Abstract
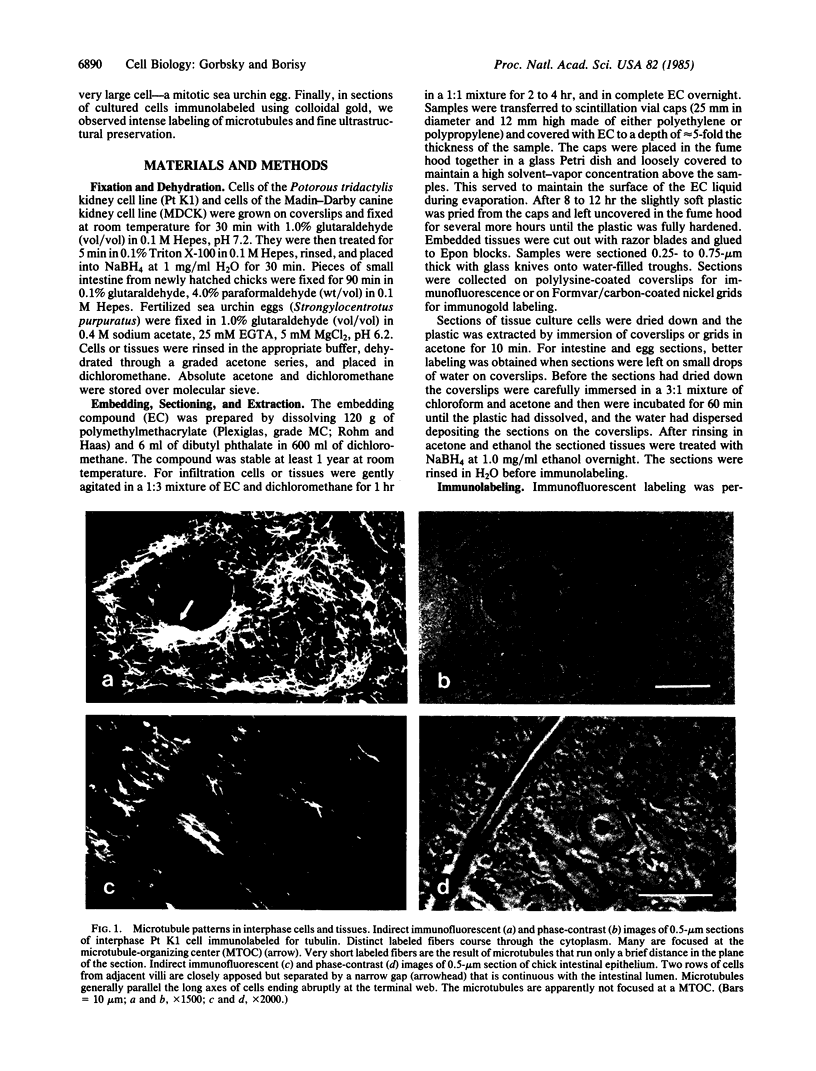
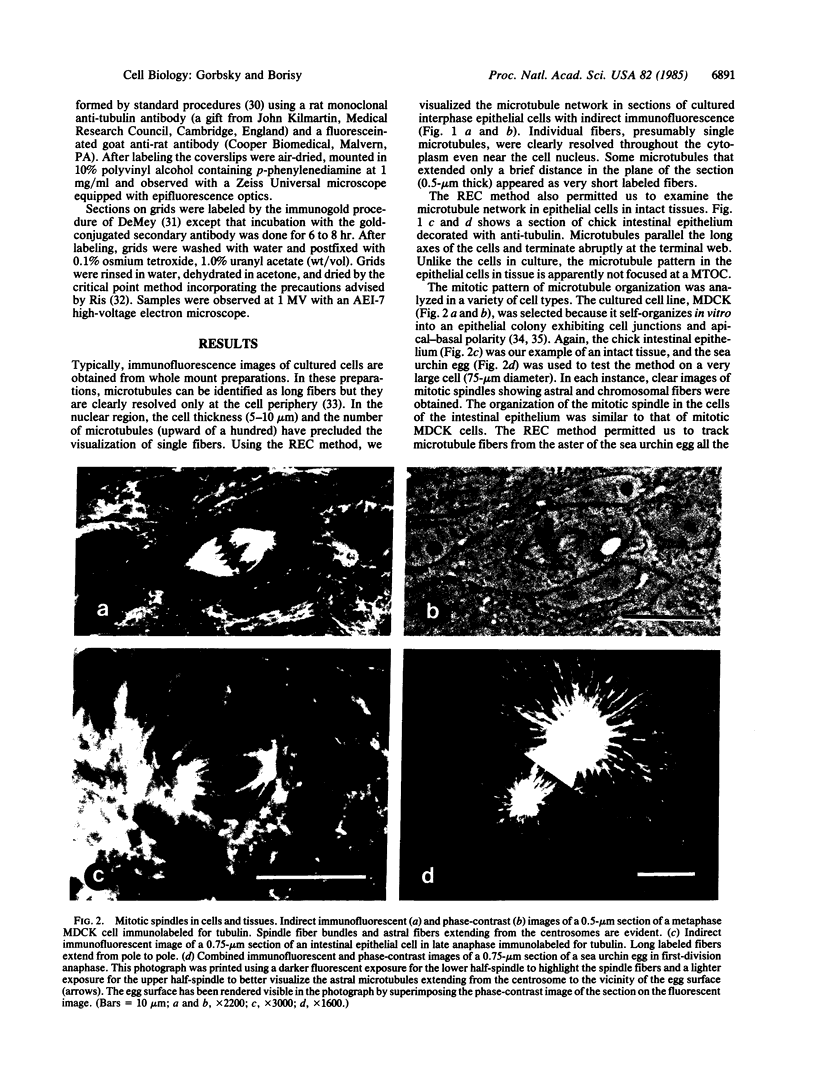
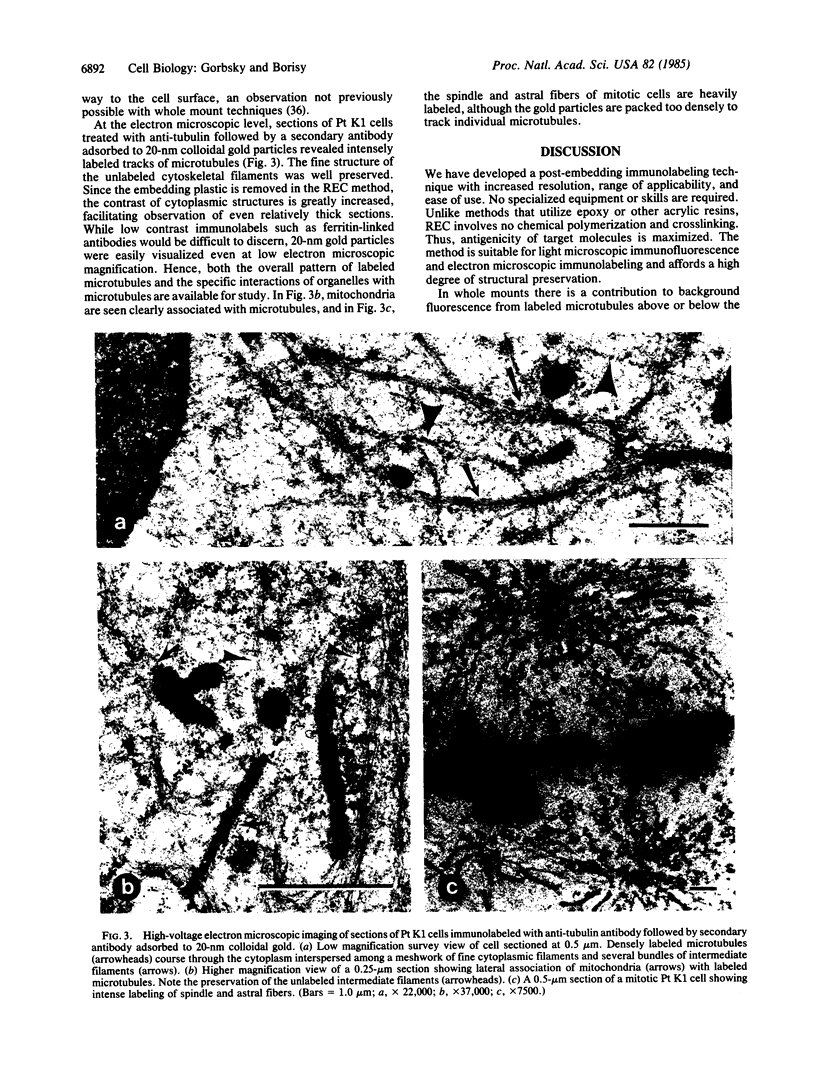
To investigate the detailed distributions of microtubules in cultured cells and intact tissues we developed a reversible embedment method for antibody labeling of sectioned material. Fixed tissues were infiltrated with fully polymerized polymethylmethacrylate dissolved in an organic solvent. Evaporation of the solvent left the tissue embedded in hard plastic. After sectioning by conventional methods, the plastic was extracted and sections were processed for indirect immunofluorescence to label microtubules. Clear images of microtubules were observed in sections of cultured epithelial cells, intact chick intestinal epithelium, and dividing sea urchin eggs. Microtubules in the differentiated epithelium of the chick intestine generally paralleled the long axis of the cells and did not focus on a microtubule-organizing center. Mitotic cells of the intestinal epithelium appeared similar to the mitotic cells of epithelial lines in culture. In sections of dividing sea urchin eggs detailed images of spindle and astral fibers were revealed. Immunoelectron microscopic labeling for tubulin was performed on sections of Pt K1 cells using secondary antibodies adsorbed to 20-nm gold particles. Semi-thick sections viewed by high-voltage electron microscopy showed both the overall distribution of microtubules and their detailed interactions with other cellular organelles. Mitochondria were often aligned along labeled microtubules. Reversible embedment cytochemistry should provide a general method for high resolution labeling of cells and tissues with affinity probes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendayan M., Roth J., Perrelet A., Orci L. Quantitative immunocytochemical localization of pancreatic secretory proteins in subcellular compartments of the rat acinar cell. J Histochem Cytochem. 1980 Feb;28(2):149–160. doi: 10.1177/28.2.7354212. [DOI] [PubMed] [Google Scholar]
- Bendayan M., Zollinger M. Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A-gold technique. J Histochem Cytochem. 1983 Jan;31(1):101–109. doi: 10.1177/31.1.6187796. [DOI] [PubMed] [Google Scholar]
- Brown W. J., Farquhar M. G. The mannose-6-phosphate receptor for lysosomal enzymes is concentrated in cis Golgi cisternae. Cell. 1984 Feb;36(2):295–307. doi: 10.1016/0092-8674(84)90223-x. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Robbins E. S., Dolan W. J., Rotunno C. A., Sabatini D. D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978 Jun;77(3):853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S., Miller C. LR white resin and improved on-grid immunogold detection of vicilin, a pea seed storage protein. Cell Biol Int Rep. 1984 Oct;8(10):879–886. doi: 10.1016/0309-1651(84)90072-9. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., van der Ley P. A., Scheffer R. C. Use of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sections. J Cell Biol. 1981 Jun;89(3):653–665. doi: 10.1083/jcb.89.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Brands R., Burke B., Louvard D., Warren G. Viral membrane proteins acquire galactose in trans Golgi cisternae during intracellular transport. J Cell Biol. 1982 Dec;95(3):781–792. doi: 10.1083/jcb.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P., Osborn M., Weber K. Distribution of tubulin-containing structures in the egg of the sea urchin Strongylocentrotus purpuratus from fertilization through first cleavage. J Cell Biol. 1980 Mar;84(3):668–679. doi: 10.1083/jcb.84.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarai Y., Nakane P. K. Localization of tissue antigens on the ultrathin sections with peroxidase-labeled antibody method. J Histochem Cytochem. 1970 Mar;18(3):161–166. doi: 10.1177/18.3.161. [DOI] [PubMed] [Google Scholar]
- Knecht E., Hernández J., Wallace R., Grisolía S. Immunoferritin location of carbamoyl phosphate synthetase in rat liver. J Histochem Cytochem. 1979 May;27(5):975–981. doi: 10.1177/27.5.90072. [DOI] [PubMed] [Google Scholar]
- Lundgren B., Westin M. Intracellular antigen of sea urchin eggs and embryos studies on ultrathin sections. J Ultrastruct Res. 1974 Feb;46(2):230–238. doi: 10.1016/s0022-5320(74)80058-4. [DOI] [PubMed] [Google Scholar]
- Misfeldt D. S., Hamamoto S. T., Pitelka D. R. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty G. C., Halmi N. S. Electron microscopic study of the adrenocorticotropin-producing cell with the use of unlabeled antibody and the soluble peroxidase-antiperoxidase complex. J Histochem Cytochem. 1972 Aug;20(8):590–603. doi: 10.1177/20.8.590. [DOI] [PubMed] [Google Scholar]
- Newman G. R., Jasani B., Williams E. D. A simple post-embedding system for the rapid demonstration of tissue antigens under the electron microscope. Histochem J. 1983 Jun;15(6):543–555. doi: 10.1007/BF01954145. [DOI] [PubMed] [Google Scholar]
- Painter R. G., Tokuyasu K. T., Singer S. J. Immunoferritin localization of intracellular antigens: the use of ultracryotomy to obtain ultrathin sections suitable for direct immunoferritin staining. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1649–1653. doi: 10.1073/pnas.70.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J. A., Erlandsen S. L. Ultrastructural immunocytochemical localization of prolactin in rat anterior pituitary by use of the unlabeled antibody enzyme method. J Histochem Cytochem. 1974 May;22(5):340–351. doi: 10.1177/22.5.340. [DOI] [PubMed] [Google Scholar]
- Parysek L. M., Wolosewick J. J., Olmsted J. B. MAP 4: a microtubule-associated protein specific for a subset of tissue microtubules. J Cell Biol. 1984 Dec;99(6):2287–2296. doi: 10.1083/jcb.99.6.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekut D. T., Casey S. M. Penetration of immunoreagents in Vibratome-sectioned brain: a light and electron microscopic study. J Histochem Cytochem. 1983 May;31(5):669–674. doi: 10.1177/31.5.6341457. [DOI] [PubMed] [Google Scholar]
- Rieder C. L., Bowser S. S. Correlative immunofluorescence and electron microscopy on the same section of epon-embedded material. J Histochem Cytochem. 1985 Feb;33(2):165–171. doi: 10.1177/33.2.3881520. [DOI] [PubMed] [Google Scholar]
- Ris H. The cytoplasmic filament system in critical point-dried whole mounts and plastic-embedded sections. J Cell Biol. 1985 May;100(5):1474–1487. doi: 10.1083/jcb.100.5.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Shahrabadi M. S., Yamamoto T. A method for staining intracellular antigens in thin sections with ferritin-labeled antibody. J Cell Biol. 1971 Jul;50(1):246–250. doi: 10.1083/jcb.50.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamiya H., Batsford S., Vogt A. An approach to postembedding staining of protein (immunoglobulin) antigen embedded in plastic: prerequisites and limitations. J Histochem Cytochem. 1980 Oct;28(10):1041–1049. doi: 10.1177/28.10.6158534. [DOI] [PubMed] [Google Scholar]
- Thomson R. O., Walker P. D., Batty I., Baillie A. Post-embedding staining with ferritin labelled antibodies. Nature. 1967 Jul 22;215(5099):393–394. doi: 10.1038/215393b0. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Visualization of longitudinally-oriented intermediate filaments in frozen sections of chicken cardiac muscle by a new staining method. J Cell Biol. 1983 Aug;97(2):562–565. doi: 10.1083/jcb.97.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Bibring T., Osborn M. Specific visualization of tubulin-containing structures in tissue culture cells by immunofluorescence. Cytoplasmic microtubules, vinblastine-induced paracrystals, and mitotic figures. Exp Cell Res. 1975 Oct 1;95(1):111–120. doi: 10.1016/0014-4827(75)90615-1. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Yamada S. S., Pastan I. Ultrastructural antibody localization of alpha2-macroglobulin in membrane-limited vesicles in cultured cells. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4359–4363. doi: 10.1073/pnas.75.9.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosewick J. J., De Mey J., Meininger V. Ultrastructural localization of tubulin and actin in polyethylene glycol-embedded rat seminiferous epithelium by immunogold staining. Biol Cell. 1983;49(3):219–226. doi: 10.1111/j.1768-322x.1984.tb00240.x. [DOI] [PubMed] [Google Scholar]
- da Silva P. P., Kachar B., Torrisi M. R., Brown C., Parkison C. Freeze-fracture cytochemistry: replicas of critical point-dried cells and tissues after fracture-label. Science. 1981 Jul 10;213(4504):230–233. doi: 10.1126/science.7244630. [DOI] [PubMed] [Google Scholar]