Abstract
Behavioral approach and avoidance are fundamental to the experience of emotion and motivation, but the motivational system associated with anger is not well established. Some theories posit that approach motivational processes underlie anger, whereas others posit that avoidance motivational processes underlie anger. The current experiment sought to address whether traits related to behavioral approach or avoidance influence responses to anger stimuli using multiple measures: ERP, electroencephalographic (EEG) α-asymmetry and self-report. After completing the behavioral inhibition system/behavioral approach system (BIS/BAS) scales, participants viewed anger pictures and neutral pictures. BAS predicted larger late positive potentials (LPPs) to anger pictures, but not to neutral pictures. In addition, BAS predicted greater left-frontal asymmetry to anger pictures. Moreover, larger LPPs to anger pictures related to greater left-frontal EEG asymmetry during anger pictures. These results suggest that trait approach motivation relates to neurophysiological responses of anger.
Keywords: Behavioral inhibition system (BIS), behavioral approach system (BAS), anger, late positive potential, approach motivation, frontal α-suppression asymmetry
INTRODUCTION
One of the fundamental systems in organisms is the motivation to go toward stimuli vs the motivation to freeze or move away from stimuli. The distinct motivational responses to approach or avoid are inherent in affective responses, and trait motivational responses are embedded into personality. In affective responses such as fear or excitement, motivational responses to avoid or approach (respectively) are apparent. However, the system associated with anger is less clear; anger responses may be associated with either behavioral approach or withdrawal systems. The current experiment sought to clarify the motivational system associated with anger responses by investigating the relationship of trait motivational systems with multiple measures [ERP, electroencephalographic (EEG) α-asymmetry and self-report] of anger responses.
Approach motivation has been associated with the behavioral approach system (BAS; Gray, 1970, 1987; Gray and McNaughton, 2000), behavioral activation system (also BAS; Fowles, 1987) or a behavioral facilitation system (Depue and Collins, 1999). Functionally, the approach motivational system is thought to modulate reactions to appetitive or rewarding stimuli and generate anticipatory positive affect (Gray, 1994). In contrast, withdrawal motivation has been associated with the behavioral inhibition system (BIS; Gray, 1970, 1987). This system is thought to modulate reactions to aversive stimuli and generate negative affective states such as fear and anxiety.
Related to Gray’s (1987) theory of motivation, Carver and White (1994) developed a scale designed to measure the individual difference in trait sensitivity of these two systems (BAS and BIS). [Carver (2009) refers to BAS and BIS as incentive and threat sensitivity. However, these trait measures are empirically related to approach and withdrawal systems, respectively.] BAS is examined along three different expressions of behavioral approach: BAS Drive, BAS Reward Responsiveness and BAS Fun-Seeking. Drive measures persistent pursuit of desired goals. Reward Responsiveness measures positive responses to the occurrence or anticipation of reward. Fun-Seeking measures a desire for potential new rewards. Expressions of trait BAS relate to achievement goals (Elliot and Thrash, 2002), mania (Meyer et al., 2001), extraversion, (Harmon-Jones et al., 2002), novelty seeking and optimism (Carver and White, 1994). BIS measures reactions to the expectation of punishment. Individuals high in BIS sensitivity report more nervousness before and during an uncomfortable task (Carver and White, 1994) and high levels of anxiety in response to fear-related stimuli (Leen-Feldner et al., 2004).
Because of the strong relationship between motivation and affective states, BAS has been predominantly associated with positive-approach affect, and BIS has been predominantly associated with negative-withdrawal affect (Balconi et al., 2012). However, anger does not completely fit within a positive-approach or negative-withdrawal framework. This raises the question: what is the relationship between anger and trait motivation?
Behavioral approach/avoidance and anger
Some models of emotion posit that anger is related to an avoidance motivational tendency and stems from avoidant systems like fear (Lang et al., 1998). Factor-analytical studies based on retrospective self-reported emotion suggest that anger and other negative affects (e.g. fear) may have the same common source (Watson et al., 1988; Watson, 2009). Situations associated with anxiety may activate more behavioral avoidance in an angry state. For example, the subjective experience of anger may be associated with avoidance, especially under conditions where expressions of anger are related to other negative affects, such as fear from a socially unacceptable situation (Zinner et al., 2008), or when individuals internalize their anger (Stewart et al., 2010).
In contrast, some models suggest that anger relates to the functioning of an approach system (Carver and Harmon-Jones, 2009). For example, anger may arise when approach toward a goal is interrupted or an anticipated reward is blocked (Berkowitz, 1993; Carver and Scheier, 1998, 2008; Rolls, 1999). Functionally, anger is thought to facilitate attempts to remove whatever is impeding goal pursuit (Frijda, 1986; Fischer and Roseman, 2007). For example, in an anger state, anger may relate to behavioral measures of approach, such as aggression and goal pursuit (Mikulincer, 1988; Wingrove and Bond, 1998; Carver, 2004). Moreover, neural regions associated with approach and aggression are activated during situational anger (see Harmon-Jones et al., 2010, for a review).
At the trait level, studies have found that both behavioral approach and avoidance relate to anger. Smits and Kuppens (2005) and Cooper et al. (2007) found that BIS and BAS were related to trait anger. However, these traits were differentially associated with the way anger is expressed. BAS was positively associated with external expressions of anger, but BIS was inversely related to outward expressions. Other research has also found self-reported anger is related to both BIS and BAS, but controlling for anxiety-related traits eliminates the association between BIS and anger (Harmon-Jones, 2003; Carver, 2004).
If anger is associated with approach or withdrawal motivation, then trait motivation sensitivity should relate to multiple affective responses to stimuli designed to evoke anger. The current experiment assessed how trait motivation relates to multiple anger responses as measured by ERP, EEG α-asymmetry and self-report when viewing anger (vs neutral) pictures. Specifically, trait motivation should relate to anger responses assessed by the late positive potential (LPP) and frontal-cortical asymmetry.
Motivation and the LPP
The LPP evoked by picture stimuli is a reliable index of motivated attentional processing (for reviews, see Ferrari et al., 2008; Olofsson et al., 2008; Hajcak et al., 2012). Highly arousing affective pictures evoke larger LPPs than low arousing and non-affective pictures (Cuthbert et al., 2000; Schupp et al., 2000, 2004b), because highly arousing emotional pictures are motivationally relevant (Lang, 1995; Bradley, 2009). Thus, the LPP is thought to reflect motivational intensity rather than a specific motivational direction. Modulation of the LPP by affective pictures also occurs regardless of perceptual differences in complexity, brightness, contrast or spatial frequency that modulate early ERP components (Bradley et al., 2007). Because the LPP is ‘the most reliable ERP component modulated by stimulus significance in a passive picture viewing context’ (Bradley, 2009, p. 5), the current experiment will focus on the LPP component of the ERP. [Because early ERP components (e.g. N1) are sensitive to affective images, it is likely ERP components occurring earlier than the LPP may be larger to anger than neutral pictures.]
BIS and BAS have been associated with LPP activation. Specifically, LPPs to aversive pictures have been associated with BIS, and LPPs to appetitive pictures have been associated with BAS (Gable and Harmon-Jones, 2010; Balconi et al., 2012). Nijs et al. (2007) also found that BAS related to LPPs elicited by non-affective, goal-relevant targets in an oddball paradigm. However, no past research has examined whether the LPP is sensitive to anger pictures, or whether the LPP to anger pictures is associated with trait approach or withdrawal motivation.
Frontal asymmetry and motivation
Frontal cortical asymmetry is one of the most prominent neurophysiological measures of motivational direction. Over 70 years of research using a variety of methods indicate that the left and right frontal cortical regions are asymmetrically involved in positive affect/approach motivation and negative affect/withdrawal motivation, respectively (Goldstein, 1939; Rossi and Rosadini, 1967; for reviews, see Pizzagalli et al., 2003; Spielberg et al., 2008; Harmon-Jones et al., 2010). This research often uses asymmetric activity in right- vs left-frontal cortical areas as measured by suppression of the α-frequency band activity derived from EEG recordings (Coan and Allen, 2003a, 2004). α-Power is inversely related to regional brain activity as evidenced by hemodynamic measures (Cook et al., 1998) and behavioral tasks (Davidson et al., 1990).
Greater left frontal-cortical activation is involved in state approach motivation (Harmon-Jones and Sigelman, 2001; Gable and Harmon-Jones, 2008). In contrast, greater right frontal-cortical activation is involved in state withdrawal motivation (Silva et al., 2002; Buss et al., 2003; Stewart et al., 2010). Moreover, frontal asymmetry relates to motivation at the trait level (Harmon-Jones and Sigelman, 2001; Harmon-Jones, 2006; Gable and Harmon-Jones, 2008; Harmon-Jones et al., 2009, 2010), such that greater BAS is associated with greater left-frontal activation (Harmon-Jones and Allen, 1997; Coan and Allen, 2003b; Amodio et al., 2008), and greater BIS is associated with greater right-frontal activation (Sutton and Davidson, 1997; Balconi and Mazza, 2009; Balconi, 2011).
The current experiment
The current experiment sought to clarify the motivational system associated with anger responses by investigating the relationship of trait motivational sensitivity with the LPP and frontal asymmetry. Trait motivation was assessed using Carver and White’s (1994) BIS/BAS scales. To manipulate anger using picture stimuli, we used personally relevant scenes designed to evoke anger (Harmon-Jones et al., 2006). Consistent with the idea that anger is evoked when an expected standard has been violated (Ortony et al., 1988), we selected a series of anti-American pictures (e.g. flag-burning), because pictures of threats toward the United States should evoke greater subjective anger. Previous studies using similar pictures have demonstrated that such stimuli reliably evoke anger (Harmon-Jones et al., 2011).
Despite much evidence associating motivation and frontal asymmetry, some past research has failed to find predicted frontal asymmetry using picture stimuli (Hagemann et al., 1998; Elgavish et al., 2003; see reviews by Murphy et al., 2003; Pizzagalli et al., 2003). This inconsistency may have been due to the pictures evoking different levels of motivation across participants; some participants may have responded with little to no motivation, and others with strong motivation (Harmon-Jones, 2007). In support of this hypothesis, Gable and Harmon-Jones (2008) found that individual differences in motivation (e.g. liking for dessert and time since participants had last eaten) interacted with picture type (desserts vs neutral objects) to predict greater left-frontal asymmetry toward dessert pictures. Individual differences in trait motivation sensitivity, as measured by the BIS/BAS scale, should predict frontal asymmetry and LPP amplitudes to anger pictures, but not neutral pictures.
In order to more fully investigate the motivational system associated with anger, several hypotheses were generated. First, because past research is mixed on whether anger is associated with BIS or BAS, we predicted that BIS and/or BAS may relate to more self-reported anger. [Carver and White (1994) conceptualized BAS Drive, BAS Reward Responsiveness and BAS Fun-seeking as being different manifestations of the behavioral approach system. Consistent with this view, we consider BAS to be assessed by any of the three BAS scales and/or a combination of all three scales (i.e. overall BAS). Past research has found that Drive and Reward Responsiveness have been most closely associated with anger responses (Wingrove and Bond, 1998; Harmon-Jones, 2003; Carver, 2004).] Second, if anger evoked by the picture stimuli is associated with approach or withdrawal motivation, then trait BIS or BAS should predict greater LPP amplitudes to anger pictures. Specifically, if anger is related to greater approach motivation, then BAS should relate to LPP amplitudes. In contrast, if anger is related to withdrawal motivation, then BIS should relate to LPP amplitudes. Third, because frontal asymmetry to affective pictures is influenced by individual differences in motivational direction, we predicted that BIS or BAS would predict EEG asymmetry when viewing anger pictures. Specifically, if anger is related to greater approach motivation, then BAS should relate to greater left-frontal activation. In contrast, if anger is related to greater withdrawal motivation, then BIS should relate to greater right-frontal activation. Finally, because the LPP and frontal asymmetry are related to motivational processes, we predicted that if the LPP and frontal asymmetry are related to the same motivation system, then the LPP should relate to measures of frontal asymmetry.
METHOD
Thirty-two (15 female) right-handed introductory psychology students participated in exchange for partial course credit. Handedness was assessed based on participant self-report of hand dominance and behavioral inspection (e.g. writing and button presses). After completing informed consent, participants completed individual difference measures of approach motivation before EEG electrodes were applied. Two participants were excluded from analyses because they had outlying (>3 s.d. from the mean) BAS scores.
Trait BIS/BAS
The BIS/BAS scales contain 20 items and comprise BAS Drive, BAS Reward Responsiveness, BAS Fun-Seeking and BIS. Drive contains four items that pertain ‘to the persistent pursuit of desired goals’. Reward Responsiveness contains five items that ‘focus on positive responses to the occurrence or anticipation of reward’. Fun-Seeking has four items ‘reflecting both a desire for new rewards and a willingness to approach a potentially rewarding event on the spur of the moment’ (Carver and White, 1994, p. 322). Items from all BAS scales were averaged to form an index of overall BAS. BIS comprises seven items that measure reactions to the expectation of punishment.
Procedure
Participants viewed 32 anger pictures taken from the Internet and 32 neutral pictures taken from the Internet and the International Affective Picture System (Lang et al., 2005). [Thirty-two anger images were chosen from a larger collection of images because pilot testing showed they evoked the most anger. Also, past studies examining LPP amplitudes and frontal asymmetry to affective pictures have used 30 or 32 images (Gable and Harmon-Jones, 2008, 2010; Dennis and Hajcak, 2009; Harmon-Jones and Gable, 2009). IAPS picture numbers: 2190, 2210, 2215, 2396, 2440, 2441, 2493, 2499, 2516 and 2595.] Anger pictures depicted anti-American scenes (e.g. flag-burning, 9/11 events) and were selected from a larger sample because they evoked the most anger. Each anger picture was matched with a neutral picture, such that objects (e.g. buildings) were matched by shape and size and scenes were matched for people presence and direct gaze or face presence. Picture sizes (1024 × 768) were equivalent. All pictures were presented in the center of a 20 inch computer monitor and superimposed over a black background. Each trial consisted of a fixation cross (500 ms) followed by an anger or neutral picture (6000 ms). Intertrial interval was 3 s.
Following picture viewing, participants reported affective reactions to the pictures, indicating how positive (vs negative) and arousing (vs calming) each picture was (1 = positive/excited; 9 = negative/calm) by pressing the corresponding numbers on a computer keyboard. Arousal ratings were reverse coded so that high ratings indicated high arousal. Participants also indicated how angry they felt when they viewed the picture (1 = no emotion; 9 = strongest feeling).
EEG assessment and processing
EEG, recorded with 64 tin electrodes mounted in a stretch lycra cap (Electro-Caps, Eaton, OH, USA), was referenced to the left earlobe. A ground electrode was mounted midway between FPZ and FZ. Electrode impedances were <5 000 Ω and homologous sites were within ≤1000 Ω of each other. Signals were amplified with Neuroscan SynAmps RT amplifier unit (El Paso, TX, USA), low-pass filtered at 100 Hz, high-pass filtered at 0.05 Hz, notch filtered at 60 Hz and digitized at 2000 Hz. Artifacts (e.g. aberrant signals due to muscle movement or large non-blink eye movements) were removed by hand. Then, a regression-based eye movement correction was applied to remove blinks (Semlitsch et al., 1986), after which the data were again visually inspected to ensure proper correction. Because of equipment malfunction, data from site CZ on two participants were not included in analyses.
ERP assessment
Data were epoched 100 ms before picture onset until 1200 ms after picture onset and re-referenced using a common average reference. Data were filtered with a low pass of 35 Hz. Aggregated waveforms for each picture type were created and baseline corrected using the pre-stimulus interval.
LPP amplitude was measured as the mean EEG activity within a window of 500–1000 ms (Hajcak and Dennis, 2009; Gable and Harmon-Jones, 2010). An average of 27.75 (86.7%) anger picture trials per participant (888 total anger trials) and 27.53 (86.0%) neutral picture trials per participant (881 total neutral trials) were included in analyses. No participant had fewer than 20 trials per condition.
Based on visual inspection of the aggregated waveforms, it appeared that there were prominent N1 and N2 differences between conditions. Therefore, we also assessed N1 and N2 amplitudes. N1 amplitudes were measured as the minimum amplitude within a window of 60–160 ms and N2 amplitudes were measured as the minimum amplitude within a window of 200–300 ms.
Frontal asymmetry assessment
Consistent with past studies measuring state frontal-cortical activation to affective pictures using α-band power (see Coan and Allen, 2004, for a review; Jackson et al., 2003; Hewig et al., 2004; Harmon-Jones et al., 2006), power spectra epochs 1.024 s in duration were extracted through a Hamming window (50% taper of distal ends). Data were re-referenced using a common average reference. Consecutive epochs were overlapped by 50% to minimize data loss due to windowing. Consistent with previous research showing that high α-power is most reflective of the inverse of cortical activity (Pizzagalli et al., 2005) and emotive processing (Klimesch et al., 1997; Harmon-Jones et al., 2011), power values within the high α-band (10–12.75 Hz) were obtained using a fast Fourier transformation and aggregated across picture type for all 6 s of picture viewing. Asymmetry indexes (log right − log left) were computed for all homologous sites. Because α-power is inversely related to cortical activity (Lindsley and Wicke, 1974), higher scores indicate greater left hemisphere activity. An average of 336.38 anger picture sweeps per participant (10 764 total anger picture sweeps) and 331.69 neutral picture sweeps per participant (10 614 total neutral picture sweeps) were included in analyses. No participant had fewer than 226 sweeps per picture type.
RESULTS
Anger pictures were rated as being more negative (mean = 6.67, s.d. = 1.55) and arousing (mean = 4.87, s.d. = 2.05) than neutral pictures (mean = 3.97, s.d. = 1.13; mean = 2.64, s.d. = 1.53), ts > 5.97, Ps < 0.0001. Participants also reported feeling more anger toward anger pictures (mean = 4.84, s.d. = 2.27) than neutral pictures (mean = 1.24, s.d. = 0.30), t(27) = 9.12, P < 0.0001. Anger ratings to anger pictures related to greater negativity (r = 0.64, P < 0.001) and arousal (r = 0.72, P < 0.001) to anger pictures. Greater arousal to anger pictures related to greater negativity to anger pictures (r = 0.55, P = 0.002). Ratings to neutral pictures were unrelated, rs < 0.31, Ps > 0.11. These results indicate that anger pictures strongly evoked highly arousing negative anger.
Correlations between BIS/BAS and picture ratings revealed that Drive related to greater anger and arousal ratings to anger pictures (Table 1). Overall BAS predicted greater ratings of anger toward anger pictures. BIS also related to greater anger ratings to anger pictures. Because Drive was the most sensitive to anger ratings, we regressed Drive and BIS onto anger and arousal picture ratings. To control for ratings to neutral pictures, difference scores were created between affective and neutral picture ratings for subsequent analyses. Drive significantly predicted more anger toward anger pictures, partial r = 0.41, P = 0.03, controlling for BIS. In contrast, BIS marginally predicted more anger toward anger pictures partial r = 0.37, P = 0.06, controlling for Drive. These results indicate that BAS was significantly related to subjective anger.
Table 1.
Correlation coefficients for picture type, picture ratings and BAS/BIS scales
| Anger |
Neutral |
|||||
|---|---|---|---|---|---|---|
| Valence | Arousal | Anger | Valence | Arousal | Anger | |
| Drive | 0.35 | 0.36* | 0.47* | −0.13 | 0.05 | 0.17 |
| Reward Responsiveness | 0.09 | 0.31 | 0.24 | −0.03 | −0.14 | 0.04 |
| Fun-Seeking | −0.06 | −0.04 | 0.09 | 0.10 | 0.51* | 0.27 |
| Overall BAS | 0.18 | 0.30 | 0.38* | −0.02 | 0.20 | 0.24 |
| BIS | 0.21 | 0.32 | 0.46* | −0.12 | −0.12 | 0.18 |
Correlations marked by asterisks are significant (P < 0.05). Higher scores indicate greater negativity, arousal and anger.
LPP amplitude
Because past research indicates that LPP amplitudes are most prominent at midline sites (Olofsson et al., 2008; Weinberg et al., 2012, unpublished data) and to reduce the number of statistical tests, we examined LPP amplitudes aggregated across picture type at indices of frontal, central-parietal and occipital midline sites (frontal: F1, FZ, F2, FC1, FCZ and FC2; central-parietal: C1, CZ, C2, CP1, CPZ, CP2, P1, PZ and P2; occipital: PO3, POZ, PO4, O1, OZ and O2; see Figure 1). Data were normally distributed as assessed by Shapiro–Wilk test of normality, Ws > 0.97, Ps > 0.66. A 3 (frontal, central-parietal and occipital indices) × 2 (picture type) repeated-measures ANOVA revealed a significant interaction, F(2, 58) = 3.37, P= 0.04, 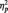 = 0.10. LPP amplitudes were maximal at central-parietal midline sites, F(2, 58) = 17.25, P < 0.0001,
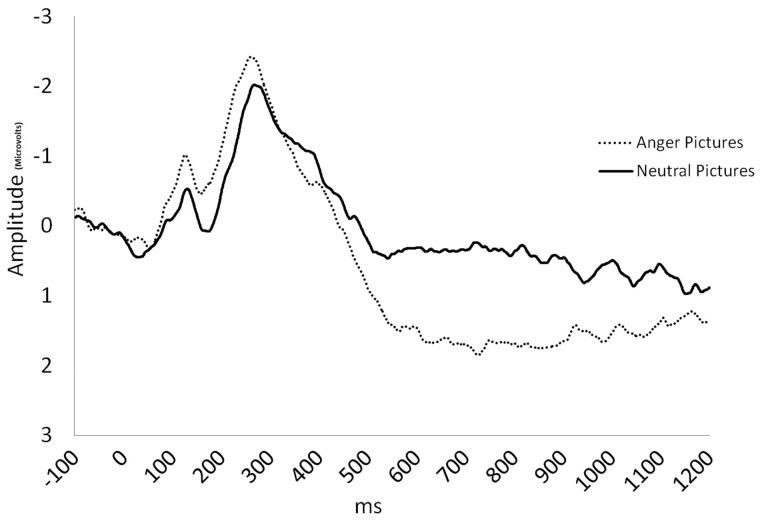
= 0.10. LPP amplitudes were maximal at central-parietal midline sites, F(2, 58) = 17.25, P < 0.0001, 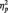 = 0.37. Anger pictures evoked larger LPPs than neutral pictures, F(1, 29) = 13.01, P = 0.001,
= 0.37. Anger pictures evoked larger LPPs than neutral pictures, F(1, 29) = 13.01, P = 0.001, 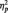 = 0.31 (Figure 2). However, follow-up analyses indicated that this was only in the central-parietal index (P< 0.001), but not at frontal or occipital indices, Ps > 0.43. These results indicate that the LPP is sensitive to affective pictures evoking anger. Because LPP amplitudes at central-parietal sites were maximal and most sensitive to affective pictures, the central-parietal index was used for subsequent LPP amplitude analyses.
= 0.31 (Figure 2). However, follow-up analyses indicated that this was only in the central-parietal index (P< 0.001), but not at frontal or occipital indices, Ps > 0.43. These results indicate that the LPP is sensitive to affective pictures evoking anger. Because LPP amplitudes at central-parietal sites were maximal and most sensitive to affective pictures, the central-parietal index was used for subsequent LPP amplitude analyses.
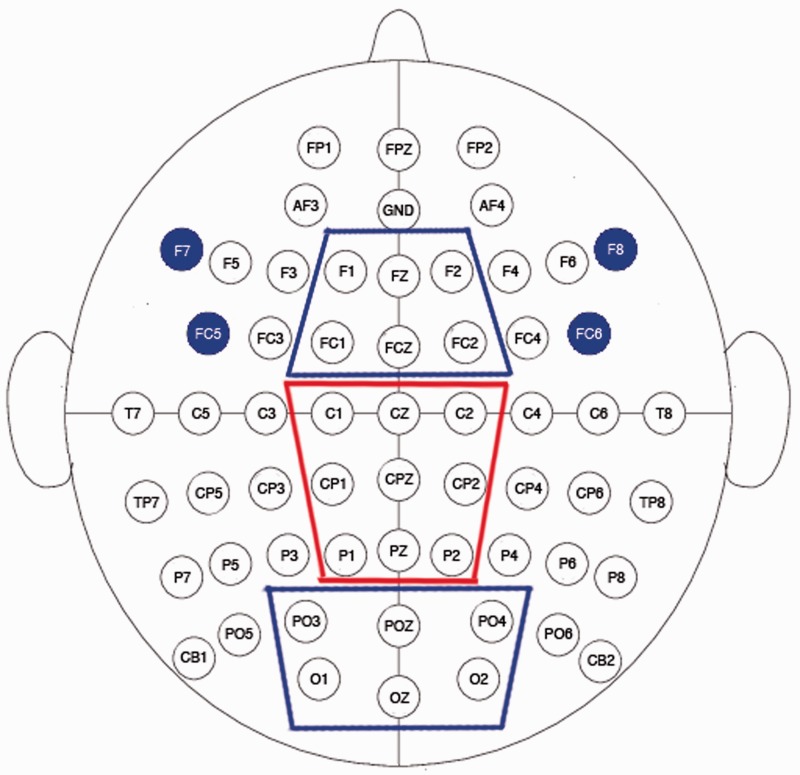
Fig. 1.
Electrode configuration with sites of interest. Outlines indicate sites used for LPP indices. Highlighted sites indicate sites used for frontal asymmetry analyses.
Fig. 2.
ERP waveform depicting LPP amplitudes to anger and neutral pictures at the central-parietal index from 100 ms before picture onset to 1200 ms after picture onset.
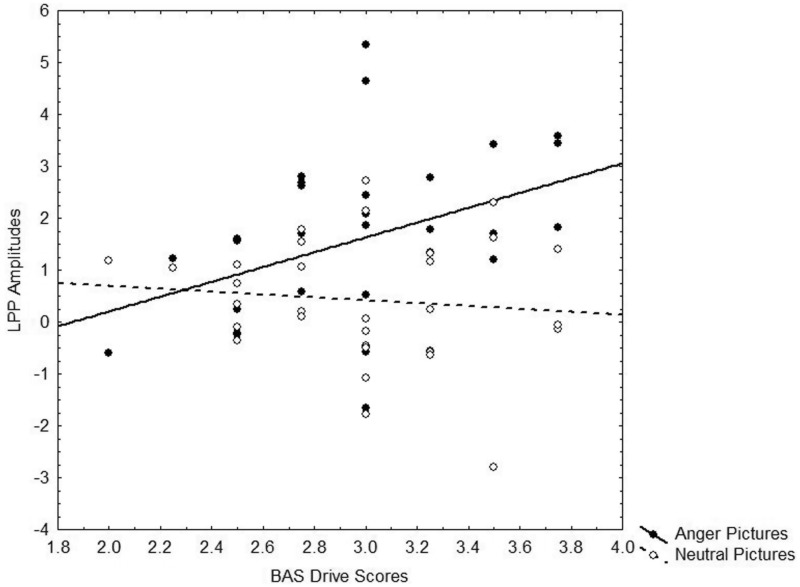
To test the prediction that trait motivation would relate to LPP amplitudes to anger pictures, we conducted a regression analysis following the methods of Aiken and West (1991) in which the BIS/BAS scales and picture type were used to interactively predict LPP amplitudes. Drive interacted with picture type to predict larger LPPs to anger pictures than to neutral pictures, F(1, 28) = 9.04, P = 0.01, 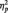 = 0.24 (Figure 3). Analyses of the interaction slopes revealed that greater Drive predicted larger LPP amplitudes to anger pictures, partial r = 0.49, P = 0.01, controlling for LPP amplitudes to neutral pictures (Figure 4). In contrast, Drive was marginally related to smaller LPP amplitudes to neutral pictures, partial r = −0.35, P = 0.06, controlling for LPP amplitudes to anger pictures. Regression interactions with Reward Responsiveness and Fun-Seeking were not significant, Fs < 1.84, Ps > 0.18. The interaction between overall BAS and picture type predicting LPP amplitudes was marginally significant, F(1, 28) = 3.60, P = 0.06,
= 0.24 (Figure 3). Analyses of the interaction slopes revealed that greater Drive predicted larger LPP amplitudes to anger pictures, partial r = 0.49, P = 0.01, controlling for LPP amplitudes to neutral pictures (Figure 4). In contrast, Drive was marginally related to smaller LPP amplitudes to neutral pictures, partial r = −0.35, P = 0.06, controlling for LPP amplitudes to anger pictures. Regression interactions with Reward Responsiveness and Fun-Seeking were not significant, Fs < 1.84, Ps > 0.18. The interaction between overall BAS and picture type predicting LPP amplitudes was marginally significant, F(1, 28) = 3.60, P = 0.06, 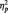 = 0.11. Analyses of the interaction slopes revealed that greater overall BAS marginally predicted larger LPPs to anger pictures, partial r = 0.33, P = 0.07, controlling for LPPs to neutral pictures. In contrast, overall BAS did not relate to LPPs to neutral pictures, partial r = −0.23, P = 0.24, controlling for LPP amplitudes to anger pictures. BIS did not interact with picture type to predict larger LPPs to anger pictures than to neutral pictures, F(1, 28) = 0.07, P = 0.79. These results indicate that LPP amplitudes to anger pictures were related to trait BAS, but not trait BIS.
= 0.11. Analyses of the interaction slopes revealed that greater overall BAS marginally predicted larger LPPs to anger pictures, partial r = 0.33, P = 0.07, controlling for LPPs to neutral pictures. In contrast, overall BAS did not relate to LPPs to neutral pictures, partial r = −0.23, P = 0.24, controlling for LPP amplitudes to anger pictures. BIS did not interact with picture type to predict larger LPPs to anger pictures than to neutral pictures, F(1, 28) = 0.07, P = 0.79. These results indicate that LPP amplitudes to anger pictures were related to trait BAS, but not trait BIS.
Fig. 3.
Relationship between BAS Drive and LPP amplitudes as a function of picture type.
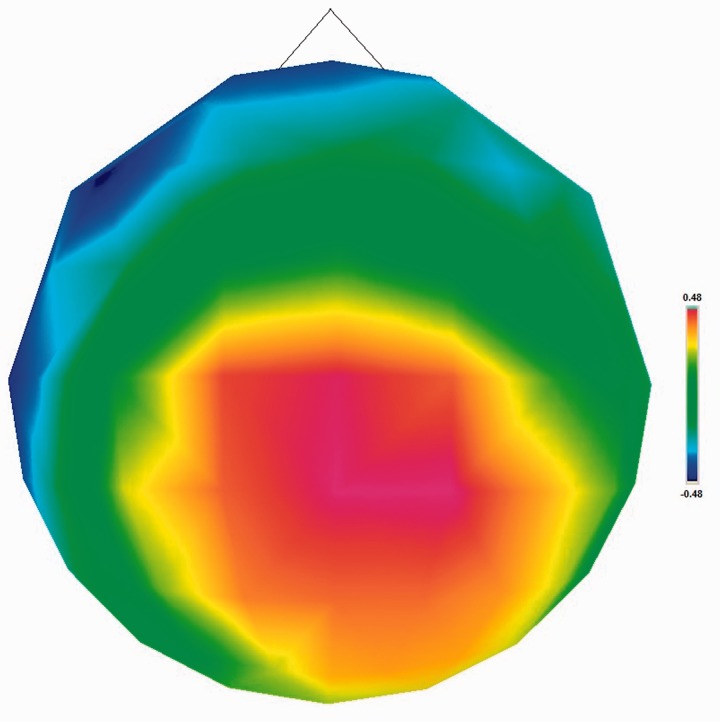
Fig. 4.
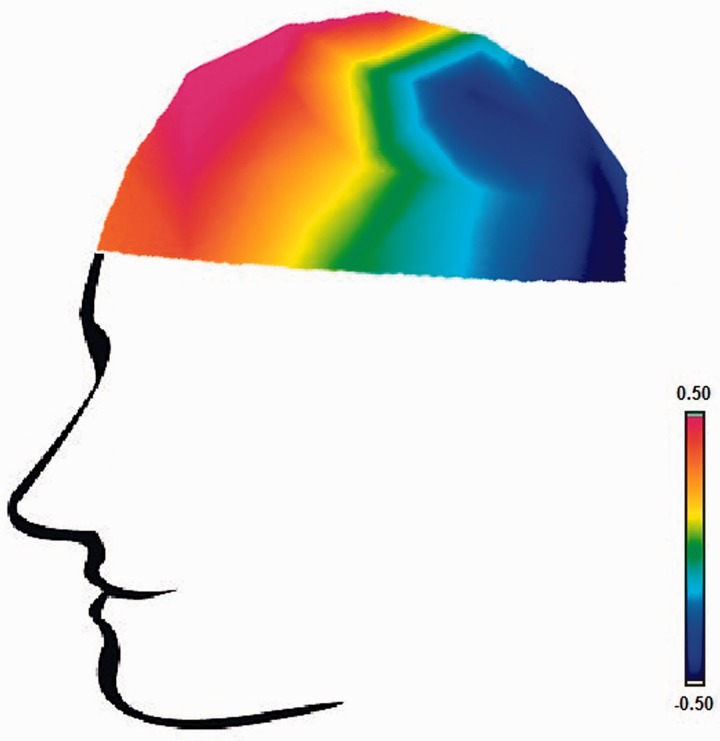
Topographical map illustrating correlations between BAS Drive and LPP amplitude to anger pictures, controlling for LPP amplitude to neutral pictures.
Larger LPP amplitudes to anger pictures marginally predicted greater arousal to anger pictures, partial r = 0.33, P = 0.08, controlling for LPP amplitudes and arousal to neutral pictures. Valence and anger ratings did not relate to LPP amplitudes to anger pictures, partial rs < 0.29, P’s > 0.13, controlling for LPP amplitudes and ratings to neutral pictures.
N1 and N2 amplitude
Because past research indicates that N1 and N2 amplitudes are most prominent at central-parietal midline sites (Foti et al., 2009; Weinberg and Hajcak, 2010; Gable and Harmon-Jones, 2011, 2013), we examined N1 and N2 amplitudes aggregated across picture type at an index of central-parietal sites (C1, CZ, C2, CP1, CPZ and CP2). Analyses revealed that anger pictures elicited greater N1 and N2 amplitudes than neutral pictures at central sites, Fs > 9.10, Ps < 0.01.
Valence, arousal and anger ratings did not relate to N1 or N2 amplitudes to anger pictures, partial rs < 0.17, Ps > 0.38, controlling for N1 and N2 amplitudes and ratings to neutral pictures. N1 and N2 amplitudes did not relate to the BAS or BIS scales, partial rs < 0.14, Ps > 0.51, controlling for N1 and N2 amplitudes to neutral pictures.
Frontal asymmetry
Based on previous research examining asymmetrical EEG activity at lateral-frontal and lateral-frontal-central sites (Harmon-Jones et al., 2002; Harmon-Jones and Sigelman, 2001; Harmon-Jones and Gable, 2009), frontal asymmetry was assessed using an index at lateral-frontal sites (F8/F7, FC6/FC5). Consistent with past research examining the effect of picture type on frontal asymmetry (Harmon-Jones, 2006, 2007; Gable and Harmon-Jones, 2008), comparison of picture type on frontal asymmetry revealed no significant results within the 6 s of picture viewing, t(29) = 0.49, P = 0.63. However, based on this past work, we tested the prediction that trait motivation and picture type would interactively relate to frontal asymmetry activation to anger pictures. We conducted a regression analysis in which the BIS/BAS scales and picture type were used to interactively predict left-frontal activation. Drive interacted with picture type to predict greater left-frontal activation to anger pictures than to neutral pictures, F(1, 28) = 5.99, P = 0.02, 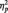 = 0.18. [Results were also examined using power values within the low α-band (8–10.25 Hz). There was a marginal interaction between BAS and picture type predicting low α-band left-frontal activation, F(1, 28) = 3.20, P = 0.08.] Analyses of the interaction slopes revealed that increased Drive predicted greater left-frontal activation to anger pictures, partial r = 0.40, P = 0.03, controlling for responses to neutral pictures. Drive was not related to frontal asymmetry to neutral pictures, r = −0.20, P = 0.28. Reward Responsiveness also interacted with picture type to predict greater left-frontal activation to anger pictures than to neutral pictures, F(1, 28) = 7.53, P = 0.01,
= 0.18. [Results were also examined using power values within the low α-band (8–10.25 Hz). There was a marginal interaction between BAS and picture type predicting low α-band left-frontal activation, F(1, 28) = 3.20, P = 0.08.] Analyses of the interaction slopes revealed that increased Drive predicted greater left-frontal activation to anger pictures, partial r = 0.40, P = 0.03, controlling for responses to neutral pictures. Drive was not related to frontal asymmetry to neutral pictures, r = −0.20, P = 0.28. Reward Responsiveness also interacted with picture type to predict greater left-frontal activation to anger pictures than to neutral pictures, F(1, 28) = 7.53, P = 0.01, 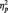 = 0.21. Analyses of the interaction slopes revealed that increased Reward Responsiveness predicted greater left-frontal activation to anger pictures, partial r = 0.45, P = 0.02, controlling for responses to neutral pictures. Reward Responsiveness was not related to frontal asymmetry to neutral pictures, r = −0.15, P = 0.43. Fun-Seeking did not interact with picture type to predict greater left-frontal activation to anger pictures than to neutral pictures, F(1, 28) = 0.43, P = 0.52.
= 0.21. Analyses of the interaction slopes revealed that increased Reward Responsiveness predicted greater left-frontal activation to anger pictures, partial r = 0.45, P = 0.02, controlling for responses to neutral pictures. Reward Responsiveness was not related to frontal asymmetry to neutral pictures, r = −0.15, P = 0.43. Fun-Seeking did not interact with picture type to predict greater left-frontal activation to anger pictures than to neutral pictures, F(1, 28) = 0.43, P = 0.52.
Overall BAS also interacted with picture type to predict greater left-frontal activation to anger pictures than to neutral pictures, F(1, 28) = 8.64, P = 0.01, 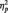 = 0.24. Analyses of the interaction slopes revealed that increased overall BAS predicted greater frontal asymmetry to anger pictures, partial r = 0.47, P = 0.01, controlling for responses to neutral pictures. Overall BAS was marginally related to frontal asymmetry to neutral pictures, r = −0.34, P = 0.06. BIS did not interact with picture type to predict greater frontal asymmetry to anger pictures than to neutral pictures, F(1, 28) = 0.72, P = 0.40. These results indicate that greater left-frontal activation to anger pictures was related to trait BAS, but not trait BIS.
= 0.24. Analyses of the interaction slopes revealed that increased overall BAS predicted greater frontal asymmetry to anger pictures, partial r = 0.47, P = 0.01, controlling for responses to neutral pictures. Overall BAS was marginally related to frontal asymmetry to neutral pictures, r = −0.34, P = 0.06. BIS did not interact with picture type to predict greater frontal asymmetry to anger pictures than to neutral pictures, F(1, 28) = 0.72, P = 0.40. These results indicate that greater left-frontal activation to anger pictures was related to trait BAS, but not trait BIS.
Greater frontal asymmetry to anger pictures predicted greater arousal to anger pictures, partial r = 0.37, P = 0.05, controlling for greater left-frontal activity and arousal to neutral pictures. Valence and anger ratings did not relate to left-frontal activity to anger pictures, partial rs < 0.19, Ps > 0.36, controlling for LPP amplitudes and ratings to neutral pictures.
To further test the relationship between the LPP and frontal asymmetry, LPP amplitudes were regressed onto frontal asymmetry scores. To control for individual differences in LPP amplitudes, we created a difference score between LPP amplitudes to anger pictures and neutral pictures (larger scores indicate larger LPP amplitudes toward anger pictures than to neutral pictures). LPP amplitudes to anger pictures interacted with picture type to predict greater left-frontal activity to anger pictures, F(1, 28) = 8.17, P = 0.01, 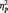 = 0.23. Larger LPP amplitudes toward anger pictures predicted greater left-frontal activation toward anger pictures, partial r = 0.71, P < 0.001, controlling for frontal activation to neutral pictures (Figure 5). LPP amplitudes to anger pictures were not related to left-frontal activation toward neutral pictures, partial r = −0.31, P = 0.10, controlling for frontal activation to anger pictures. These results indicate that LPP amplitudes to anger pictures were related to greater left-frontal activation, a state measure of approach motivation.
= 0.23. Larger LPP amplitudes toward anger pictures predicted greater left-frontal activation toward anger pictures, partial r = 0.71, P < 0.001, controlling for frontal activation to neutral pictures (Figure 5). LPP amplitudes to anger pictures were not related to left-frontal activation toward neutral pictures, partial r = −0.31, P = 0.10, controlling for frontal activation to anger pictures. These results indicate that LPP amplitudes to anger pictures were related to greater left-frontal activation, a state measure of approach motivation.
Fig. 5.
Topographical map illustrating correlations between LPP amplitude difference scores (anger–neutral) and left-frontal activity difference scores (anger–neutral).
DISCUSSION
The current experiment found that trait approach motivation related to neurophysiological anger responses when viewing pictures. Anger pictures evoked larger LPP amplitudes than neutral pictures, and BAS related to LPP amplitudes to anger pictures. These results suggest that the LPP to anger pictures is related to BAS sensitivity. In addition, trait BAS predicted greater left-frontal activation to anger pictures, suggesting that individuals high in behavioral approach sensitivity had greater state approach motivation during anger pictures. Moreover, LPP amplitudes to anger pictures predicted greater left-frontal activation toward anger pictures but not neutral pictures, suggesting that the LPP to anger pictures is related to state neurophysiological measures of approach motivation. Notably, trait approach motivation related to relatively early (i.e. <1 s) neurophysiological responses as measured by the LPP ERP component to anger pictures, as well as neurophysiological assessments of motivational relevance throughout the entire 6 s of anger picture display.
The current experiment is the first to demonstrate that the LPP is sensitive to pictures evoking anger. These findings extend much past research investigating the LPP to negative affects other than anger. These results also extend previous research on the LPP by suggesting that the LPP to anger pictures is related to approach motivation. Much past research has shown that the LPP is sensitive to aversive as well as appetitive stimuli (Schupp et al., 2004a; Briggs and Martin, 2009; MacNamara and Hajcak, 2009; Gable and Harmon-Jones, 2010; Weinberg and Hajcak, 2010; Harmon-Jones et al., 2011). These past findings suggest that the LPP is related to motivational intensity. In conjunction with these past findings, the current results suggest that the LPP may reflect intensity of the motivational direction engaged by affective stimuli. Recent research has found that BAS relates to LPPs evoked by approach-motivation positive pictures and BIS relates to LPPs evoked by withdrawal-motivation negative pictures (Balconi et al., 2012). The current results suggest that the LPP to anger pictures is related to approach motivation and not to withdrawal motivation. In sum, the LPP may reflect intensity of the motivational direction engaged by affective stimuli.
In the current experiment, BAS and BIS related to subjective ratings of anger. This relationship is consistent with prior research showing that self-reported anger is associated with BAS and BIS (Harmon-Jones, 2003; Carver, 2004; Smits and Kuppens, 2005). However, when controlling for anger responses to neutral pictures, Drive remained a significant predictor of subjective anger, but BIS did not. Past studies have found that after controlling for trait fear (e.g. anxiety or nervousness), BIS no longer related to anger. In the current experiment, the subjective experience of anger toward threats to the United States may cause avoidant-anger in individuals high in BIS. Future research should incorporate self-report measures of withdrawal-related emotions.
Findings in the current experiment may help tease apart the dimensions of motivational direction as opposed to general arousal in anger. For example, arousal is inherent in both strong approach and strong avoidant affects. Both high BAS and high BIS individuals should experience greater arousal to anger pictures. However, the relationship between BAS Drive and subjective arousal suggests that BAS, but not BIS, was associated with arousal toward anger pictures. Consistent with prominent models of emotion which propose that emotional arousal is an index of motivational intensity (Bradley and Lang, 2007), LPP amplitudes and greater left-frontal activation to anger pictures related to subjective arousal to anger pictures.
Furthermore, by manipulating anger states, the current experiment helps disentangle approach motivation from affective valence. That is, affective valence is typically thought to control motivational direction (De Cesarei amd Codispoti, 2011), and positive affects are generally associated with approach motivational intensity. However, results of the current experiment help further link anger with approach motivation (Harmon-Jones and Sigelman, 2001; Harmon-Jones, 2004, 2007; Harmon-Jones et al., 2004, 2008).
Conflict of Interest
None declared.
REFERENCES
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. London: Sage Publications; 1991. [Google Scholar]
- Amodio DM, Master SL, Yee CM, Taylor SE. Neurocognitive components of the behavioral inhibition and activation systems: implications for theories of self-regulation. Psychophysiology. 2008;45:11–9. doi: 10.1111/j.1469-8986.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Balconi M. Frontal brain oscillation modulation in facial emotion comprehension: the role of reward and inhibitory systems in subliminal and supraliminal processing. Journal of Cognitive Psychology. 2011;23(6):723–35. [Google Scholar]
- Balconi M, Falbo L, Conte VA. BIS and BAS correlates with psychophysiological and cortical response systems during aversive and appetitive emotional stimuli processing. Motivation and Emotion. 2012;36:218–31. [Google Scholar]
- Balconi M, Mazza G. Brain oscillations and BIS/BAS (behavioral inhibition/activation system) effects on processing masked emotional cues. ERS/ERD and coherence measures of alpha band. International Journal of Psychophysiology. 2009;74:158–65. doi: 10.1016/j.ijpsycho.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. Aggression: Its Causes, Consequences, and Control. New York: McGraw-Hill; 1993. [Google Scholar]
- Bradley MM. Natural selective attention: orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Hamby S, Löw A, Lang PJ. Brain potentials in perception: picture complexity and emotional arousal. Psychophysiology. 2007;44:364–73. doi: 10.1111/j.1469-8986.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Emotion and motivation. In: Cacioppo JT, Tassinary LG, Berntson G, editors. Handbook of Psychophysiology. 3rd edn. New York: Cambridge University Press; 2007. pp. 581–607. [Google Scholar]
- Briggs KE, Martin FH. Affective picture processing on motivational relevance: arousal and valence effects on ERPs in an oddball task. International Journal of Psychophysiology. 2009;72:299–306. doi: 10.1016/j.ijpsycho.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Buss KA, Malmstadt Schumacher JR, Dolski I, Kalin NH, Goldsmith HH, Davidson RJ. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Behavioral Neuroscience. 2003;117:11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Carver CS. Negative affects deriving from the behavioral approach system. Emotion. 2004;77(1):125–38. doi: 10.1037/1528-3542.4.1.3. [DOI] [PubMed] [Google Scholar]
- Carver CS. Threat sensitivity, incentive sensitivity and the experience of relief. Journal of Personality. 2009;4(1):3–22. doi: 10.1111/j.1467-6494.2008.00540.x. [DOI] [PubMed] [Google Scholar]
- Carver CS, Harmon-Jones E. Anger is an approach-related affect: evidence and implications. Psychological Bulletin. 2009;135(2):183–204. doi: 10.1037/a0013965. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF. On the Self-regulation of Behavior. New York: Cambridge University Press; 1998. [Google Scholar]
- Carver CS, Scheier MF. Feedback processes in the simultaneous regulation of action and affect. In: Shah JY, Gardner WL, editors. Handbook of Motivation Science. New York: Guilford Press; 2008. pp. 308–24. [Google Scholar]
- Carver CS, White TL. Behavioral-inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67(2):319–33. [Google Scholar]
- Coan JA, Allen JJB. The state and trait nature of frontal EEG asymmetry in emotion. In: Hugdahl K, Davidson RJ, editors. The Asymmetrical Brain. 5th edn. Cambridge, MA: MIT Press; 2003a. pp. 565–615. [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003b;40:106–14. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cook IA, O’Hara RO, Uijtdehaage SHJ, Mandelkern M, Leuchter AF. Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalography and Clinical Neurophysiology. 1998;107(6):408–14. doi: 10.1016/s0013-4694(98)00092-3. [DOI] [PubMed] [Google Scholar]
- Cooper A, Gomez R, Buck E. The relationship between the BIS and BAS, anger and responses to anger. Personality and Individual Differences. 2007;44:403–13. [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Chapman JP, Chapman LJ, Henriques JB. Asymmetrical brain electrical activity discriminates between psychometrically-matched verbal and spatial cognitive tasks. Psychophysiology. 1990;27(5):528–43. doi: 10.1111/j.1469-8986.1990.tb01970.x. [DOI] [PubMed] [Google Scholar]
- De Cesarei A, Codispoti M. Affective modulation of the LPP and α-ERD during picture viewing. Psychophysiology. 2011;48:1397–404. doi: 10.1111/j.1469-8986.2011.01204.x. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Hajcak G. The late positive potential: a neurophysiological marker for emotion regulation in children. The Journal of Child Psychology and Psychiatry. 2009;50(11):1373–83. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Elgavish E, Halpern D, Dikman Z, Allen JJB. Does frontal EEG asymmetry moderate or mediate responses to the international affective picture system (IAPS)? Psychophysiology. 2003;40(Suppl. 1):s38. [Google Scholar]
- Elliot AJ, Thrash TM. Approach-avoidance motivation in personality: approach and avoidance temperaments and goals. Journal of Personality and Social Psychology. 2002;82(5):804–18. doi: 10.1037//0022-3514.82.5.804. [DOI] [PubMed] [Google Scholar]
- Ferrari V, Codispoti M, Cardinale R, Bradley MM. Directed and motivated attention during processing of natural scenes. Journal of Cognitive Neuroscience. 2008;20(10):1753–61. doi: 10.1162/jocn.2008.20121. [DOI] [PubMed] [Google Scholar]
- Fischer AH, Roseman IJ. Beat them or ban them: the characteristics and social functions of anger and contempt. Journal of Personality and Social Psychology. 2007;93:103–15. doi: 10.1037/0022-3514.93.1.103. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology. 2009;46(3):521–30. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC. Application of a behavioral theory of motivation to the concepts of anxiety and impulsivity. Journal of Research in Personality. 1987;21:417–35. [Google Scholar]
- Frijda NH. The Emotions. Cambridge; New York: Cambridge University Press; 1986. [Google Scholar]
- Gable PA, Harmon-Jones E. Relative left frontal activation to appetitive stimuli: considering the role of individual differences. Psychophysiology. 2008;45:275–8. doi: 10.1111/j.1469-8986.2007.00627.x. [DOI] [PubMed] [Google Scholar]
- Gable PA, Harmon-Jones E. Late positive potential to appetitive stimuli and local attentional bias. Emotion. 2010;10(3):441–6. doi: 10.1037/a0018425. [DOI] [PubMed] [Google Scholar]
- Gable PA, Harmon-Jones E. Attentional states influence early neural responses associated with motivational processes: local vs. global attentional scope and N1 amplitude to appetitive stimuli. Biological Psychology. 2011;87:303–5. doi: 10.1016/j.biopsycho.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Gable PA, Harmon-Jones E. Trait behavioral approach sensitivity (BAS) relates to early (< 150 ms) electrocortical responses to appetitive stimuli. Social Cognitive and Affective Neuroscience. 2013;8(7):795–8. doi: 10.1093/scan/nss072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G. Emotional behavior and hemispheric side of the lesion. Cortex. 1972;8:41–55. doi: 10.1016/s0010-9452(72)80026-1. [DOI] [PubMed] [Google Scholar]
- Goldstein K. The Organism: An Holistic Approach to Biology, Derived from Pathological Data in Man. New York: American Book; 1939. [Google Scholar]
- Gray JA. The psychophysiological basis of introversion-extraversion. Behaviour Research and Therapy. 1970;8:249–66. doi: 10.1016/0005-7967(70)90069-0. [DOI] [PubMed] [Google Scholar]
- Gray JA. The Psychology of Fear and Stress. 2nd edn. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- Gray JA. Three fundamental emotion systems. In: Ekman P, Davidson RJ, editors. The Nature of Emotion: Fundamental Questions. New York: Oxford University Press; 1994. pp. 243–7. [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of Anxiety. Oxford: Oxford University Press; 2000. [Google Scholar]
- Hagemann D, Ewald N, Becker G, Maier S, Bartussek D. Frontal brain asymmetry and affective style: a conceptual replication. Psychophysiology. 1998;35:372–88. [PubMed] [Google Scholar]
- Hajcak G, Dennis TA. Brain potentials during affective picture processing children. Biological Psychology. 2009;80:333–8. doi: 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, Foti D. ERPs and the study of emotion. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of ERP Components. New York: Oxford University Press; 2012. [Google Scholar]
- Harmon-Jones E. Anger and the behavioral approach system. Personality and Individual Differences. 2003;34:1–11. [Google Scholar]
- Harmon-Jones E. On the relationship of frontal brain activity and anger: examining the role of attitude toward anger. Cognition and Emotion. 2004;18(3):337–61. [Google Scholar]
- Harmon-Jones E, Lueck L, Fearn M, Harmon-Jones C. The effect of personal relevance and approach-related action expectation on relative left frontal cortical activity. Psychological Science. 2006;17:434–40. doi: 10.1111/j.1467-9280.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Trait anger predicts relative left frontal cortical activation to anger-inducing stimuli. International Journal of Psychophysiology. 2007;66:154–60. doi: 10.1016/j.ijpsycho.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Sigelman J, Bohlig A, Hogan ME, Harmon-Jones C. Proneness to hypomania/mania symptoms or depression symptoms and asymmetrical frontal cortical responses to an anger-evoking event. Journal of Personality and Social Psychology. 2002;82(4):610–8. [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJB. Behavioral activation sensitivity and resting frontal EEG asymmetry: covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology. 1997;106(1):159–63. doi: 10.1037//0021-843x.106.1.159. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA. Neural activity underlying the effect of approach-motivated positive affect on narrowed attention. Psychological Science. 2009;20(4):406–9. doi: 10.1111/j.1467-9280.2009.02302.x. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biological Psychology. 2010;84:451–62. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Price TF. Toward an understanding of the influence of affective states on attentional tuning: comment on Friedman and Förster (2010) Psychological Bulletin. 2011;137(3):508–12. doi: 10.1037/a0022744. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Harmon-Jones C, Amodio DM, Gable PA. Attitudes toward emotions: conceptualization and measurement of evaluations of specific emotions. Journal of Personality and Social Psychology. 2011;101(6):1332–50. doi: 10.1037/a0024951. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Harmon-Jones C, Fearn M, Sigelman JD, Johnson P. Left frontal cortical activation and spreading of alternatives: tests of the action-based model of dissonance. Journal of Personality and Social Psychology. 2008;94(1):1–15. doi: 10.1037/0022-3514.94.1.1. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Harmon-Jones C, Serra R, Gable PA. The effect of commitment on relative left frontal cortical activity: tests of the action-based model of dissonance. Personality and Social Psychology Bulletin. 2011;37(3):395–408. doi: 10.1177/0146167210397059. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Lueck L, Fearn M, Harmon-Jones C. The effect of personal relevance and approach-related action expectation on relative left frontal cortical activity. Psychological Science. 2006;17(5):434–40. doi: 10.1111/j.1467-9280.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Peterson CK, Harris CR. Jealousy: novel methods and neural correlates. Emotion. 2009;9(1):113–7. doi: 10.1037/a0014117. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman J. State anger and prefrontal brain activity: evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. Journal of Personality and Social Psychology. 2001;80(5):797–803. [PubMed] [Google Scholar]
- Harmon-Jones E, Vaughn-Scott K, Mohr S, Sigelman J, Harmon-Jones C. The effect of manipulated sympathy and anger on left and right frontal cortical activity. Emotion. 2004;4(1):1–7. doi: 10.1037/1528-3542.4.1.95. [DOI] [PubMed] [Google Scholar]
- Hewig J, Hagemann D, Seifert J, Naumann E, Bartussek D. On the selective relation of frontal cortical asymmetry and anger-out versus anger-control. Journal of Personality and Social Psychology. 2004;87(6):926–39. doi: 10.1037/0022-3514.87.6.926. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Mueller CJ, Dolski I, et al. Now you feel it, now you don’t: frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological Science. 2003;14(6):612–7. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Ripper B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neuroscience Letters. 1997;238:9–12. doi: 10.1016/s0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe. American Psychologist. 1995;50:372–85. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Digitized photographs, instruction manual and affective ratings. Technical Report A-6. University of Florida, Gainesville: FL; 2005. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biological Psychiatry. 1998;44:1248–63. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Leen-Feldner EW, Zvolensky MJ, Feldner MT. Behavioral inhibition sensitivity and emotional response suppression: a laboratory test among adolescents in a fear-relevant paradigm. Journal of Clinical Child and Adolescent Psychology. 2004;33:783–91. doi: 10.1207/s15374424jccp3304_13. [DOI] [PubMed] [Google Scholar]
- Lindsley DB, Wicke JD. The electroencephalogram: autonomous electrical activity in man and animals. In: Thompson R, Patterson MN, editors. Bioelectric Recording Techniques. New York: Academic Press; 1974. pp. 3–79. [Google Scholar]
- MacNamara A, Hajcak G. Anxiety and spatial attention moderate the electrocortical response to aversive pictures. Neuropsychologia. 2009;47(13):2975–80. doi: 10.1016/j.neuropsychologia.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Ambady N, Kleck RE. The effects of fear and anger facial expressions on approach- and avoidance-related behaviors. Emotion. 2005;5(1):119–24. doi: 10.1037/1528-3542.5.1.119. [DOI] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: relations of the BIS/BAS scales with symptoms. Journal of Psychopathology and Behavioral Assess. 2001;23(3):133–43. doi: 10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulincer M. Reactance and helplessness following exposure to unsolvable problems: the effects of attributional style. Journal of Personality and Social Psychology. 1988;54:679–86. doi: 10.1037//0022-3514.54.4.679. [DOI] [PubMed] [Google Scholar]
- Mühlberger A, Wieser MJ, Herrmann MJ, Weyers P, Tröger C, Pauli P. Early cortical processing of natural and artificial emotional faces differs between lower and higher socially anxious persons. Journal of Neural Transmission. 2008;116(6):735–46. doi: 10.1007/s00702-008-0108-6. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Nijs IMT, Franken IHA, Smulders FTY. BIS/BAS sensitivity and the P300 event-related brain potential. Journal of Psychophysiology. 2007;21(2):83–90. [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: an integrative review of ERP findings. Biological Psychology. 2008;77(3):247–65. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortony A, Clore GL, Collins A. The Cognitive Structure of Emotions. New York: Cambridge University Press; 1988. [Google Scholar]
- Pizzagalli D, Shackman AJ, Davidson RJ. The functional neuroimaging of human emotion: asymmetric contributions of cortical and subcortical circuitry. In: Hugdahl K, Davidson RJ, editors. The Asymmetrical Brain. Cambridge, MA: MIT Press; 2003. pp. 511–32. [Google Scholar]
- Piazzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness: a source-localization study. Psychological Science. 2005;16(10):805–13. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Robinson RG, Price TR. Post-stroke depressive disorders: a follow-up study of 103 patients. Stroke. 1982;13:635– 41. doi: 10.1161/01.str.13.5.635. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The Brain and Emotion. Oxford: Oxford University Press; 1999. [Google Scholar]
- Rossi GF, Rosadini GR. Experimental analyses of cerebral dominance in man. In: Millikan DH, Darley FL, editors. Brain Mechanisms Underlying Speech and Language. New York: Grune & Stratton; 1967. pp. 167–84. [Google Scholar]
- Sackeim H, Greenberg MS, Weimen AL, Gur RC, Hungerbuhler JP, Geschwind N. Hemispheric asymmetry in the expression of positive and negative emotions: neurologic evidence. Archives of Neurology. 1982;39:210–8. doi: 10.1001/archneur.1982.00510160016003. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–61. [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004a;41(3):441–9. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Öhman A, Junghöfer M, Weike AI, Stockburger J, Hamm AO. The facilitated processing of threatening faces: an ERP analysis. Emotion. 2004b;4(2):189–200. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Silva JR, Pizzagalli DA, Larson CL, Jackson DC, Davidson RJ. Frontal brain asymmetry in restrained eaters. Journal of Abnormal Psychology. 2002;111(4):676–81. doi: 10.1037//0021-843x.111.4.676. [DOI] [PubMed] [Google Scholar]
- Smits DJM, Kuppens P. The relations between anger, coping with anger, and aggression, and the BIS/BAS system. Personality and Individual Differences. 2005;39:783–93. [Google Scholar]
- Spielberg JM, Stewart JL, Levin RL, Miller GA, Heller W. Prefrontal cortex, emotion, and approach/withdrawal motivation. Social Personnel Psychology Compass. 2008;2(1):135–53. doi: 10.1111/j.1751-9004.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Towers DN, Coan JA, Allen JJB. The oft-neglected role of parietal EEG asymmetry and risk for major depressive disorder. Psychophysiology. 2011;48(1):82–95. doi: 10.1111/j.1469-8986.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Silton RL, Sass SM, et al. Attentional bias to negative emotion as a function of approach and withdrawal anger styles: an ERP investigation. International Journal of Psychophysiology. 2010;76(1):9–18. doi: 10.1016/j.ijpsycho.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: a biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8(3):204–10. [Google Scholar]
- Vallortigara G, Rogers L. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behavioral and Brain Sciences. 2005;28:575–633. doi: 10.1017/S0140525X05000105. [DOI] [PubMed] [Google Scholar]
- Watson D. Locating anger in the hierarchical structure of affect: comment on Carver and Harmon-Jones (2009) Psychological Bulletin. 2009;135(2):205–8. doi: 10.1037/a0014413. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Ferri J, Hajcak G. Interactions Between Attention and Emotion: Reflections on the Late Positive Potential. 2012. Unpublished manuscript. [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion. 2010;10(6):767–82. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Wingrove J, Bond AJ. Angry reactions to failure on a cooperative computer game: the effect of trait hostility. Aggressive Behavior. 1998;24(1):27–36. [Google Scholar]
- Zinner L, Brodish A, Devine PG, Harmon-Jones E. Anger and asymmetrical frontal cortical activity: evidence for an anger-withdrawal relationship. Cognition and Emotion. 2008;22:1081–93. doi: 10.1080/02699930701622961. [DOI] [PMC free article] [PubMed] [Google Scholar]