asignificant at P < 0.1).
bSignificant finding for females.
POS
Given the significant results found for temperament, analyses for symptoms were restricted to frequency of right POS in the whole sample (see Table 5). There was a significant effect of right POS for depressive symptoms, whereby individuals with two POS in the right hemisphere had higher depressive symptoms than those with zero POS (P = 0.012) and tended to have higher depressive symptoms than those with one POS (P = 0.074).
Cortical thickness
Given the significant results found for temperament, analyses for thickness were restricted to left pattern type in females, right pattern type in the whole sample and frequency of right POS in the whole sample (see Table 5). There was an effect of right sulcogyral pattern type on thickness in both the right gyrus rectus and the right lateral orbital sulcus. For both regions, Type I had thinner cortex than Type III (P = 0.001 and P = 0.045, respectively). For the former region, Type II also had thinner cortex than Type III (P = 0.015). For the latter region, Type I also had thinner cortex than Type II (P = 0.026). There were no significant effects for right POS.
For females, there was an effect of left sulcogyral pattern type on thickness in the left orbital gyrus and on thickness in the left medial orbital sulcus. For both regions, Type I had thinner cortex than Type II (P = 0.050 and P = 0.017, respectively). For the latter region, Type III also had thinner cortex than Type II (P = 0.023).
DISCUSSION
This study comprehensively assessed the influence of individual differences in orbitofrontal sulcogyral folding patterns, and their interaction with sex, on temperament, psychopathology symptoms and cortical thickness, in a large community sample of adolescents. Contrary to our hypotheses, we showed that Type I folding pattern, the most common folding type, may be associated with particular risk for internalizing symptomatology. That is, Type I folding pattern was associated with lower temperamental Surgency (i.e. introversion), higher Negative Affectivity (in girls), higher depressive symptoms and thinner cortex. Furthermore, we found that the absence of POS, the most common POS configuration in our sample, was associated with fewer depressive symptoms and lower temperamental Affiliation.
Our temperament results did not support our hypotheses, which were based on results obtained for healthy adults by Nakamura et al. (2007) and Roppongi et al. (2010). Table 6 shows a summary of our results in comparison to those of Nakamura et al. (2007) and Roppongi et al. (2010). Specifically, Nakamura et al. (2007) found that Type II pattern was associated with higher Positive Emotionality. In our data, Type III was associated with higher Surgency (i.e. a temperament dimension usually associated with Positive Emotionality) compared with Type I, and Type I was associated with higher Negative Affectivity compared with Types II and III. Regarding the POS, Roppongi et al. (2010) found that healthy controls with absent POS had lower Extraversion (i.e. comparable with Positive Emotionality) and higher Openness (i.e. comparable with Affiliation), those with one POS had lower Openness and those with two POS had higher Conscientiousness (i.e. comparable with Effortful Control). In our data, those with absent POS had lower temperamental Affiliation.
Table 6.
Summary of associations between temperament and OFC sulcogyral folding patterns across our data and existing literature
| Whittle et al. | Nakamura et al. | Roppongi et al. | |
|---|---|---|---|
| Sulcogyral type | |||
| Effortful control | −a | − | NA |
| Negative affectivity | I > II & III | − | NA |
| Affiliation | − | NA | NA |
| Surgency | III > I | II > I & III | NA |
| POS | |||
| Effortful control | − | NA | − |
| Negative affectivity | − | NA | − |
| Affiliation | 1 > 0 & 2 | NA | 0 > 1 & 2 |
| Surgency | − | NA | 1 & 2 > 0 |
Note that for simplicity, the nomenclature of Ellis and Rothbart is used for the temperament/personality labels; these labels correspond to the actual dimensions assessed by each study as suggested in the text.
a ‘−’ denotes a null finding; NA indicates that the association was not tested.
The reason for the dissimilarities in findings might be due to the fact that our instruments differed and that our sample was younger in age (early adolescent vs adult). As mentioned above, adult personality may be shaped by experience (Strelau, 1987), and thus may not map cleanly onto biological predispositions such as early appearing neural folding patterns when compared with temperament in a young population. Additionally, our sample size [152 compared with 50 and 28 healthy controls in Nakamura et al. (2007) and Roppongi et al.’s (2010) studies, respectively] should provide a more representative and powerful sample than in previous studies.
In this study, Type I folding pattern in the right hemisphere was associated with lower temperamental Surgency. This dimension is characterized by sensation seeking or extraversion at the one end of the dimension and neuroticism, or tendency toward experiencing fear and shyness, at the other. Individuals low in Surgency are thought to experience higher arousal, acquire conditioned responses more easily, and extinguish them more slowly, than do individuals high in Surgency (Eysenck, 1965). Thus, our finding is consistent with the known role of the OFC in emotion processing, and in particular in the learning and extinction of stimulus–outcome associations.
Left hemisphere Type I folding pattern was also associated with higher temperamental Negative Affectivity, but only in females. Individuals high in Negative Affectivity have a tendency to experience frustration or irritability in the face of limitations; thus, our finding is also consistent with the role of the OFC in responding to reward and punishment stimuli. For example, lesion studies show that damage to the OFC is associated with frustration when rewards are delayed (Berlin et al., 2004). The reason behind the observed sex difference in this finding is unclear; however, evidence for sex differences in OFC development and function (Overman, 2004) suggests that further work is needed to understand the influence of sex on OFC structure and function.
Both low Surgency and high Negative Affectivity have been associated with risk for internalizing psychopathology, and it has been suggested that this risk might be mediated by neurobiological mechanisms (Whittle et al., 2006). Our results suggest that Type I folding pattern might represent one such neurobiological mechanism. While no hypotheses were made regarding symptoms due to the exploratory nature of this study aim, we found that Type I folding pattern was associated with increased depressive (but not anxiety or externalizing) symptoms in our sample, consistent with our suggestion that Type I folding pattern represents a risk for internalizing (particularly depressive) psychopathology. Furthermore, given that sulcogyral patterns are thought to be largely established by birth and genetically influenced, a Type I folding pattern may represent a neurodevelopmental risk marker for such psychopathology.
The specific neurodevelopmental processes that lead to different sulcogyral patterns in the OFC are unknown. Gyri and sulci formation have been linked to cytoarchitecture of underlying structures (Chance et al., 2004; Xu et al., 2010) and neuronal connectivity (Hilgetag, 2006; Herculano-Houzel et al., 2010). It has recently been suggested that cortical folding is most likely to result from differential growth of cortical regions (Xu et al., 2010). Further work is needed to investigate the processes giving rise to OFC sulcogyral folding patterns, including genetics and pre- and perinatal factors.
Given the existing literature linking Type III folding pattern with risk for schizophrenia, and our lack of findings regarding Type III, our results suggest some specificity of neurodevelopmental risk for different psychopathologies, with Type III indicating risk for psychotic disorders and Type I potentially indicating risk for internalizing psychopathology. Interestingly, based on findings that patients with schizophrenia have relative decreased Type I frequency, and that those patients with Type I folding pattern have better cognitive function and milder positive symptoms, it has been suggested that Type I folding pattern in schizophrenia might represent a protective factor (Nakamura et al., 2007). Thus, while Type I folding pattern may be protective for some symptoms, our findings suggest that Type I folding pattern may confer vulnerability to other psychological problems. Future prospective work is needed to confirm this hypothesis.
The mechanisms by which OFC folding patterns influence behavior are not clear, but they likely involve alterations in other aspects of OFC structure and function. Only two studies to date have investigated the relationship between OFC folding type and regional gray matter properties (Nakamura et al., 2008; Takayanagi et al., 2010), neither of which found an effect of folding type on OFC volume. Our results are not necessarily inconsistent with these findings, given that cortical thickness and volume are not comparable; volume is a complex measure that is determined by both cortical thickness and area (Fornito et al., 2008). We found that Type I folding pattern (associated with low Surgency, high Negative Affectivity and high depressive symptoms) was associated with reduced thickness in both gyri (left orbital, right gyrus rectus) and sulci (left medial orbital, right lateral orbital) in the orbitofrontal region. Although we made no hypotheses regarding cortical thickness due to the exploratory nature of this aim, our thickness findings are consistent with our suggestion that Type I folding pattern may represent risk for internalizing problems, given evidence that adults low in extraversion (i.e. adults that may be at higher risk for internalizing problems) tend to have thinner OFC gray matter (Rauch et al., 2005). It has been suggested that thinner OFC in these individuals might be associated with deficits in extinction retention (Rauch et al., 2005), which may be one mechanism by which OFC structure influences risk for internalizing problems. However, given the dynamic nature of brain maturation during adolescence (i.e. gray matter thickness shows non-linear patterns of growth across adolescence, Giedd et al., 1999), interpretation of thickness in our participants is complicated. Thus, further research investigating associations between OFC folding patterns and different aspects of OFC structure across development are needed to corroborate our interpretations.
Our results regarding POS are somewhat consistent with Roppongi et al. (2010). While they found a single POS to be associated with trait anxiety, we found this POS pattern to be associated with temperamental Affiliation, which refers to the tendency to desire warmth and closeness with others, independent of shyness or extraversion. There is some evidence that Affiliation and anxiety are related. For example, highly affiliative individuals may seek out social closeness as a means of buffering high levels of trait anxiety (Teichman, 1974). We also found that individuals with absent POS, the most frequent configuration in our sample, showed lower depressive symptoms. This is a novel finding, and given that (i) sulcogyral pattern type was not related to POS frequency and (ii) the posterior OFC is thought to develop earlier than the anterior OFC, and responds more to primary reinforcers (e.g. taste, smell) as compared with secondary or abstract reinforcers (e.g. loss of money) (Kringelbach and Rolls, 2004), these results suggest potentially separate neurodevelopmental risk factors for depressive symptoms. It is worth note that we found the distribution of POS in our sample to differ significantly from that originally reported by Chiavaras and Petrides (2000). While zero POS was the most common pattern in both hemispheres in our sample, one POS was the most common pattern in both hemispheres in Chiavaras and Petrides’ sample. It is unknown whether these differences may be due to measurement differences or sample characteristics. Further work is needed to explore these possibilities.
While our study has some strengths, including a large sample size and broad measurement of both temperament and clinical symptoms, our results must be interpreted in the context of some limitations. While our participants were drawn from a community sample, they were selected on the basis of temperamental risk for psychopathology. This has implications for the generalizability of our findings. We did not include measurement of all classes of psychiatric symptoms, so we cannot comment on associations between folding patterns and subthreshold psychotic symptoms for example. We did not correct for multiple comparisons; thus, future studies are needed to replicate findings. We were not able to comprehensively investigate the mechanisms linking folding patterns with temperament or symptoms; future work employing functional imaging may be informative in this regard. Furthermore, it is possible that the same genetic factors may be responsible for both cortical folding patterns and individual differences in temperament and symptoms, and as such, folding patterns may not represent risk mechanisms per se. However, although cortical folding has been suggested to be partly genetically determined, there is some evidence that environmental factors may be more influential (Bartley et al., 1997), and also that both development (White et al., 2010) and experience (Luders et al., 2012) may shape folding patterns. Thus, further work is needed to examine the combined influence of these factors on folding patterns and risk for psychopathology.
CONCLUSIONS
Our results provide evidence that OFC sulcogyral patterns may have relevance for affective functioning, and suggest that individual differences in these patterns may be associated with neurodevelopmental risk for internalizing psychopathology. These findings add to the current work on OFC folding that has to date, been primarily focused on psychosis and suggest potentially differential neurodevelopmental pathways for psychotic vs internalizing disorders. We found some evidence for sex effects, and although future work is needed to clarify the causes and consequences of these effects, these results support the literature documenting sex differences in the relationship between neurobiology and behavior (Whittle et al., 2011).
FUNDING
This research was supported by grants from the Colonial Foundation, the National Health and Medical Research Council (NHMRC; Australia; Program Grant 350241) and the Australian Research Council (ARC; Discovery Grant DP0878136). S.W. is supported by an NHMRC Career Development Fellowship (ID: 1007716). C.B. is supported by an NHMRC Postdoctoral Fellowship (ID: 567042). M.D. and N.V. are supported by Australian Postgraduate Awards. M.Y. is supported by an NHMRC Clinical Career Development Award (ID: 509345).
Conflict of Interest
None declared.
Acknowledgments
Neuroimaging analysis was facilitated by the Neuropsychiatry Imaging Laboratory at the Melbourne Neuropsychiatry Centre and supported by Neurosciences Victoria. The authors would like to thank the Brain Research Institute for support in acquiring the neuroimaging data and the families who participated in the study.
REFERENCES
- Achenbach TM. Manual for the Youth Self-report and 1991 Profiles. Burlington, VT: Department of Psychiatry, University of Vermont; 1991. [Google Scholar]
- Bartley AJ, Jones DW, Weinberger DR. Genetic variability of human brain size and cortical gyral patterns. Brain. 1997;120:257–69. doi: 10.1093/brain/120.2.257. [DOI] [PubMed] [Google Scholar]
- Beck AT, Brown G, Epstein N, Steer RA. An inventory for measuring clinical anxiety - psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56(6):893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127(5):1108–26. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Nazeer A, et al. Reduced volume of orbitofrontal cortex in major depression. Biological Psychiatry. 2002;51:273–9. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- Chakirova G, Welch KA, Moorhead TWJ, et al. Orbitofrontal morphology in people at high risk of developing schizophrenia. European Psychiatry. 2010;25(6):366–72. doi: 10.1016/j.eurpsy.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Chance SA, Tzotzoli PM, Vitelli A, Esiri MM, Crow TJ. The cytoarchitecture of sulcal folding in Heschl’s sulcus and the temporal cortex in the normal brain and schizophrenia: lamina thickness and cell density. Neuroscience Letters. 2004;367(3):384–8. doi: 10.1016/j.neulet.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Chiavaras MM, Petrides M. Orbitofrontal sulci of the human and macaque monkey brain. Journal of Comparative Neurology. 2000;422(1):35–54. [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology. 1994;103(1):103–16. [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO PI-R) and NEO Five-Factor Inventory (NEO-FFI) Professional Manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Ellis LK, Rothbart MK. Revision of the Early Adolescent Temperament Questionnaire. 2001 Biennial Meeting of the Society for Research in Child Development, Minneapolis, Minnesota..2001. [Google Scholar]
- Eysenck HJ. Extraversion and the acquisition of eyeblink and GSR conditioned responses. Psychological Bulletin. 1965;63(4):258–70. doi: 10.1037/h0021921. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fornito A, Wood SJ, Whittle S, et al. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: associations with cortical thickness, surface area, volume, and sulcal depth. Human Brain Mapping. 2008;29(2):222–36. doi: 10.1002/hbm.20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a lingitudinal MRI study. Nature Neuroscience. 1999;2(10):861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S, Mota B, Wong P, Kaas JH. Connectivity-driven white matter scaling and folding in primate cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(44):19008–13. doi: 10.1073/pnas.1012590107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgetag CC. Principles of brain connectivity organization. Behavioral and Brain Sciences. 2006;29(1):18–19. [Google Scholar]
- Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kang DH, Kim JJ, Choi JS, et al. Volumetric investigation of the frontal-subcortical circuitry in patients with obsessive-compulsive disorder. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16(3):342–9. doi: 10.1176/jnp.16.3.342. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neurophysiology. Progress in Neurobiology. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Fornito A, Yücel M. Structural brain abnormalities in major depressive disorder: A selective review of recent MRI studies. Journal of Affective Disorders. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Luders E, Kurth F, Mayer EA, Toga AW, Narr KL, Gaser. C. The unique brain anatomy of meditation practitioners: alterations in cortical gyrification. Frontiers in Human Neuroscience. 2012;6:34. doi: 10.3389/fnhum.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. The Journal of Abnormal Psychology. 2001;110(1):40–8. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, Levitt JJ, et al. Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain. 2008;131(1):180–95. doi: 10.1093/brain/awm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, McCarley RW, et al. Altered orbitofrontal sulcogyral pattern in schizophrenia. Brain. 2007;130(3):693–707. doi: 10.1093/brain/awm007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the edinburgh handedness inventory. Neuropsychologia. 1971;9:97–114. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich . 1994. Schedule for affective disorders and schizophrenia for school age children: epidemiologic version. Unpublished Manual. [Google Scholar]
- Overman WH. Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain and Cognition. 2004;55(1):134–47. doi: 10.1016/S0278-2626(03)00279-3. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen E, Cohen P, Brook JS. Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder? American Journal of Psychiatry. 1999;156:133–5. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Ellis LK, Rothbart MK. The structure of temperament from infancy through adolescence. In: Eliasz A, Angleitner A, editors. Advances in Research on Temperament. Lengerich, Germany: Pabst Science Publishers; 2001. pp. 165–82. [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Rauch SL, Milad MR, Orr SR, Quinn BT, Fischl B, Pitman RK. Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport. 2005;16(17):1909–12. doi: 10.1097/01.wnr.0000186599.66243.50. [DOI] [PubMed] [Google Scholar]
- Riffkin J, Yucel M, Maruff P, et al. A manual and automated MRI study of anterior cingulate and orbito-frontal cortices, and caudate nucleus in obsessive-compulsive disorder: Comparison with healthy controls and patients with schizophrenia. Psychiatry Research-Neuroimaging. 2005;138(2):99–113. doi: 10.1016/j.pscychresns.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery and Psychiatry. 1994;57(12):1518–24. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roppongi T, Nakamura M, Asami T, et al. Posterior orbitofrontal sulcogyral pattern associated with orbitofrontal cortex volume reduction and anxiety trait in panic disorder. Psychiatry and Clinical Neurosciences. 2010;64(3):318–26. doi: 10.1111/j.1440-1819.2010.02085.x. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Lerner R, editors. Handbook of Child Psychology: Social, Emotional, and Personality Development. New York: Wiley; 2006. pp. 99–166. [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelau J. The concept of temperament in personality research. European Journal of Personality. 1987;1(2):107–17. [Google Scholar]
- Takayanagi Y, Takahashi T, Orikabe L, et al. Volume reduction and altered sulco-gyral pattern of the orbitofrontal cortex in first-episode schizophrenia. Schizophrenia Research. 2010;121(1–3):55–65. doi: 10.1016/j.schres.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Tebartz van Elst L, Hesslinger B, Thiel T, et al. Frontolimbic brain abnormalities in patients with borderline personality disorder: a volumetric magnetic resonance imaging study. Biological Psychiatry. 2003;54(2):163–71. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- Teichman Y. Predisposition for anxiety and affiliation. Journal of Personality and Social Psychology. 1974;29(3):405–10. doi: 10.1037/h0035920. [DOI] [PubMed] [Google Scholar]
- Tellegen A, Waller N. Exploring personality through test construction: development of the Multidimensional Personality Questionnaire. In: Briggs SR, Cheek JM, editors. Personality Measures: Development and Evaluation. Greenwich, CT: JAI Press; 1994. pp. 172–208. [Google Scholar]
- Uehara-Aoyama K, Nakamura M, Asami T, et al. Sexually dimorphic distribution of orbitofrontal sulcogyral pattern in schizophrenia. Psychiatry and Clinical Neurosciences. 2011;65(5):483–9. doi: 10.1111/j.1440-1819.2011.02229.x. [DOI] [PubMed] [Google Scholar]
- White T, Su S, Schmidt M, Kao C-Y, Sapiro G. The development of gyrification in childhood and adolescence. Brain and Cognition. 2010;72(1):36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Allen NB, Fornito A, et al. Variations in prenatally determined cortical folding patterns are related to individual differences in temperament. Psychiatry Research: Neuroimaging. 2009;172(1):68–74. doi: 10.1016/j.pscychresns.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Whittle S, Allen NB, Lubman DI, Yucel M. Neurobiological basis of temperament: towards a better understanding of psychopathology. Neuroscience and Biobehavioral Reviews. 2006;30(4):511–25. doi: 10.1016/j.neubiorev.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Whittle S, Yap M, Yücel M, et al. Prefrontal and amygdala volumes are related to adolescents’ affective behaviors during parent-adolescent interactions. Proceedings of the National Academy of Sciences of the United States of America; 2008a. pp. 3652–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Yücel M, Fornito A, et al. Neuroanatomical correlates of temperament in early adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2008b;47(6):682–93. doi: 10.1097/CHI.0b013e31816bffca. [DOI] [PubMed] [Google Scholar]
- Whittle S, Yücel M, Yap MBH, Allen NB. Sex differences in the neural correlates of emotion: evidence from neuroimaging. Biological Psychology. 2011;87(3):319–33. doi: 10.1016/j.biopsycho.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Xu G, Knutsen AK, Dikranian K, Kroenke CD, Bayly PV, Taber LA. Axons pull on the brain, but tension does not drive cortical folding. Journal of Biomechanical Engineering. 2010;132(7):071013. doi: 10.1115/1.4001683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap MBH, Allen NB, Ladouceur CD. Maternal socialization of positive affect: the impact of invalidation on adolescent emotion regulation and depressive symptomatology. Child Development. 2008;79(5):1415–31. doi: 10.1111/j.1467-8624.2008.01196.x. [DOI] [PubMed] [Google Scholar]
- Yücel M, Stuart GW, Maruff P, et al. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: an MRI morphometric study. Cerebral Cortex. 2001;11(1):17–25. doi: 10.1093/cercor/11.1.17. [DOI] [PubMed] [Google Scholar]
- Zheng W, Chee MWL, Zagorodnov V. Improvement of brain segmentation accuracy by optimizing non-uniformity correction using N3. Neuroimage. 2009;48(1):73–83. doi: 10.1016/j.neuroimage.2009.06.039. [DOI] [PubMed] [Google Scholar]