Abstract
Background
Reperfusion is associated with good functional outcome after stroke. However, minimal data are available regarding the effect of reperfusion on clinical outcome and infarct growth in patients with distal MCA branch occlusions.
Aim
The aim of this study was to evaluate this association and to determine the impact of the perfusion-diffusion mismatch.
Methods
Individual patient data from three stroke studies (EPITHET, DEFUSE and DEFUSE 2) with baseline MRI profiles and reperfusion status were pooled. Patients were included if they had a single cortical perfusion lesion on their baseline MRI that was consistent with a distal MCA branch occlusion. Good functional outcome was defined as a score of 0–2 on the modified Rankin Scale at day 90 and infarct growth was defined as change in lesion volume between the baseline DWI and the final T2/FLAIR.
Results
Thirty patients met inclusion criteria. Eighteen (60%) had a good functional outcome and twenty (67%) had reperfusion. Reperfusion was not associated with good functional outcome in the overall cohort (OR: 1.0, 95%CI 0.2–4.7) and also not in the subset of patients with a PWI-DWI mismatch (n=17; OR: 0.7, 95% CI 0.1–5.5). Median infarct growth was modest and not significantly different between patients with (0 ml) and without reperfusion (6 ml); p=0.2.
Conclusions
The overall high rate of good outcomes in patients with distal MCA perfusion lesions might obscure a potential benefit from reperfusion in this population. A larger pooled analysis evaluating the effect of reperfusion in patients with distal MCA branch occlusions is warranted as confirmation of our results could have implications for the design of future stroke trials.
Keywords: Reperfusion, Functional outcome, distal MCA, Perfusion imaging, Mismatch
Introduction
Reperfusion is associated with improved functional outcome after ischemic stroke.1 Our group and others have shown that patients with a perfusion-diffusion mismatch (small DWI lesions relative to PWI lesions) are more likely to benefit from reperfusion than patients without a mismatch.2–4 The site of the arterial occlusion is also a predictor of clinical outcome with distal lesions showing more favorable outcomes than proximal arterial occlusions.5 However, the relationship between reperfusion and good outcome in patients with more distal perfusion lesions has not yet been assessed. This association is of interest as it could affect the design of clinical trials that study the effect of reperfusion therapy outside the currently established time-window. We pooled data from three studies to determine the effect of reperfusion on clinical outcome and infarct growth in patients with single perfusion lesions distal to the first branch of the MCA (M1).
Methods
We combined individual patient data from the EPITHET (Echoplanar Imaging Thrombolysis Evaluation Trial), DEFUSE (Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution) and DEFUSE 2 datasets for this pooled analysis. The full methodology of the three studies has been described previously.2–4 In brief, EPITHET was a prospective randomized trial in which patients received alteplase or placebo 3 to 6 hours after stroke onset.3 DEFUSE was a prospective cohort study of patients treated with alteplase between 3 and 6 hours after symptom onset.2 DEFUSE 2 was a prospective cohort of ischemic stroke patients who underwent a baseline MRI scan before endovascular therapy.4 All studies required a baseline MRI with PWI to assess mismatch status and a follow-up MRI with perfusion imaging to assess reperfusion. Patients with single perfusion deficits, corresponding to vascular territories of MCA branches distal to the M1 segment, were included in this pooled analysis. Tmax maps were used to identify the PWI lesion volumes. PWI volumes were measured on Tmax>6s maps. Patients were excluded if they had 1) evidence of a DWI lesion outside of the perfusion deficit; 2) a lacunar pattern or a perfusion lesion without cortical involvement; or 3) an M1 or ICA occlusion on Magnetic Resonance Angiography (MRA). Target Mismatch was determined according to criteria from DEFUSE 2: A ratio between the volumes of critically hypoperfused tissue (Tmax >6s) and the ischemic core (ADC<600) of 1.8 or more, with an absolute difference of 15 ml or more; ischemic core volume of less than 70 ml; and less than 100 ml of tissue with a severe delay in bolus arrival (Tmax >10 s).
Reperfusion status was primarily assessed based on criteria reported in the original studies. In EPITHET this was defined as 90% or more reduction in the volume of PWI (Tmax ≥2 s) between baseline and days 3 to 5 3; in DEFUSE as 30% or greater volume reduction (Tmax ≥2 s) at 6 hours compared to baseline2; and in DEFUSE 2 as a more than 50% reduction in the volume of the PWI (Tmax >6 s) lesion between baseline and early follow-up at 12 hours or, when the early follow-up scan was unavailable, as TICI 2B perfusion at the completion of the endovascular procedure.4 A secondary analysis was performed, with reperfusion defined per DEFUSE 2 study criteria for all patients in the pooled analysis. Infarct growth was determined by subtracting the baseline DWI volumes from the final FLAIR or T2 volumes (at day 90 in EPITHET, day 30 in DEFUSE and day 5 in DEFUSE 2). Good functional outcome was defined as a Modified Rankin Scale (mRS) score of 0–2 and excellent outcome as a mRS score of 0–1 at day 90. Symptomatic intracranial hemorrhage was defined according to the definition in the Safe Implementation of Thrombolysis in Stroke Monitoring Study 2. 6 Approval for all three studies was obtained from local institutional review boards. Each patient or—if the patient was unable—a relative provided written informed consent. Statistical analyses were performed with SPSS 20.0.
Results
Thirty patients (19 from EPITHET, 6 from DEFUSE and 5 patients from DEFUSE 2) who had perfusion lesions consistent with single distal MCA branch occlusions were included (Figure 1A). MRA demonstrated the distal branch occlusion in six of these 30 patients (20%). Baseline characteristics are listed in Table 1; none of the patients had PWI lesions smaller than 5 ml. Seventeen patients (57%) had a target mismatch on baseline MRI and 20 patients (67%) had evidence of reperfusion on their follow-up MRI (Table 1). Baseline clinical and radiological characteristics were similar in patients with and without reperfusion. However the proportion of patients receiving tPA was greater in patients with reperfusion and the time from symptom onset to tPA administration was longer in patients with reperfusion (Table 1).
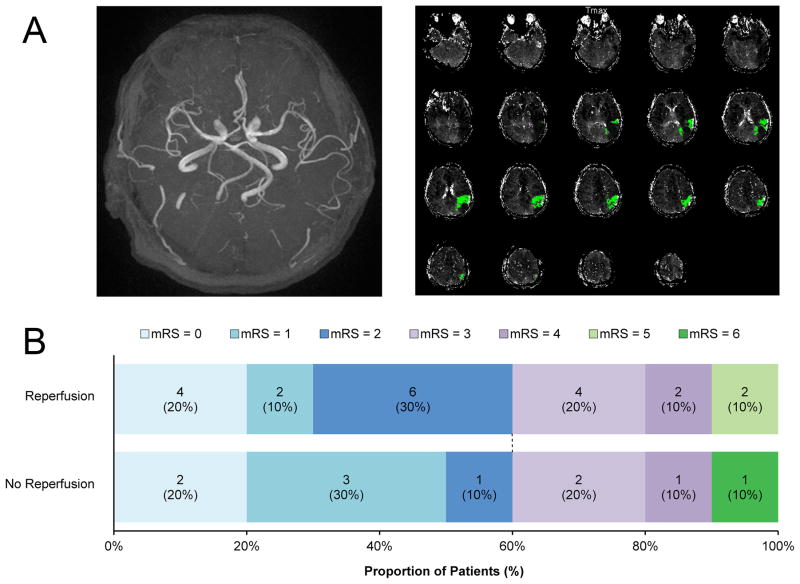
Figure 1. Example of imaging and functional outcome according to mRS at 90 days.
A An example of a patient included in the pooled analysis. MRA did not reveal vessel occlusion (left panel), but a single distal MCA perfusion lesion (right panel) was visualized on PWI (Tmax>6 sec).
B Good functional outcome (score 0–2) did not differ between patients in relation to reperfusion (p=1.0). Defining outcome more strictly as an mRS of 0–1 (excellent outcome) did not alter the results (30% of patients with reperfusion versus 50% of patients without reperfusion; p=0.4)
mRS= modified Rankin scale.
Table 1.
Baseline characteristics in patients with single distal MCA perfusion lesions with and without reperfusion
| All patients (n=30) | No reperfusion (n=10) | Reperfusion (n=20) | P-value* | |
|---|---|---|---|---|
| Mean age (years) | 75 (12) | 72 (13) | 76 (11) | 0.5 |
| Women | 14 (47%) | 5 (50%) | 9 (45%) | 1.0 |
| Atrial fibrillation | 10 (33%) | 3 (30%) | 7 (35%) | 1.0 |
| Diabetes mellitus | 8 (27%) | 1 (10%) | 7 (35%) | 0.2 |
| Hypertension | 18 (60%) | 5 (50%) | 13 (65%) | 0.5 |
| Median baseline NIHSS score | 9 (7 – 11) | 8 (6–11) | 9 (8–12) | 0.4 |
| Median Baseline DWI volume – ml | 15 (8 – 25) | 14 (6–18) | 17 (11–34) | 0.3 |
| Target Mismatch | 17 (57%) | 5 (50%) | 12 (60%) | 0.7 |
| Median baseline PWI volume – ml | 24 (13 – 38) | 21 (10–38) | 24 (15–38) | 0.8 |
| IV tPA | 18 (60%) | 3 (30%) | 15 (75%) | 0.02 |
| Median time to tPA treatment – min** | 295 (255 – 318) | 180 (143–216) | 309 (282–322) | 0.02 |
| Endovascular treatment | 5 (17%) | 3 (30%) | 2 (10%) | 0.4 |
| Time to MRI for final T2/FLAIR – days*** | 91 (27–95) | 93 (24–95) | 91 (28–93) | 0.9 |
Data are n (%), mean (SD) or median (IQR).
DWI: Diffusion-weighted Imaging; IV: Intravenous; NIHSS: National Institutes of Health Stroke Scale; PWI: Perfusion-weighted Imaging; tPA: Tissue Plasminogen Activator
P-values are given for the comparison between reperfusion versus no reperfusion
Median reported for n=18: Patients without (3) versus patients with reperfusion (15)
Median reported for n=26: Patients without (9) versus patients with reperfusion (17)
None of the patients had a symptomatic intracranial hemorrhage. The mortality rate at day 90 was 3% (Table 1). Sixty percent of the patients experienced a good functional outcome (Figure 1B). Reperfusion was not associated with functional outcome; the percentages of good functional outcome (mRS 0–2) were identical (60%) in patients with reperfusion and patients without reperfusion (OR: 1.0, 95% CI 0.2–4.7). Re-analyzing this association, using a uniform definition of reperfusion by DEFUSE 2 criteria in all patients, did not alter the results (OR for the association between reperfusion and good functional outcome: 0.3; 95% CI 0.03–3.3).
The findings were analogous in the small subset of patients in whom a vessel occlusion had been identified on MRA (OR: 1.0, 95% CI 0.1–96). Stratification according to target mismatch status on baseline imaging did also not alter the results. In target mismatch patients 6 of 12 patients with reperfusion had good functional outcome (50%) versus 3 of 5 patients without reperfusion (60%) (OR: 0.7, 95% CI 0.1–5.5). There was also no significant association between reperfusion and good functional outcome after excluding patients who were treated with endovascular therapy (the DEFUSE 2 cohort) from the pooled analysis (OR: 2.1, 95% CI 0.4–12.3). There was no association between reperfusion and excellent functional outcome (mRS 0–1; OR: 0.4, 95% CI 0.1–2.1). In the EPITHET cohort (n=19), tPA treatment was associated with reperfusion (four of ten placebo patients, 40%, experienced reperfusion compared to all nine, 100%, who were treated with tPA, p=0.01), but there was no association between reperfusion and good functional outcome (OR: 1.2, p=1.0) nor between tPA and good functional outcome (OR: 1.3, p=1.0).
Infarcts grew little between the baseline (DWI) and the late follow-up MRI, and final infarct volumes were small (Table 2). Median infarct growth was not significantly different between patients with reperfusion (0 ml) and patients without reperfusion (6 ml; p=0.2). Results were similar when using the uniform definition of reperfusion according to DEFUSE 2 criteria: Median infarct growth was 0 ml in patients with reperfusion versus 11 ml in patients without reperfusion (p=0.1).
Table 2.
Clinical and radiological outcomes (n=30)
| Reperfusion | 20 (67%) |
| Median final infarct volume – ml* | 14 (6 – 33) |
| Median lesion growth – ml | 1 (−4 – 11) |
| With reperfusion, n=17 – ml | 0 (−8 – 11) |
| Without reperfusion, n=9 – ml | 6 (4 – 11) |
| Mortality at day 90 | 1 (3%) |
| Symptomatic intracranial hemorrhage | 0 (0%) |
Data are n (%) or median (IQR)
Median reported for patients in whom final MRI was performed: n=26
Discussion
Patients with single distal MCA perfusion lesions often experienced reperfusion and often had a good functional outcome. However, reperfusion was not associated with good functional outcome in our study cohort. Several previous studies of intra-arterial therapy in patients with distal MCA occlusions have also reported high rates of recanalization and good functional outcomes, both exceeding 50%, but they did not report the relationship between these two variables.7–9
There are some limitations to our study. Firstly, the association between reperfusion and good functional outcome, if present, might be less marked in patients with single distal MCA perfusion lesions than in the overall cohort. Larger sample sizes would therefore be required to identify an association. To mitigate this limitation we included data from three studies, but the absolute number of patients remains modest and limits our power to demonstrate a potential effect of reperfusion. Secondly, as with any pooled analysis, combining data from multiple studies introduces variability due to differences in study design. However, the benefit of pooling data to increase the overall sample size outweighs, in our opinion, the downside of introducing variability. In this pooled analysis variability included the assessment of reperfusion at different time points and differences in the definition of reperfusion among the three studies. Late assessment of reperfusion in EPITHET may have weakened the association between reperfusion and outcome. This formed the rationale for the use of study-specific reperfusion criteria as they were used in the original studies, but this may not have fully accounted for the variability. To assess the impact of the variability in reperfusion definitions, we ran a secondary analysis with a uniform definition of reperfusion according to DEFUSE 2 study criteria (>50% reduction in PWI lesion volume). The results of this secondary analysis were comparable to the primary analysis which used study-specific reperfusion criteria.
Another area of variability involves the treatment regimens. Patients were treated with intravenous tPA in DEFUSE, intravenous tPA or placebo in EPITHET, and endovascular therapy in DEFUSE 2. The association between reperfusion and clinical outcome might differ based on treatment strategy. For example, reperfusion could occur later and may therefore be less effective in patients treated endovascularly. Excluding the DEFUSE 2 dataset from the analysis, however, did not alter our results. A third limitation is that high rates of good outcomes in patients without reperfusion, as documented here and by others7–9, might lead to a ceiling effect that is difficult to improve upon by reperfusion strategies. A stricter definition of favorable outcome (eg a mRS score of 0–1 at day 90) could potentially address this limitation, but our results remained unchanged when this stricter definition was applied. Finally, digital subtraction angiography is the gold standard for identification of distal MCA occlusions but this was not performed in DEFUSE or EPITHET. Instead we selected patients based on a combination of MRA data to exclude patients with M1 obstructions, and PWI data to identify patients with single distal MCA perfusion deficits. We chose this approach because MRA lacks sensitivity for diagnosing distal MCA lesions.10
Previous studies have clearly established that reperfusion is associated with good functional outcome.1 Our results do not contradict these studies, but we failed to demonstrate such an association in the subgroup of patients with single distal MCA perfusion lesion who were treated beyond three hours from symptom onset. In addition, final infarct volumes were small and lesion growth was modest irrespective of reperfusion status. These findings suggest that the association between reperfusion and good functional and radiological outcome is weaker in this subgroup than in the overall population of acute stroke patients, including those who are treated earlier and/or have larger PWI lesions. In the patients from EPITHET the expected association between tPA treatment and reperfusion existed in patients with distal MCA branch occlusions, yet an association between reperfusion and good functional outcome did not; suggesting that tPA induced reperfusion might lack efficacy in patients with distal MCA branch occlusions who are treated outside the current therapeutic time-window.
Considering the small sample size in this and other studies of patients with distal MCA lesions, further pooling of data from multiple studies is required to determine, with sufficient power, whether the response to reperfusion in patients with distal MCA obstructions indeed differs from the response in the overall population. Larger studies would also be needed to explore the relationship between clinical outcome and reperfusion according to the side and/or location of the distal MCA branch occlusion.
Our results do not imply that intravenous thrombolysis should be withheld in patients with distal MCA perfusion lesions who meet established criteria for this treatment. They should only be considered as potential evidence that the response to reperfusion might differ when the distal MCA is involved. If confirmed by a larger pooled analysis, these results could be of relevance when deciding on patient selection criteria for future stroke studies. Specifically, trials that do not exclude patients with single distal MCA lesions may require larger sample sizes since these patients might have a high rate of good outcome regardless of reperfusion.
Acknowledgments
The DEFUSE study was supported by the National Institutes of Health (NINDS, RO1 NS39325; K24 NS044848). The EPITHET study was supported by National Health and Medical Research Council, Australia; National Stroke Foundation, Australia; Heart Foundation of Australia. The DEFUSE 2 study was supported by the National Institutes of Health (NINDS, K23 NS051372; R01 NS03932505). RL is a senior clinical investigator of FWO Flanders.
Footnotes
Conflict of interest
BC has received speaker’s honoraria from Novartis and Boehringer Ingelheim and has consulted for Lundbeck. SD has served on advisory boards or given lectures for Boehringer Ingelheim, Pfizer and Sanofi Aventis. RB and GWA have equity interest in iSchemaView. GWA has served on advisory boards for Genentech and Lundbeck.
All other authors rep ort no conflict of interest.
References
- 1.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke; a journal of cerebral circulation. 2007;38(3):967–73. doi: 10.1161/01.STR.0000258112.14918.24. Epub 2007/02/03. [DOI] [PubMed] [Google Scholar]
- 2.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Annals of neurology. 2006;60(5):508–17. doi: 10.1002/ana.20976. Epub 2006/10/27. [DOI] [PubMed] [Google Scholar]
- 3.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet neurology. 2008;7(4):299–309. doi: 10.1016/S1474-4422(08)70044-9. Epub 2008/02/26. [DOI] [PubMed] [Google Scholar]
- 4.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet neurology. 2012;11(10):860–7. doi: 10.1016/S1474-4422(12)70203-X. Epub 2012/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saqqur M, Uchino K, Demchuk AM, Molina CA, Garami Z, Calleja S, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke; a journal of cerebral circulation. 2007;38(3):948–54. doi: 10.1161/01.STR.0000257304.21967.ba. Epub 2007/02/10. [DOI] [PubMed] [Google Scholar]
- 6.Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275–82. doi: 10.1016/S0140-6736(07)60149-4. Epub 2007/01/30. [DOI] [PubMed] [Google Scholar]
- 7.Rahme R, Abruzzo TA, Martin RH, Tomsick TA, Ringer AJ, Furlan AJ, et al. Is Intra-Arterial Thrombolysis Beneficial for M2 Occlusions? Subgroup Analysis of the PROACT-II Trial. Stroke; a journal of cerebral circulation. 2013;44(1):240–2. doi: 10.1161/STROKEAHA.112.671495. Epub 2012/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi ZS, Loh Y, Walker G, Duckwiler GR. Clinical outcomes in middle cerebral artery trunk occlusions versus secondary division occlusions after mechanical thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI trials. Stroke; a journal of cerebral circulation. 2010;41(5):953–60. doi: 10.1161/STROKEAHA.109.571943. Epub 2010/04/10. [DOI] [PubMed] [Google Scholar]
- 9.Tomsick T, Broderick J, Carrozella J, Khatri P, Hill M, Palesch Y, et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR American journal of neuroradiology. 2008;29(3):582–7. doi: 10.3174/ajnr.A0843. Epub 2008/03/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prabhakaran S, Romano JG. Current diagnosis and management of symptomatic intracranial atherosclerotic disease. Current opinion in neurology. 2012;25(1):18–26. doi: 10.1097/WCO.0b013e32834ec16b. Epub 2011/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]