Abstract
Many cardiovascular risk factors, associated with an increased incidence of Alzheimer’s disease, are characterized by reduced bioavailability of nitric oxide (NO). Recently, we demonstrated in endothelial nitric oxide synthase deficient (eNOS-/-) mice loss of endothelial NO led to increased protein levels of amyloid-β protein precursor (AβPP), β-site AβPP cleaving enzyme 1 (BACE1) and amyloid-β (Aβ) peptide. Therefore, our aim was to determine if NO supplementation in vivo would attenuate AβPP and BACE1 protein levels. AβPP and BACE1 protein levels were statistically lower in cerebral microvessels from nitroglycerin treated eNOS-/- mice as compared to vehicle treated. Nitroglycerin treatment significantly increased cGMP levels in the cerebral microvessels of nitroglycerin treated eNOS-/- animals compared to vehicle treated. Our findings support the concept that preservation of NO/cGMP signaling is an important modulator of expression and processing of AβPP.
Keywords: amyloid-beta protein precursor, amyloid-beta, vascular endothelial cells, nitric oxide, endothelial nitric oxide synthase, Alzheimer’s disease
Introduction
Endothelial nitric oxide (NO) is an important signaling molecule within the blood vessel wall. Loss of NO can lead to endothelial dysfunction, including impaired vasomotor function and increased oxidative stress[1]. Many cardiovascular risk factors are associated with a reduction in the bioavailability of NO[2]. Importantly, individuals with cardiovascular risk factors also have a higher incidence of developing Alzheimer’s disease[3, 4]. While the role of the cerebral vasculature as a contributing cause to developing AD was proposed in the early 1990’s[5], the link between Alzheimer’s disease (AD) and cardiovascular risk factors remains to be determined. Importantly, our previous studies suggest that one possible mechanism is the loss of endothelial NO[6].
In the cerebral circulation there is close proximity between the vascular endothelial cells and neurons and thus endothelial NO may be an important signaling molecule between these two cell types[7, 8]. Indeed, Gutsaeva and colleagues demonstrated that basal endothelial NO production contributes to the expression of mitochondrial proteins in cortical neurons[9]. Furthermore, we reported that endothelial nitric oxide synthase deficient (eNOS-/-) mice showed increased levels of amyloid-beta protein precursor (AβPP) and beta-site AβPP cleaving enzyme (BACE)1 in both cerebral microvasculature and brain tissue[6].
In the present study we hypothesized that supplementation of NO in vivo could suppress AβPP and BACE1 protein expression. We provide evidence that treatment with nitroglycerin suppressed AβPP and BACE1 protein levels in the cerebral microvessels of eNOS-/- mice.
Materials and Methods
Animals
Male wild type (C57BL6) and eNOS-/- (Nos3tm1Unc/J) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice had free access to food and water. Wild type and eNOS-/- mice, 4 months of age, were treated with 30 mg/kg nitroglycerin b.i.d. or vehicle, via subcutaneous injections, for 3 days. The nitroglycerin dose was chosen based on a previous study in eNOS-/- mice[10]. This dosing regimen is known to induce minimal tolerance to nitroglycerin[10]. Mice were sacrificed by lethal dose of pentobarbital. All animal care and use were approved by Mayo institutional Animal Care and Use Committee.
Tissue collection
Brains were carefully removed and immediately placed in ice cold modified Krebs-Ringer bicarbonate solution containing 118.6 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 25.1 mM NaHCO3, 0.026 mM EDTA, 10.1 mM glucose plus protease inhibitors. Large arteries, including the basilar and cerebral arteries, were removed prior to homogenization.
Cerebral microvessel isolation
Cerebral microvessels were isolated from brain tissue as previously described[6]. Briefly, brain tissue, devoid of large vessels, was homogenized in ice cold PBS with Dounce homogenizer. Microvessels were isolated by layering over 15% Dextran/PBS solution and filtering using a 40 μm filter[6].
Western blot
Brain or microvessel tissue homogenates were lysed in ice cold Triton lysis buffer (10 mM Hepes, 50 mM NaF, 50 mM NaCl, 5 mM EDTA, 5 mM EGTA, 100 μM Na3VO4, 50 mM Na pyrophosphate and 1% Triton X-100). Equal protein were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Blots were probed with AβPP (Invitrogen [Life Technologies]; Grand Island, NY), PS2, Neprilysin (Millipore; Billerica, MA), BACE1, nNOS, ADAM9 (Cell Signaling; Danvers, MA), ECE1, IDE (Abcam; Cambridge, MA), Aph1, PS1, Nicastrin (ProSci Incorporated; Poway, CA), ADAM10 (Chemicon [Millipore]; Billerica, MA), and Actin (Sigma-Aldrich; St. Louis, MO) (loading control) specific primary antibodies.
cGMP
Brain and microvessel tissue was collected as described above. Tissue cGMP levels were measured using a colorimetric immunoassay according to the acetylated protocol provided in the manufacturer’s instructions (Cell BioLabs, Inc; San Diego, CA).
Statistical analysis
Data are represented as mean ± SD. Statistical analysis was performed using two-way ANOVA with Tukey-Kramer post hoc comparison or the unpaired Student’s t-test.
Additional and more detailed methods information is provided in the online supplement which can be accessed at http://www.j-alz.com.
Results
Consistent with the literature eNOS-/- mice had a significantly elevated blood pressure as compared to wild type mice (Supplemental Table 1; n=6-7, P<0.001). Body weights of eNOS-/- mice were significantly lower than those of wild type mice (Supplemental Table 1; n=7-17, P<0.001). Circulating levels of cholesterol, HDL, triglycerides and glucose were not different between wild type and eNOS-/- mice and no difference was observed between vehicle and nitroglycerin treated animals in either wild type or eNOS-/- background (Supplemental Table 1). Consistent with our previous findings, AβPP and BACE1 protein levels were increased in the microvessels and brains of eNOS-/- mice as compared to wild type control (Figure 1)[6]. AβPP and BACE1 protein levels were significantly lower in the cerebral microvessels of eNOS-/- mice treated with nitroglycerin as compared to vehicle treated mice (Figure 1A-C, n=6-10, P<0.05). No differences were observed between treatment groups in the wild type mice (n=6). AβPP and BACE1 levels within the brains of mice treated with nitroglycerin did not differ from vehicle treated mice in either wild type or eNOS-/- mice (Figure 1D-F). Furthermore, Aβ levels were not significantly different in the brains of nitroglycerin treated animals as compared to vehicle treated animals (data not shown). Importantly, nitroglycerin treatment did not alter the expression levels of alpha or gamma secretase enzymes or Aβ degradation enzymes in the cerebral microvessels of eNOS-/- mice (data not shown).
Figure 1.
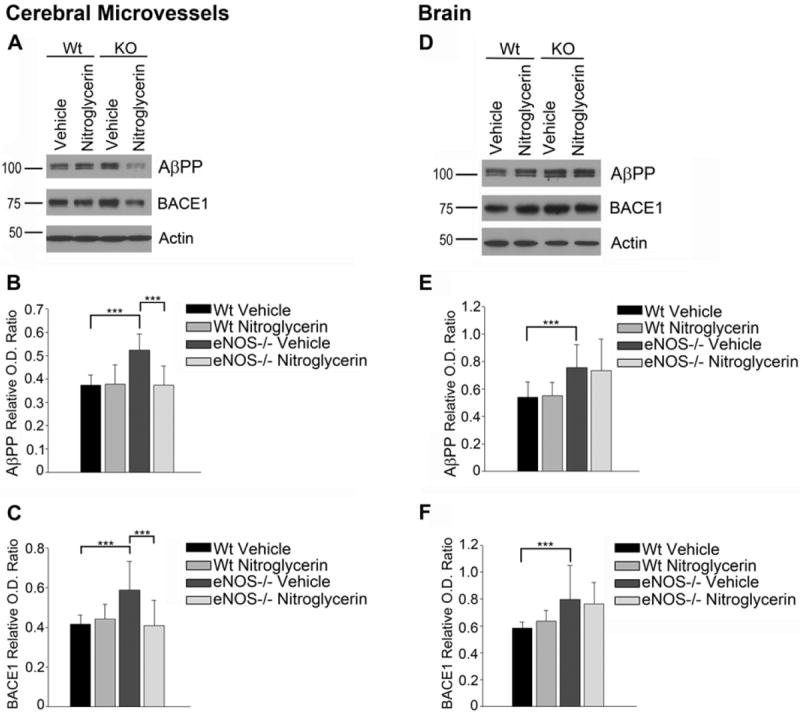
Cerebral microvessel and brain AβPP and BACE1 protein levels in wild type and eNOS-/- mice treated with nitroglycerine or vehicle. (A) Western blot and densitometric analysis of (B) AβPP and (C) BACE1 were performed in cerebral microvessel tissue. (n=6-8, P<0.001). (D) Western blot and densitometric analysis of (E) AβPP and (F) BACE1 were performed in brain tissue. (n=6-10, P<0.001).
To determine if nitroglycerin treatment resulted in an activation of soluble guanylyl cyclase (sGC), we measured levels of cGMP in both cerebral microvessel and whole brain tissue in wild type and eNOS-/- mice treated with vehicle or nitroglycerin. cGMP levels were significantly lower in the cerebral microvessels of eNOS-/- mice as compared to wild type mice (Figure 2A-B). Nitroglycerin treatment significantly increased the cGMP levels in the cerebral microvessels of eNOS-/- mice but not in wild type mice (Figure 2A-B). Nitroglycerin treatment was unable to increase cGMP in whole brain tissue in either wild type or eNOS-/- mice (Figure 2C-D)
Figure 2.
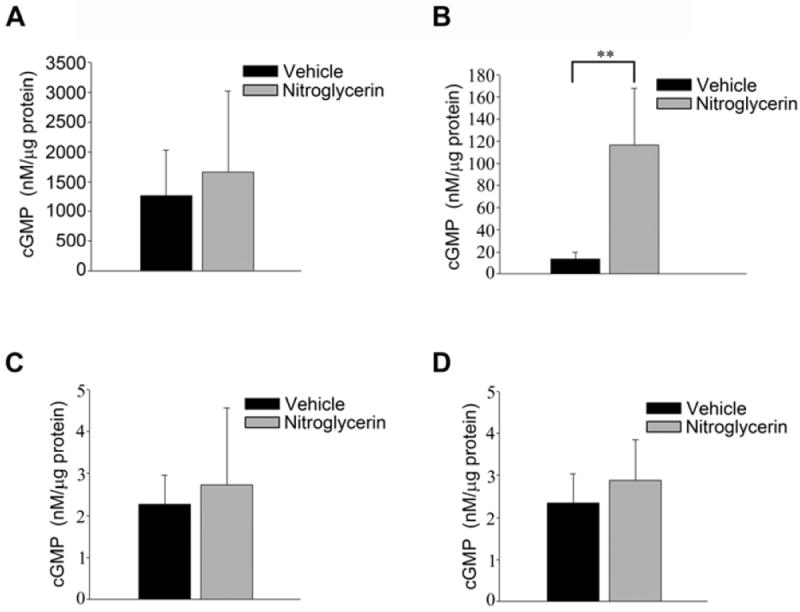
cGMP levels in the cerebral microvessels and brains of wild type and eNOS-/- mice treated with nitroglycerine or vehicle. cGMP was measured via immunoassay in the cerebral microvessel of (A) wild type and (B) eNOS-/- mice. cGMP measured in the brain tissue of (C) wild type and (D) eNOS-/- animals. Data is presented as mean ± SD (n=6-7, P<0.01).
Discussion
We recently reported that in a mouse model of eNOS deficiency, levels of AβPP and BACE1 were increased in brain and cerebral microvessel tissue[6]. In addition, we also observed increased Aβ formation in the brain tissue of eNOS-/- mice (but could not detect Aβ in the microvessels)[6]. This led us to determine if supplementation with NO was able to attenuate AβPP and BACE1 levels in the eNOS-/- model. We report here, that nitroglycerin treatment does indeed lower levels of AβPP and BACE1 in the cerebral microvessels of eNOS-/- mice. This effect was independent of alterations in metabolic parameters and blood pressure as nitroglycerin did not significantly change these peripheral parameters. Nitroglycerin treatment was unable to normalize AβPP and BACE1 levels in the brains of eNOS-/- mice. Consistent with these observations, cGMP levels were increased in the cerebral microvessels of the nitroglycerin treated eNOS-/- mice but were unaltered in the brains of these animals. We suspect that the local concentration of NO generated by nitroglycerin treatment was insufficient to activate brain sGC. The inability of nitroglycerin to increase cGMP levels in the brain appears to be the most likely explanation for unaltered brain levels of AβPP and BACE1.
The endothelial NO/cGMP pathway could be an important link between cardiovascular risks and AD. Recently, it has been demonstrated that treating cultured neurons with low levels of NO suppressed BACE1 expression and activity[11]. Furthermore, in a mouse model of AD, treatment with sildenafil, a phosphodiesterase 5 inhibitor, which increases cGMP levels, attenuated AD-related pathologies, including: Aβ load and synaptic function and memory loss[12]. Lastly, the protective role of exercise could be mediated by a shear-stress induced increase in the production of endothelial NO[13, 14]. While further investigation is required, modulation of the endothelial NO/cGMP pathway may provide novel targets for prevention of sporadic AD.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health grants HL-91867 and HL-111062, the Mayo Alzheimer’s Disease Research Center (Z.S.K.), AHA scientist development award 07-30133N (to L.V.d.), AHA postdoctoral fellowship 12POST8550003 (S.A.A.), Clinical Pharmacology Training Grant (T32 GM08685) (Trainee S.A.A.) and the Mayo Foundation.
References
- 1.Ignarro LJ. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990;16:477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- 2.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 3.Purnell C, Gao S, Callahan CM, Hendrie HC. Cardiovascular risk factors and incident Alzheimer disease: a systematic review of the literature. Alzheimer Dis Assoc Disord. 2009;23:1–10. doi: 10.1097/WAD.0b013e318187541c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Torre JC, Mussivand T. Can disturbed brain microcirculation cause Alzheimer’s disease? Neurol Res. 1993;15:146–153. doi: 10.1080/01616412.1993.11740127. [DOI] [PubMed] [Google Scholar]
- 6.Austin SA, Santhanam AV, Katusic ZS. Endothelial nitric oxide modulates expression and processing of amyloid precursor protein. Circ Res. 2010;107:1498–1502. doi: 10.1161/CIRCRESAHA.110.233080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garthwaite G, Bartus K, Malcolm D, Goodwin D, Kollb-Sielecka M, Dooldeniya C, Garthwaite J. Signaling from blood vessels to CNS axons through nitric oxide. J Neurosci. 2006;26:7730–7740. doi: 10.1523/JNEUROSCI.1528-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pawlik G, Rackl A, Bing RJ. Quantitative capillary topography and blood flow in the cerebral cortex of cats: an in vivo microscopic study. Brain Res. 1981;208:35–58. doi: 10.1016/0006-8993(81)90619-3. [DOI] [PubMed] [Google Scholar]
- 9.Gutsaeva DR, Carraway MS, Suliman HB, Demchenko IT, Shitara H, Yonekawa H, Piantadosi CA. Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J Neurosci. 2008;28:2015–2024. doi: 10.1523/JNEUROSCI.5654-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otto A, Fontaine J, Tschirhart E, Fontaine D, Berkenboom G. Rosuvastatin treatment protects against nitrate-induced oxidative stress in eNOS knockout mice: implication of the NAD(P)H oxidase pathway. Br J Pharmacol. 2006;148:544–552. doi: 10.1038/sj.bjp.0706738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwak YD, Wang R, Li JJ, Zhang YW, Xu H, Liao FF. Differential regulation of BACE1 expression by oxidative and nitrosative signals. Mol Neurodegener. 2011;6:17. doi: 10.1186/1750-1326-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puzzo D, Staniszewski A, Deng SX, Privitera L, Leznik E, Liu S, Zhang H, Feng Y, Palmeri A, Landry DW, Arancio O. Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer’s disease mouse model. J Neurosci. 2009;29:8075–8086. doi: 10.1523/JNEUROSCI.0864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y. Physical activity, diet, and risk of Alzheimer disease. Jama. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


