Abstract
An individual’s affective style is influenced by many things, including the manner in which an individual responds to an emotional challenge. Emotional response is composed of a number of factors, two of which are the initial reactivity to an emotional stimulus and the subsequent recovery once the stimulus terminates or ceases to be relevant. However, most neuroimaging studies examining emotional processing in humans focus on the magnitude of initial reactivity to a stimulus rather than the prolonged response. In this study, we use functional magnetic resonance imaging to study the time course of amygdala activity in healthy adults in response to presentation of negative images. We split the amygdala time course into an initial reactivity period and a recovery period beginning after the offset of the stimulus. We find that initial reactivity in the amygdala does not predict trait measures of affective style. Conversely, amygdala recovery shows predictive power such that slower amygdala recovery from negative images predicts greater trait neuroticism, in addition to lower levels of likability of a set of social stimuli (neutral faces). These data underscore the importance of taking into account temporal dynamics when studying affective processing using neuroimaging.
Keywords: amygdala, fMRI, emotion, time course, chronometry
INTRODUCTION
The ability to regulate emotion and to recover from emotionally evocative stimuli is critical for maintaining well-being and its dysregulation could lead to various forms of psychopathology (Cicchetti et al., 1995; John and Gross, 2004). Many neuroimaging studies have sought to understand the role of neural circuitry supporting individual differences in the regulation of emotion by examining the relationship between the magnitude of activation in particular brain regions and an individual’s reported trait and state affect. However, it is not only one’s initial reaction to an event but also the sustained response that define one’s ability to regulate emotions, and ultimately one’s affective style (Davidson, 2004). In light of this, it is equally important to take into account the chronometry of the neural response in addition to its magnitude. It is possible that one has a strong initial reaction to a negative stimulus, but can then return quickly to a baseline state, while another has a moderate reaction to a negative stimulus but requires a prolonged period of time to recover. Consequently, repeated exposure to negative stimuli could result in greater emotional dysregulation for individuals with decreased ability to recover from an emotional challenge.
Neuroticism is a personality trait that is characterized by decreased ability to regulate emotion (Kokkonen and Pulkkinen, 2001), negative emotionality (Pervin and John, 1999), increased experienced negative affect (Mroczek and Almeida, 2004) and greater emotional perseveration following negative events (Suls and Martin, 2005). The amygdala is a region that is well known to be involved in emotional processing (Phelps and LeDoux, 2005). Indeed, high neuroticism predicts greater amygdala activation in a number of tasks involving emotional processing (Haas et al., 2007; Stein et al., 2007; Hooker et al., 2008; Harenski et al., 2009; Brück et al., 2011; Cunningham et al., 2011) and decreased connectivity between the amygdala and prefrontal regions involved in regulation, specifically the anterior cingulate cortex (Cremers et al., 2010).
This study represents a first effort to ascertain the importance of individual differences in amygdala chronometry in a non-clinical population to the expression of trait affect. We collected functional magnetic resonance imaging (fMRI) data while presenting emotionally evocative images to participants and analyzed amygdala activity in response to those images. To distinguish reactivity and recovery in the amygdala time course, we split the neural response into two separate time periods—an initial reactivity period in response to the presentation of the stimulus and a recovery period of equal duration after the offset of the stimulus. We computed an area under the curve (AUC) metric to measure amygdala activity in these periods. Because neuroticism is associated with greater perseveration of emotional events, we hypothesized that greater neuroticism would be specifically related to slower amygdala recovery. In addition to self-reported neuroticism, we investigated an implicit measure of affective style. Several studies have used participant ratings of unfamiliar faces on a number of metrics as measures of social evaluation (Engell et al., 2007; Todorov and Engell, 2008; Schiller et al., 2009; Mende-Siedlecki et al., 2012). In this study, we used participants’ average likability ratings of a set of novel neutral faces as an implicit measure of the participant’s positive or negative bias when evaluating novel social information. We hypothesized that in addition to predicting higher neuroticism scores, slower amygdala recovery would predict more negative evaluation of novel social information. In the study of emotion and affective style, reactivity and recovery are distinct constructs that must be distinguished to more fully understand the role of each.
MATERIALS AND METHODS
Participants
We recruited 127 healthy human subjects (81 female) in Madison, WI, and the surrounding community using flyers, online advertisements and advertisements in local media. Recruitment materials requested participation in a study of ‘health and well-being’ or the ‘benefits of health wellness classes’. Participants were excluded if they had used medication for anxiety, depression or other psychological issues, or had a psychiatric diagnosis in the past year. Participants were also excluded if they had any history of bipolar or schizophrenic disorders, brain damage or seizures. This task was one of a number of tasks administered during a 24 h laboratory visit as part of a larger study. UW–Madison’s Health Sciences Institutional Review Board approved the study paradigm, and all participants were given monetary compensation for their participation. Two participants were excluded due to brain abnormalities, one participant dropped out of the study before the task was completed, three were not able to complete the task due to technical difficulties and one participant was excluded due to excessive motion in the scanner. This left a total of 120 participants (76 female, 117 right-handed) with average age of 48.4 ± 10.8 years and a range of 25–65 years.
Experimental task
Seventy-two images selected from the International Affective Picture Set (IAPS) (Lang et al., 2005) were presented in this task, evenly split between negative, neutral and positive images. This resulted in 24 images in each of the three valence categories. The average normative valence (V) and arousal (A) ratings of the images in the three categories were negative (V = 2.87 ± 0.87, A = 5.51 ± 0.47), neutral (V = 5.08 ± 0.60, A = 3.86 ± 0.63) and positive (V = 7.10 ± 0.47, A = 5.36 ± 0.37), where both valence and arousal are measured on nine-point scales (where 1 = most unpleasant or least arousing and 9 = most pleasant or most arousing, respectively). Valence order was pseudo-randomized and image order was completely randomized within the task. The task also included the presentation of neutral faces after the offset of the image in two-thirds of the trials. The faces were intended to serve as a potential behavioral measure of recovery after the emotional image offset, as described below. Both male and female faces were included and appeared either 1 s (8× per valence) or 3 s (8× per valence) post-image offset. Eight images within each block were not followed by a face. Faces were chosen from the Extended Multimodal Face Database (Messer et al., 1999) and randomly allocated to each of the valence and time conditions across subjects. Each face was presented twice over the course of the session, always paired with an image of the same valence category and lag time for each subject. In total, 24 unique faces were presented in a total of 48 trials. Each image was presented for a total of 4 s, and each face was presented for 500 ms. Participants were not explicitly instructed to regulate their emotional response to each image, but to ensure they were paying attention to each image they were instructed to press a button indicating the valence category of the image (either Negative, Neutral or Positive). Button order was counterbalanced across subjects. Participants were instructed to passively view the faces following the images and not to rate them. All stimuli were presented using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA, USA) and participants viewed these images with a fiber-optic goggle system (Avotec, Inc., Stuart, FL, USA) while inside the MRI scanner. Eye movements were tracked using a SensoMotoric Instruments tracking system. The task consisted of four runs approximately 5 min each. An illustration of the paradigm is presented in Figure 1. In order to familiarize participants with the task and the scanning environment, they engaged in a simulation session in a mock scanner prior to beginning the experiment.
Fig. 1.
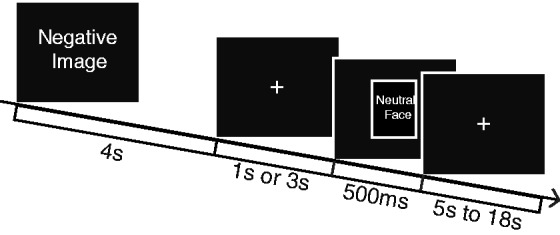
Experimental paradigm. Image is presented for 4 s, and participants press a button indicating the valence of the image (Negative, Neutral or Positive). After image offset, a neutral face is presented at 1 s or 3 s after image offset or not at all.
Behavioral measures
In order to look at effects of the preceding image on likability of the neutral faces, participants were asked to complete likability ratings of the 24 faces they had seen during the task and a set of 24 age- and gender-matched unfamiliar faces 3 days after the fMRI scan. Participants were not reminded which faces they had previously seen or with which images the faces were paired. Participants rated likability of each face on a continuous scale from −1 (Really Dislike) to 1 (Really Like). The ratings were either completed in the laboratory using E-Prime software or online outside of the laboratory, with participants instructed to complete the ratings in a quiet area and in one sitting. The order of the faces was completely randomized. Gaze fixations during the scanner task were calculated using in-house software and trials in which participants viewed the face for <100 ms of the 500 ms duration were excluded from the behavioral analysis. Participants were completely excluded from the behavioral analysis if they had missed more than two of the eight faces in each valence category or both presentations of any unique neutral face. Out of the eight possible faces presented following images of each valence, participants viewed an average of 5.73 ± 3.01 faces following negative, 5.66 ± 2.98 faces following neutral images and 5.77 ± 2.98 faces following positive images. In total, 112 participants completed Day 3 ratings of unfamiliar and previously presented faces. However, 39 participants were removed from analyses involving faces presented in the scanner due to the eye-tracking exclusion criteria described above, leaving a total n of 73 for those analyses. Participants also completed a battery of questionnaires as part of a larger study in which this experiment was embedded. The questionnaire of interest for this study was the Neuroticism subscale of the Big Five Inventory (Messer et al., 1999).
Image acquisition
Images were acquired on a GE X750 3.0 Tesla MRI scanner device with an eight-channel head coil. Anatomical scans consisted of a high-resolution 3D T1-weighted inversion recovery fast gradient echo image (inversion time = 450 ms, 256 × 256 in-plane resolution, 256 mm FOV, 124 × 1.0 mm axial slices). Four functional scan runs were acquired using a gradient echo EPI sequence (64 × 64 in-plane resolution, 240 mm FOV, TR/TE/Flip = 2000 ms/25 ms/60°, 40 × 4 mm interleaved sagittal slices and 159 3D volumes per run).
Analysis
Anatomical images were transformed to Montreal Neurological Institute (MNI) space with an affine transformation computed in Analysis of Functional NeuroImages (AFNI) (Cox, 1996), and then segmented with SPM8 (Wellcome Department of Cognitive Neurology, UCL, UK). Diffeomorphic warps to a common group-space were created for each subject using DARTEL (Ashburner, 2007) in SPM8. The anatomical image for each subject was then transformed to group space using the corresponding DARTEL warp. The anatomical images in group space were averaged together to make a group-average template, which was subsequently normalized to MNI space to create a transformation matrix by which the individual contrasts in group-template space could be transformed to MNI space.
Functional data were slice-time-corrected, then motion-corrected and transformed in AFNI to MNI space using an affine transformation. The functional data from individual subjects were analyzed using a general linear model (GLM) with stimulus presentation modeled with a canonical hemodynamic response function, as defined in SPM8 (Wellcome Department of Cognitive Neurology, UCL). Each trial was modeled as two separate parts: the 4 s presentation of the IAPS image itself and the 4 s period following the IAPS image offset. This model was used solely to functionally define the amygdala region of interest (ROI). The contrast of interest was Negative > Neutral during the 4 s IAPS presentation. This contrast resulting from the first-level GLM was further transformed non-linearly to MNI space using the DARTEL flow fields defined for each participant’s corresponding anatomical image. The individual contrasts in MNI space were then smoothed with an 8 mm smoothing kernel.
Clusters of interest from the group-level analysis were transformed from MNI space back to functional space using the inverse DARTEL flowfield and used as an amygdala mask from which to extract each participant’s amygdala time course. The average time course for each subject was extracted from the voxels within the mask which were active in the contrast Negative > Fixation at P= 0.1, uncorrected. All subsequent analyses were done using the individual subject amygdala time course, averaged over all Negative trials. Reactivity and recovery periods were chosen based on the shape of the amygdala time course averaged over all participants, with the reactivity period defined as the time from 5 to 8 s post-image onset and the recovery period defined from 9 to 12 s post-image onset (Figure 2b). Reactivity was calculated as the AUC in the reactivity period. Recovery was calculated as the AUC during the recovery period, controlling for AUC in the reactivity period. The lag observed in the amygdala time course averaged over all the participants is in accordance with the hemodynamic lag that is characteristic of the blood oxygenation level-dependent (BOLD) signal (Buxton et al., 2004). Relationships between fMRI measures and behavioral measures were analyzed in R Version 1.35-dev (http://www.r-project.org/) using ordinary least squares regressions.
Fig. 2.
Amygdala response to negative images. (a) Amygdala regions that are significantly greater in Negative > Neutral. (b) Time course of amygdala following Negative image presentation, averaged over all participants. The black rectangle above the abscissa denotes the presentation of the negative image. Reactivity is defined as the AUC in the period denoted in dark gray. Recovery is defined as AUC over the period denoted in light gray, controlling for reactivity AUC. (c) Average time course of amygdala in participants with high neuroticism (top quartile, in red) and low neuroticism (bottom quartile, in blue). (d) Average time course of amygdala in participants who rated novel faces as least likable (bottom quartile, in yellow) and most likable (top quartile, in green). Error bars represent standard deviations.
RESULTS
Behavioral results: effect of preceding image on face likability
In order to test for emotional perseveration in the period following the offset of negative and neutral images, we performed a paired t-test to look at likability of faces following negative images vs faces following neutral images. We found that faces following negative images were rated as less likable than faces following neutral images (n= 73, t= −3.95, P< 0.001). Furthermore, we found that faces following negative images were less likable than the set of novel faces (n= 73, t= −2.19, P= 0.032), whereas the faces following neutral images were rated as more likable than the novel faces (n= 73, t= 1.97, P= 0.053). These effects are particularly striking given that faces were only seen twice for 500 ms, 3 days prior to the likability ratings. Likability ratings for novel and previously seen faces are illustrated in Supplementary Figure S1. There were no significant relationships between trait neuroticism and likability of previously seen faces—those preceded by negative images (n= 72, r= −0.025, P= 0.832), those preceded by neutral images (n= 72, r= −0.102, p= 0.393) or the difference in likability between those preceded by negative vs neutral images (n= 72, r= −0.090, P= 0.449). However, greater trait neuroticism did significantly predict less likability of novel faces (n= 112, r= −0.207, P= 0.028).
fMRI results: main effect of negative vs neutral images
For the Negative > Neutral contrast, we found bilateral amygdala activation that was significant with a family-wise error rate of 0.05 (Figure 2a). Location and extent of amygdala volumes are described in Table 1. AUC metrics in the reactivity and recovery periods in response to Negative images were calculated, as described in the methods. No significant differences were found in amygdala activity in the recovery period with respect to face condition, so trials were collapsed across face conditions in all subsequent analyses.
Table 1.
Amygdala regions active in Negative > Neutral
| Cluster | x | y | z | Extent (mm3) |
|---|---|---|---|---|
| Right amygdala | 26 | −6 | −12 | 1104 |
| Left amygdala | −28 | −10 | −12 | 1008 |
Correlations between amygdala activity and neuroticism
Amygdala response to negative images during the reactivity period did not predict Neuroticism score (n= 119, r= −0.044, P= 0.636). However, amygdala recovery from negative images, controlling for reactivity, significantly predicted Neuroticism, such that activity in the amygdala remains elevated longer, relative to baseline, in individuals who endorse greater neuroticism (n= 119, r= 0.204, P= 0.026, illustrated in Figure 3a). This correlation remains significant when controlling for age and gender (df= 115, partial r= 0.203, P= 0.028). To better understand these potential differences in the temporal dynamics of the amygdala in participants with different levels of trait Neuroticism, we display the average amygdala time course in participants with Neuroticism scores in the top and bottom quartiles of the sample in Figure 2c. These time course data do not represent a formal test, but serve to illustrate that more neurotic individuals exhibited more sustained amygdala activity than less neurotic individuals in response to Negative images. Supporting specificity of the association between neuroticism and recovery from negative information, the relationship between amygdala recovery from positive images and trait neuroticism is non-significant and is reported in Supplementary Figure S2.
Fig. 3.
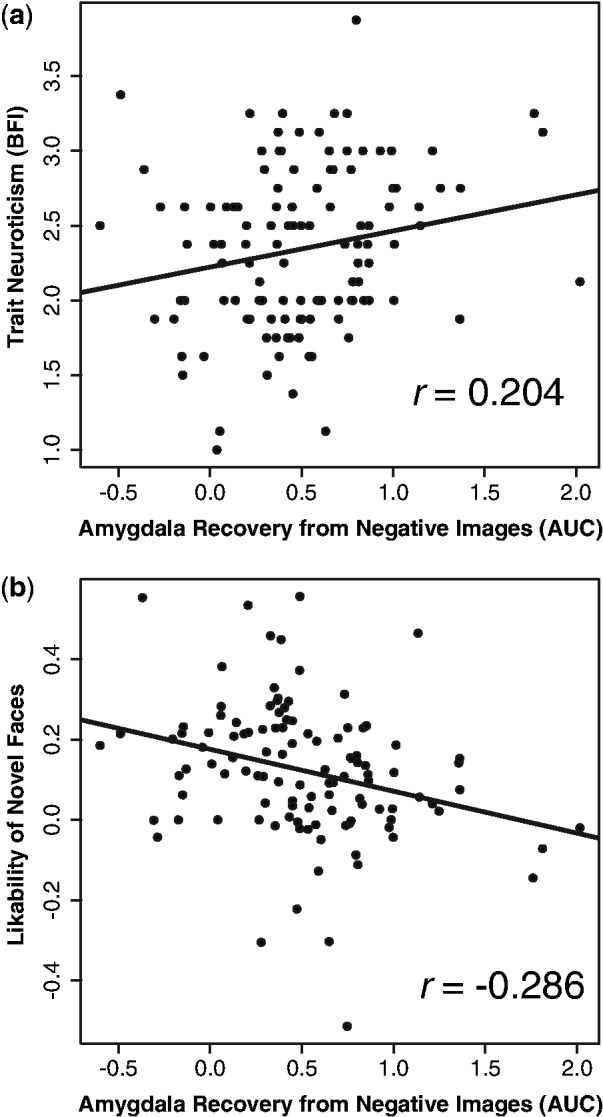
Amygdala recovery from negative images predicts emotional traits. (a) Slower amygdala return to baseline predicts greater trait neuroticism. (b) Slower amygdala return to baseline predicts lower likability ratings of novel faces.
Correlations between amygdala activity and face likability
To examine the relationship between amygdala activity and response to social stimuli, we computed the correlation between amygdala reactivity and recovery measures, and likability of novel and previously seen neutral faces. We found that amygdala response to negative images during the reactivity period did not predict likability ratings of novel faces (n= 111, r= 0.021, P= 0.828), nor did it predict likability of previously presented faces, either faces following neutral images (n= 71, r= 0.036, P= 0.762) or faces following negative images (n= 71, r= −0.079, P= 0.509). However, similar to the relationship with neuroticism, slower amygdala recovery from negative images predicted lower likability ratings of novel faces (n= 111, r= −0.286, P= 0.002, illustrated in Figure 3b). This relationship remained significant when controlling for age and gender (df= 108, partial r= −0.288, P= 0.002). Slower amygdala recovery from negative images also significantly predicted lower likability of faces following neutral images (n= 71, r= −0.240, P= 0.044) and faces following negative images (n= 71, r= −0.245, P= 0.040), though these relationships were no longer significant when controlling for age and gender for faces following neutral images (df= 71, r= −0.177, P= 0.145) and faces following negative images (df= 67, r= −0.213, P= 0.079). These relationships (and the relationship with likability of faces following positive images) are illustrated in Supplementary Figure S3a–c. The average amygdala time course in participants with the highest and lowest likability ratings of novel faces is illustrated in Figure 2d. Relationships between amygdala recovery from positive images and face likability ratings are reported in Supplementary Figure S2b and Supplementary Figure S3d–f.
DISCUSSION
The results reported here highlight the significance of considering the time course when studying trait measures and emotional response. We found that neither trait neuroticism nor evaluation of novel social stimuli were predicted by one’s initial reactivity to a negative challenge, but both were uniquely predicted by one’s ability to recover from that challenge after it ceased to be present. Greater amygdala activity during recovery, controlling for reactivity, predicted greater trait neuroticism and lower likability of an unrelated set of novel social stimuli. It is important to recall that the participants in this paradigm were not explicitly instructed to regulate their emotional response to images. Individuals may have varying abilities to voluntarily regulate emotion when explicitly instructed to do so, but in typical day-to-day living, individuals are not often required to volitionally alter their emotional response. For this reason, responses to emotionally laden stimuli in the absence of explicit regulation instructions may be more likely to reveal the neural processes that play a part in the formation of trait affect. These data could help to explain the neural mechanisms behind what has been called ‘affective inertia’, or difficulty altering a negative mood state once it has been established, associated with individuals with higher levels of trait neuroticism (Suls et al., 1998). These data are particularly interesting because they inform how trait affective measures can be related to a specific aspect of the emotional response. They imply that an individual can have either large or small initial responses to emotional stimuli, but that initial response does not predict that individual’s level of neuroticism; it is only informed by the emotional recovery. Emotion and emotion regulation are conceptualized differently based on the theoretical perspectives from which they are approached, from emotions as purely biological states to purely socially constructed mental events (Gross and Feldman Barrett, 2011) and the results reported here are but one example of how neuroscientific research can help to understand the mechanisms underlying individual differences in emotion regulation at the social and psychological levels.
This study features a large sample size, with individuals spanning a wide age range, affording us the power to detect smaller effects and draw conclusions in the population more broadly. Unlike most previous studies that explore relations between individual differences in a personality trait and BOLD signal, we used an amygdala ROI that was derived from the main effect contrast of negative vs neutral pictures, rather than conducting a voxel-wise regression with neuroticism. This enabled us to utilize an independently derived ROI for analyses on relations between amygdala reactivity and recovery and neuroticism and likeability ratings. There have been a number of studies showing differences in physiological and neural responses to negative stimuli when employing voluntary regulation strategies that intervene at early vs late stages in emotional processing (Jackson et al., 2000; Gross, 2001; Goldin et al., 2008; Urry, 2009; Walter et al., 2009; Sheppes and Gross, 2011; Thiruchselvam et al., 2011) but an individual’s regulatory behavior in the absence of explicit instruction more closely approximates what happens outside of the laboratory. Some work has been done showing that sustained activity of cortical midline regions in the absence of regulation instructions corresponds with greater reported emotional intensity following negative stimuli (Waugh et al., 2010). Also, the amygdala time course has been examined in depressed patients (Siegle et al., 2002) and spider-phobics (Larson et al., 2006), but to our knowledge, this is the first study to employ neuroimaging techniques to study individual differences in the temporal unfolding of the automatic amygdalar response and its relationship to non-clinical levels of trait affect. Previous studies showing a relationship between neuroticism and amygdala activity have differed in a few key ways from this work. In three of the studies, emotional stimuli were presented for a prolonged period of time, either 20 or 30 s blocks (Haas et al., 2007; Stein et al., 2007; Harenski et al., 2009), most likely capturing more than just initial amygdala reactivity. Hooker and colleagues (2008) examined amygdala activity during fear learning, while Brück and colleagues studied amygdala activity during processing of emotional prosody vs semantic meaning (2011), both of which paradigms are likely to elicit somewhat different amygdala dynamics than uninstructed emotion regulation. And Cunningham et al. (2011) found correlations specifically between amygdala activation and a volatility subscale of neuroticism.
In the future, it will be important to examine the specific circuitry that plays a causal role in regulating the speed of amygdala recovery. The prefrontal cortex and the amygdala are well known to have inhibitory structural connections (Ghashghaei and Barbas, 2002) as well as functional connectivity (Hariri et al., 2000; Banks et al., 2007; Wager et al., 2008) and so it will be important to study the relationship between these connections and amygdala recovery. It is also compelling to study whether training in mental regulatory strategies can result in faster amygdala recovery and ultimately, positive emotional outcomes (Davidson and McEwen, 2012). The most important conclusion we draw from this work is that it is not only the magnitude of the initial response to an emotional challenge that is important to affective style but also the subsequent recovery from that challenge. It is crucial to take these temporal considerations into account when piecing together the neural substrates of emotional response and well-being.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We would like to thank Michael Anderle, Ron Fisher, Lisa Angelos, Heather Hessenthaler, Trina Nelson, Amelia Cayo, Michael Kruepke, Jon Brumbaugh and Sarah Christens for assistance with data collection. We would also like to thank Diane E. Stodola, John Ollinger and Nate Vack for technical assistance.
This work was supported by the National Center for Complementary and Alternative Medicine (NCCAM) P01AT004952 to RJD, grants from the National Institute of Mental Health (NIMH) R01-MH43454, P50-MH084051 to RJD, grants from the Fetzer Institute and the John Templeton Foundation to RJD, a core grant to the Waisman Center from the National Institute of Child Health and Human Development [P30 HD003352-449015]. BSS was supported by a Neuroscience Training Program training grant [T32GM007507; Tom Yin PI] from the National Institute of General Medical Sciences.
REFERENCES
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2:303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brück C, Kreifelts B, Kaza E, Lotze M, Wildgruber D. Impact of personality on the cerebral processing of emotional prosody. Neuroimage. 2011;58:259–68. doi: 10.1016/j.neuroimage.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludağ K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. NeuroImage. 2004;23(Supplement 1):S220–33. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Ackerman BP, Izard CE. Emotions and emotion regulation in developmental psychopathology. Development and Psychopathology. 1995;7:1–10. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cremers HR, Demenescu LR, Aleman A, et al. Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. Neuroimage. 2010;49:963–70. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Arbuckle NL, Jahn A, Mowrer SM, Abduljalil AM. Reprint of: aspects of neuroticism and the amygdala: chronic tuning from motivational styles. Neuropsychologia. 2011;49:657–62. doi: 10.1016/j.neuropsychologia.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Well-being and affective style: neural substrates and biobehavioural correlates. Philosophical Transactions of the Royal Society B: Biological Sciences. 2004;359:1395–411. doi: 10.1098/rstb.2004.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nature Neuroscience. 2012;15:689–95. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engell AD, Haxby JV, Todorov A. Implicit trustworthiness decisions: automatic coding of face properties in the human amygdala. Journal of Cognitive Neuroscience. 2007;19:1508–19. doi: 10.1162/jocn.2007.19.9.1508. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–79. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation in adulthood: timing is everything. Current Directions in Psychological Science. 2001;10:214–19. [Google Scholar]
- Gross JJ, Feldman Barrett L. Emotion generation and emotion regulation: one or two depends on your point of view. Emotion Review. 2011;3:8–16. doi: 10.1177/1754073910380974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behavioral Neuroscience. 2007;121:249–56. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- Harenski CL, Kim SH, Hamann S. Neuroticism and psychopathy predict brain activation during moral and nonmoral emotion regulation. Cognitive, Affective, & Behavioral Neuroscience. 2009;9:1–15. doi: 10.3758/CABN.9.1.1. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Miyakawa A, Knight RT, D’Esposito M. The influence of personality on neural mechanisms of observational fear and reward learning. Neuropsychologia. 2008;46:2709–24. doi: 10.1016/j.neuropsychologia.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–22. [PubMed] [Google Scholar]
- John OP, Gross JJ. Healthy and unhealthy emotion regulation: personality processes, individual differences, and life span development. Journal of Personality. 2004;72:1301–33. doi: 10.1111/j.1467-6494.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- Kokkonen M, Pulkkinen L. Extraversion and Neuroticism as antecedents of motion regulation and dysregulation in adulthood. European Journal of Personality. 2001;15:407–24. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Gainesville: 2005. Tech.Rep. A-6, University of Florida. [Google Scholar]
- Larson CL, Schaefer HS, Siegle GJ, Jackson CAB, Anderle MJ, Davidson RJ. Fear is fast in phobic individuals: amygdala activation in response to fear-relevant stimuli. Biological Psychiatry. 2006;60:410–7. doi: 10.1016/j.biopsych.2006.03.079. [DOI] [PubMed] [Google Scholar]
- Mende-Siedlecki P, Said CP, Todorov A. Social Cognitive and Affective Neuroscience. 2012. The social evaluation of faces: a meta-analysis of functional neuroimaging studies. Available at: http://scan.oxfordjournals.org/content/early/2012/01/27/scan.nsr090 (accessed 13 June 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer K, Matas J, Kittler J, Luettin J, Maitre G. The Extended M2VTS Database, Proceedings 2nd Cenference on Audio and Video-base Biometric Personal Verification (AVBPA99) New York, NY: Springer Verlag; 1999. Available at: http://www.ee.surrey.ac.uk/Research/VSSP/xm2vtsdb (accessed April 6, 2012) [Google Scholar]
- Mroczek DK, Almeida DM. The effect of daily stress, personality, and age on daily negative affect. Journal of Personality. 2004;72:355–78. doi: 10.1111/j.0022-3506.2004.00265.x. [DOI] [PubMed] [Google Scholar]
- Pervin LA, John OP. Handbook of Personality: Theory and Research. 2nd edn. New York, NY: The Guilford Press; 1999. [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA. A neural mechanism of first impressions. Nature Neuroscience. 2009;12:508–14. doi: 10.1038/nn.2278. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Gross JJ. Is timing everything? Temporal considerations in emotion regulation. Personality and Social Psychology Review. 2011;15:319–31. doi: 10.1177/1088868310395778. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shakethat feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. American Journal of Psychiatry. 2007;164:318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Suls J, Green P, Hillis S. Emotional reactivity to everyday problems, affective inertia, and neuroticism. Personality and Social Psychology Bulletin. 1998;24:127–36. [Google Scholar]
- Suls J, Martin R. The daily life of the garden-variety neurotic: reactivity, stressor exposure, mood spillover, and maladaptive coping. Journal of Personality. 2005;73:1485–509. doi: 10.1111/j.1467-6494.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- Thiruchselvam R, Blechert J, Sheppes G, Rydstrom A, Gross JJ. The temporal dynamics of emotion regulation: an EEG study of distraction and reappraisal. Biological Psychology. 2011;87:84–92. doi: 10.1016/j.biopsycho.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Todorov A, Engell AD. The role of the amygdala in implicit evaluation of emotionally neutral faces. Social Cognitive and Affective Neuroscience. 2008;3:303–12. doi: 10.1093/scan/nsn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL. Using reappraisal to regulate unpleasant emotional episodes: goals and timing matter. Emotion. 2009;9:782–97. doi: 10.1037/a0017109. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, von Kalckreuth A, Schardt D, Stephan A, Goschke T, Erk S. The temporal dynamics of voluntary emotion regulation. PLoS One. 2009;4:e6726. doi: 10.1371/journal.pone.0006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Hamilton JP, Gotlib IH. The neural temporal dynamics of the intensity of emotional experience. Neuroimage. 2010;49:1699–707. doi: 10.1016/j.neuroimage.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



