Abstract
N -Formyl-methionyl peptides can specifically bind to surface receptors on phagocytic cells. A single copy of N-formyl-methionine-leucine-phenylalanine (fMLF) covalently linked to a poly(ethylene glycol)-based polymer displayed reduced binding avidity (Kd = 190 nM) for differentiated HL-60 cells relative to free fMLF (Kd = 28 nM). Increasing the number of fMLF residues (up to eight) attached to a single polymer results in enhanced avidity for these cells (Kd= 0.18 nM), which appears to be independent of whether the polymer backbone is linear or branched. However, no conjugate showed enhanced ability to activate phagocytic cells, relative to the free peptide (EC50= 5 nM), as measured by transient stimulation of release of calcium ions from intracellular stores into the cytoplasm. A polymer bearing four fMLF and four digoxigenin residues showed specific enhancement in binding to differentiated HL-60 cells and mouse peritoneal macrophages in situ relative to a polymer lacking fMLF; no such enhancement was seen in binding to receptor-negative lymphocytic Jurkat cells. These results suggest that multiple fMLF residues linked to a drug-delivery polymer can be used to target appended drugs to phagocytic cells with relatively little toxicity due to cellular activation.
INTRODUCTION
Therapeutic drugs are generally administered via oral or parenteral routes, so that they are widely distributed throughout the body, although only one organ or tissue might be affected by the disease being treated. By targeting a drug to the site of the disease, it should be possible to achieve a higher and more effective dose where needed and a lower and less toxic dose in unaffected tissues. The present study focuses on targeting drugs to macrophages and related phagocytic cells of the reticuloendothelial system, such as monocytes and dendritic cells. Macrophage targeting could be especially useful for therapy of AIDS and tuberculosis, since these cells represent a major reservoir for propagation of the causative virus and bacterium, respectively, as well as for the AIDS-associated opportunistic infection, leishmaniasis, caused by a tropical protozoan. Macrophage targeting has been studied before, examples being the use of nanoparticles for antileishmanial drugs (Venier-Julienne et al., 1995), glycoside-liposomes for antituberculosis drugs (Medda et al., 1995), and mannosylated replacement enzyme for Gaucher’s disease (Mistry et al., 1996). To achieve macrophage targeting for AIDS, reverse transcriptase inhibitors have been covalently attached to lipoproteins (Mankertz et al., 1996), noncovalently adsorbed into colloidal nanoparticles (Lobenberg and Kreuter, 1996), and incorporated into erythrocytes (Rossi et al., 1998). In this report, macrophage targeting is based on the chemotactic peptide, N-formyl-methionine-leucine-phenylalanine (fMLF),1 which is indicative of a bacterial infection since all bacterial (but not eukaryotic) proteins are synthesized with N-formyl-methionine as their N-terminal amino acid. Monocyte-derived dendritic cells (Randolph et al., 1998), which migrate to lymph nodes and which can be infected by both M-tropic and T-tropic strains of HIV-1, also bind and respond to fMLF (Benelli et al., 1998). The high affinity receptor for fMLF is found on phagocytes and some other cells, while sequence-related low affinity and orphan receptors are more widely distributed (reviewed by Prossnitz and Ye, 1997; Le et al., 2001). Expression of the fMLF receptor has been shown to be sufficient to enable normally receptor negative cells to specifically bind to fMLF (Prossnitz et al., 1993).
We have developed a novel method of targeting drug delivery to phagocytic cells based on a poly(ethylene glycol) (PEG) carrier possessing multiple sites for covalent attachment of targeting groups and drug moieties (Huang et al., 1998). Whereas the bioreversible disulfide bond had been proposed for reversibly appending the therapeutic agent to the carrier and delivering it to cellular targets (Huang et al., 1998), the present studies used the more stable amide bond for appending the reporter group, digoxigenin, as a surrogate drug substance. To achieve high affinity binding to fMLF receptors, we explored the concept of using multiple copies of this ligand on the carrier polymer. In addition to achieving strong and specific binding to the fMLF receptor, a clinically acceptable targeted drug–carrier conjugate should not activate macrophages, thereby avoiding undesired side effects. A previously proposed solution was to use a receptor antagonist as the targeting group. This could be accomplished by replacing the formyl group on the targeting peptide ligand with a branched carbamate structure, such as the tert-butyloxycarbonyl group (tBoc) commonly used as a blocking group in peptide synthesis (Derian et al., 1996). However, this substitution was found to weaken receptor binding 100-fold (Kd increased from 0.033 to 4.5 μM) (Derian et al., 1996). Based on studies using carrier polymers bearing different numbers of fMLF ligands, we now report that we have found a method by which macrophages can be targeted but not activated, by attaching multiple fMLF moieties to a single drug-carrying polymer.
EXPERIMENTAL PROCEDURES
Materials
N-Formyl-methionine-leucine-phenylalanine (fMLF) peptide was purchased from Sigma (St. Louis, MO). Radioactive [45Ca]calcium chloride (specific activity 16 mCi/mg) and fML[3H]F (40 Ci/mmol) were from DuPont-NEN Research Products (Boston, MA). The human cells used in this study (HL-60 and Jurkat T-cells) were purchased from the American Type Culture Collection (Manassas, VA). Branched amino-PEGs with three (10 kDa), four (10 kDa), or eight arms (20 kDa) and α, ω-diamino-PEG (3.4 kDa) were purchased from Shearwater Polymers (Huntsville, AL). Dichloromethane (DCM) and N,N-dimethylformamide (DMF) were purchased from Fisher Scientific (Pittsburgh, PA). Triflouroacetic acid (TFA), diiosopropylcarbodiimide (DIPC), and p-toluenethiosulfonic acid (PTSA) were obtained from Aldrich Chemical (Milwaukee, WI). N-Hydroxysuccinimide-digoxigenin (NHS-DIG) was obtained from Boehringer-Mannheim (Indianapolis, IN). Fluorescamine was a gift from S. Udenfriend. Hanks’s balanced salt solution (HBSS) and RPMI 1640 tissue culture medium were purchased from GIBCO (Grand Island, NY).
Quantitation of Primary Amino Groups
Fluorescamine (Udenfriend et al., 1972) was used to monitor conversion of primary amines to amides. A fluorescence signal is obtained only by reaction with primary amines and not with amides or with secondary or aromatic amines. To each well of the 96-well plates, 0.1 mL of sample was added. Then 0.05 mL of 0.2 M borate buffer (pH 9.3) was added to each well, and 0.05 mL of fluorescamine (0.5 mg/mL in acetonitrile) was added and mixed immediately. The fluorescence intensity was measured at an excitation wavelength of 390 nm and emission wavelength of 475 nm.
Synthesis of Linear PEG–Aspartate Copolymer
This carrier copolymer was prepared by copolymerization of tBoc-aspartic acid and α, ω-diamino-PEG (3400 Da), using a modification of previous methods (Nathan et al., 1993; Poiani et al., 1994). The tBoc group was removed by treatment with TFA, thereby providing primary amino groups (NH2) for attachment of fMLF. Copolymer size was determined by size exclusion chromatography using PEG–tyrosine molecular weight standards prepared by us. The molecular weight was estimated to be 29 kDa. The structure of the resulting copolymer (m ~ 8, n ~ 75) is
Conjugation of PEG–Aspartate Copolymer with fMLF and DIG
Linear PEG–aspartate (PEG/A) copolymer (about 10 mg) was reacted with 7-fold excess (relative to amino groups) of fMLF peptide, 3.5 molar excess of DIPC, and 0.5 equiv of PTSA and 4-(dimethylamino)-pyridine (4-DMAP) in DMF:DCM (1:1 v/v). The total volume of the reaction was adjusted to 1 mL. The reaction mixture was incubated at room temperature for 24 h with gentle stirring. Then the polymer was precipitated with 10 volumes of ice-cold ethyl ether and dissolved in 90% phosphate-buffered saline (PBS) pH = 7.4, 10% acetonitrile. The unreacted peptide was separated from polymer by ultrafiltration with Microcon 10 (molecular weight cutoff = 10 kDa Amicon/Millipore, Inc., Bedford, MA). The retentate was dried and dissolved in PBS. The extent of peptide conjugated to the polymer was estimated using the fluorescamine assay. Separately, 4 mg of PEG/A or 4 mg of PEG/A–fMLF was reacted with 1 mg of NHS-DIG, dissolved in DMSO, in 0.5 mL of PBS, adjusted with NaOH to pH 8.0, with stirring overnight. The products were precipitated with ether, dissolved in PBS, and separated from the unreacted reagent by ultrafiltration with Microcon 10. The products were PEG/A–fMLF4 and PEG/A–fMLF4–DIG4, and PEG/A–DIG8.
Conjugation of fMLF with Branched (B) PEG
Addition of fMLF to the eight-arm branched amino-PEGs was accomplished in a similar manner as above. To achieve a range of molar ratio of fMLF conjugated to the copolymer, three sets of reactions with 14-, 8-, and 2-fold excess fMLF were set up. Fluorescamine assay was used to determine the average extent of conjugation of fMLF on the polymer, which was 8 (100%), 5.5 (68%), and 1.1 (14%) fMLF residues per polymer, respectively, for each reaction. These products are denoted B8-PEG–fMLF8, B8-PEG–fMLF5.5, and B8-PEG–fMLF1.1, respectively. Similarly, a 14-fold molar excess of fMLF was used for reactions with three-arm and four-arm branched amino-PEGs to achieve100% coupling, yielding B3-PEG–fMLF3 and B4-PEG–fMLF4.
Competitive Receptor Binding Assay
HL-60 cells were propagated in RPMI 1640 supplemented with 2 mM L-glutamine and 10% (v/v) heat-inactivated fetal bovine serum and were subcultured every 3 days at a ratio of 1:4. Cells were grown at 37 °C in an atmosphere of 5% CO2. Dimethyl sulfoxide (DMSO, 1.3%) was added to the culture (3 × 105 cells/mL) 48 h before the binding assay to initiate cellular differentiation. Differentiated HL-60 cells (2 × 105) were washed with PBS, pH 7.4, and resuspended in 1.77 mM KH2PO4, 8.0 mM Na2HPO4, 0.117 M NaCl, 0.15 mM CaCl2, 0.5 mM MgCl, and 1% bovine serum albumin (binding buffer) with fML[3H]F (30,000 cpm) and unlabeled fMLF (0.1 nM–200 nM) or conjugate. The cells were incubated for 15 min at 37 °C with gentle shaking. They were collected by centrifugation at 3000g for 5 min at room temperature. The supernatant was removed, and cells were resuspended in 0.1 mL of binding buffer and carefully added to the very top of tubes (Denville Scientific, South Plainfield, NJ, catalog #C19002) that contained 0.3 mL of 10% sucrose. They were centrifuged for 5 min at 12000g. The cells settled to the bottom, and any unbound ligand remained in the middle of the tube. The tubes were placed in dry ice for 5 min, and then their bottoms were cut off to collect the cells. Cell-bound and free radioactivity were determined by liquid scintillation counting. The value of Kd for each curve was determined graphically using the Cricket Graph program.
Intracellular Calcium Assay
HL-60 cells (2 × 105 cells/mL) were grown in the presence of 1.3% DMSO for 5 days. For each time course experiment, 2 × 106 cells were resuspended in 5 mL of HBSS containing 2 mCi/mL of 45CaCl2 as the sole source of calcium. After 90 min incubation at 37 °C, cells were washed twice as above with cold HBSS. Vesicle-associated and cytoplasmic 45Ca were measured prior to and at 1, 2, 3, 4, 5, and 10 min after the addition of the fMLF or conjugated compounds. The EC50 for cellular activation is the concentration of each compound tested resulting in 50% maximal stimulation of calcium efflux by this assay. Cell pellets were treated with 0.5% Triton X-100/0.1 M NaOH and centrifuged in tubes containing 0.3 mL of 10% sucrose, and radioactivity associated with the pellets was measured; this represents vesicle-associated 45Ca. The top portion of the tube, which contained the cytoplasmic 45Ca was also measured. The peak level of transient increase in cytoplasmic calcium and decrease in vesicle-associated calcium represents efflux of calcium from intracellular storage compartments that is stimulated by fMLF interaction with membrane receptors (Montero et al., 1994; Klinker et al., 1996; Azuma et al., 1996; Anderson and Mohamed Goolam, 1997).
Uptake by HL-60, Jurkat and Peritoneal Cells
Human Jurkat T-cell growth conditions were the same as for HL-60 cells, except that the subcultivation ratio was 1:3. Jurkat cells (2 × 105) or fully differentiated HL-60 cells were washed twice with PBS and incubated in binding buffer (see above) with conjugate for 30 min at 37 °C. Each conjugate (1 μg, dissolved at a concentration of 33 nM) was used in these experiments. Cells were lysed, the membranes and cell debris were removed by centrifugation (5 min at 14000g), and the enzyme-linked immunosorbant assay (ELISA) for DIG was performed on the supernatant as described below. To study primary peritoneal cells, BALB/c mice were injected in the peritoneal cavity with 5 μg of PEG/A–DIG4-fMLF4 (n = 3 mice), or PEG/A–DIG8 (n=4 mice). After 1 h, the cavity was flushed with medium, and cells were collected and washed twice with cold PBS, as described above.
Cells were resuspended in 100 μL of PBS, containing 0.6% Tween 20, vortexed briefly, and kept on ice for 30 min. Microscopic examination confirmed complete cell lysis. Uptake of DIG was determined by ELISA of the supernatant. Anti-DIG-Fab fragment and anti-DIG-horseradish peroxidase (POD) were purchased from Boehringer Mannheim. Anti-DIG-Fab (1 mg/mL) was diluted 1:100 and anti-DIG–POD (150 U/mL) was diluted 1:10000. ELISA was performed according to the manufacturer’s procedure. DIG content was proportional to the difference in absorbance at 495 and 450 nm. A standard curve for PEG/A–DIG8 and PEG/A–fMLF4–DIG4 was included in each assay. To account for the potential different amounts of peritoneal cells from each mouse, the results were normalized to the amount of protein, as measured with the Bradford assay (BioRad, Hercules, CA).
For all cell uptake experiments, statistical significance of the differences between experimental groups was evaluated using the two-sample t-test for independent samples with unequal variance.
RESULTS
Competitive Binding of fMLF Conjugates to Differentiated HL-60 Cells
To determine the effect of different numbers of targeting groups on polymer avidity for differentiated HL-60 cells, conjugates were tested for their binding activity, measured by their ability to compete with fML[H3]F for cell binding. The HL-60 cells used for these experiments were differentiated for 48 h with DMSO. HL-60 cells treated with DMSO for 5 days yielded similar results (data not shown). However, HL-60 cells that were not subjected to a differentiation-inducing regimen did not bind to fMLF (data not shown). HL-60 cells, which are derived from human promyelocytic leukemia cells, have the capacity to respond to many stimuli to differentiate into many cell types of the monocytic–granulocytic class. The categorization of the differentiated states by the nomenclature for normal cell types is somewhat arbitrary, since the cells tend to have properties that overlap these categories. Although DMSO is considered to induce cells to differentiate along the granulocytic pathway, the final phenotype of these cells is not identical to normal granulocytes (reviewed by Collins, 1987). The functional properties of HL-60 differentiated cells are similar (although not identical) to neutrophils (Dahlgren et al., 1987). They are able to undergo azurophilic granule release and to generate O2–(Seifert et al., 1992). Expression of the receptor for fMLF is one of the early events of differentiation of HL-60 cells and its presence is the only requirement for binding of fMLF to these cells (Prossnitz et al.,. 1993). In this work these differentiated HL-60 cells are used as a model cell system; these cells have some properties of both macrophages and neutrophils, but are not identical with either cell type.
Figures 1 and 2 show the result of competitive binding studies. The conjugates tested included PEG/A–fMLF4, PEG/A–fMLF4–DIG4, PEG/A–DIG8, B8-PEG–fMLF1.1,5.5,8, B4-PEG–fMLF4, and B3-PEG–fMLF3. The amount of fML[H3]F was kept constant while the concentration of each conjugate was varied. The binding curves (Figures 1 and 2) show that the specific binding is about 75% of the total binding, since about 25% of maximum ligand binding was nonspecific in that it was not subject to competition at any concentration of any competitor. The binding constant (Kd) estimated here for fMLF is 28 nM, which is close to that (33 nM) determined by Derian et al. (1996). As can be seen in Figure 1 and Table 1, the increased Kd of B8-PEG–fMLF1.1 (190 nM) relative to the free fMLF peptide (28 nM) indicates that attachment to the polymer reduces the avidity of the peptide for binding to cellular receptors. However, polymers with multiple fMLF moieties attached showed increased avidity for differentiated HL-60 cells, with B8-PEG–fMLF8 showing the lowest Kd (0.18 nM) and the greatest avidity relative to the number of fMLF peptides [Kd × (no. of fMLF) = 0.18 nM × 8= 1.44 nM].
Figure 1.
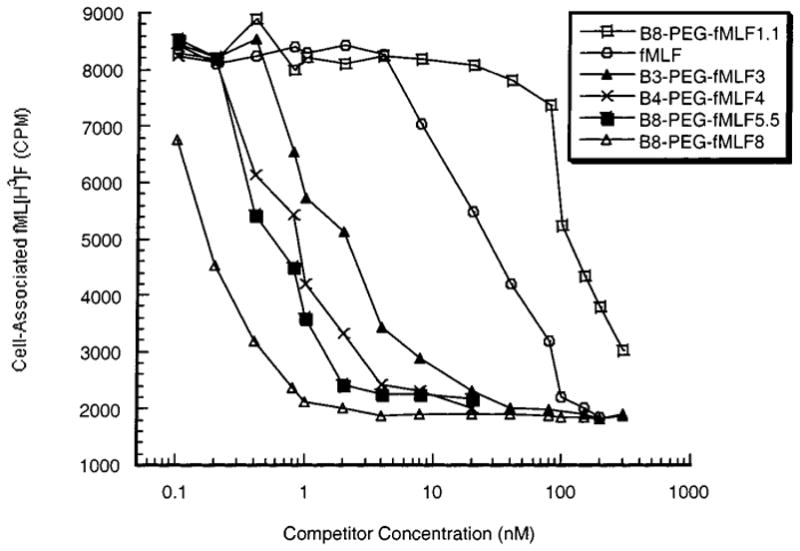
Competition of free and branched polymer-conjugated fMLF for binding to differentiated HL-60 cells. Varying concentrations of the indicated compounds were tested for their ability to compete with fML[3H]F for binding to DMSO-differentiated HL-60 cells, as described in Experimental Procedures. These results are the means of three independent assays; for each data point, the maximal deviation from the mean among these assays was 3%.
Figure 2.
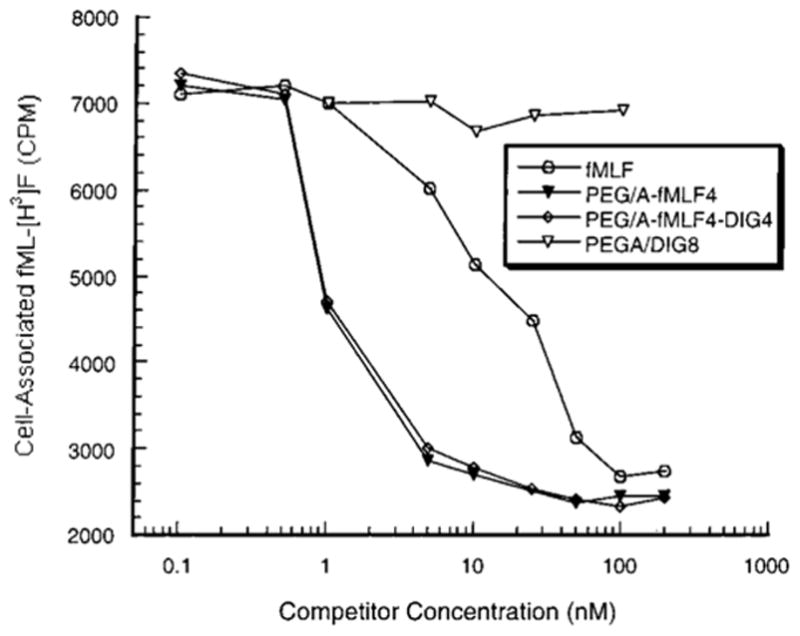
Competition of free and linear polymer-conjugated fMLF for binding to differentiated HL-60 cells. The experiment was performed as in Figure 1. The results shown are the means of three independent assays; the maximal deviation from the mean among these assays was 8%.
Table 1.
Binding and Cell Activation by Conjugates
| competitor | avidity: Kd (nM) | cell activation: EC50 (nM) | relative avidity: Kd × no. of fMLF | avidity/activation: EC50/Kd |
|---|---|---|---|---|
| fMLF | 28 | 4.7 | 28 | 0.168 |
| B8-PEG–fMLF1.1 | 190 | – | 209 | – |
| B3-PEG–fMLF3 | 1.9 | 9.6 | 5.7 | 5.05 |
| B4-PEG–fMLF4 | 0.8 | 5.0 | 3.2 | 6.25 |
| B8-PEG–fMLF5.5 | 0.5 | 5.3 | 2.75 | 10.6 |
| B8-PEG–fMLF8 | 0.18 | 5.0 | 1.44 | 27.8 |
| PEG/A–fMLF4 | 1.1 | ND | 4.4 | – |
| PEG/A–fMLF4–DIG4 | 1.2 | 5.9 | 4.8 | 4.92 |
The size or shape of the polymer does not seem to play a major role in avidity of binding, since the linear polymer with four copies of fMLF showed properties similar to its branched counterpart, both displaying a Kd of about 1 nM (Table 1). Furthermore, whether the other four potential attachment sites on the linear PEG/A were either unoccupied or were occupied with DIG made little difference in the Kd (Figure 2, Table 1). Thus, additional moieties such as therapeutic drugs could be added to the targeting polymer without compromising the binding avidity.
Cellular Activation of HL-60 Cells
Upon binding of the chemotactic peptide to the G-protein coupled receptor, one of the early events related to cellular activation is the release of Ca2+ from membrane-bound intracellular compartments into the cytoplasm (Azuma et al., 1996; Anderson and Mahomed Goolam, 1997; Prossnitz et al., 1999). This assay for cellular activation was used because it is the earliest measurable event after fMLF binding along the complex activation pathway. The release of calcium is rapid, reaching its maximum value in 2–3 min followed by slow recovery to near basal level (Figure 3). Similar kinetics of calcium release were observed in the response of differentiated HL-60 cells to free fMLF and to polymers bearing multiple fMLF moieties (Figure 3 and data not shown). As was the case for binding to these cells, B8-PEG–fMLF1.1 showed reduced ability relative to free fMLF to activate differentiated HL-60 cells, with virtually no flux of calcium even at 100 nM polymer. Activation also resembled binding in being enhanced by the presence of multiple fMLF moieties on a polymer, so that three fMLF residues on a polymer yielded readily detectable activation and four fMLF residues on a polymer were about as potent as free fMLF by this assay (Figure 4 and Table 1). However, unlike the binding assay, the Ca2+ efflux into the cytoplasm did not further increase as the copy number of fMLF per polymer increased beyond 4 (Figure 4 and Table 1). All of the conjugates with the copy number of fMLF ≥ 4 showed similar half-maximal release of intracellular calcium (EC50, as determined graphically using the Cricket Graph program) of about 5 nM of conjugate, which is approximately the same as the EC50 value (4.7 nM) for the free peptide (Table 1). Thus, the presence of multiple ligands (>3) on PEG does not increase the activation potency as much as it increases binding avidity for differentiated HL-60 cells.
Figure 3.
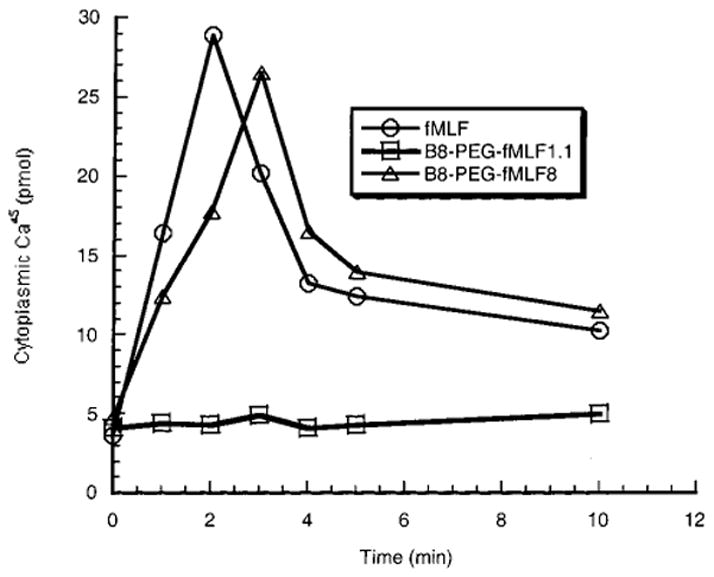
Time course of calcium release from intracellular stores in differentiated HL-60 cells. Release of 45Ca into the cytoplasmic compartment of differentiated HL-60 cells was measured at various times after addition of fMLF or the conjugated polymers. Similar time courses were observed when the added compounds were at concentrations between 1 and 100 nM.
Figure 4.
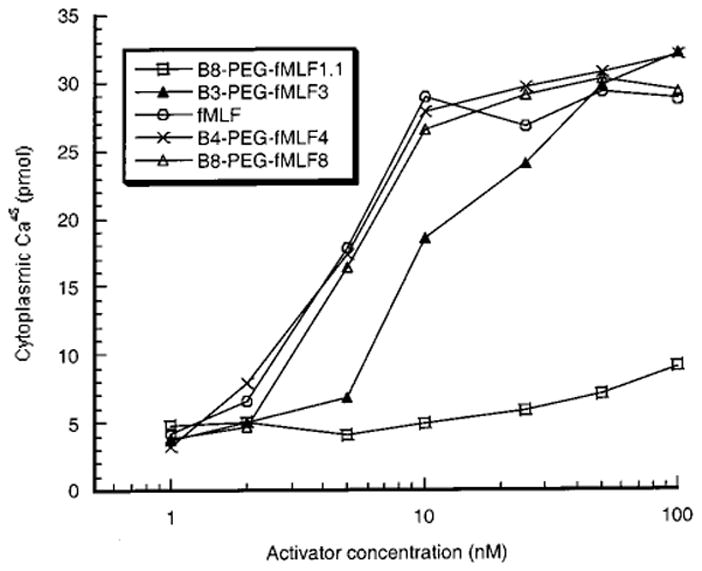
Calcium mobilization in differentiated HL-60 cells by fMLF and polymer-conjugated fMLF. The peak value of calcium release after addition of the indicated fMLF derivatives at various concentrations was measured as shown in Figure 3. Each data point is the mean of at least two independent experiments; for all data points the variation between repeat measurements was less than 8.2%.
Comparison of cellular binding and activation by various conjugates (Table 1) reveals that while no conjugate is more potent in activating differentiated HL-60 cells than is free fMLF, the avidity of conjugate for these cells increases with increasing number of fMLF moieties. This increase is most remarkable when expressed as Kd for the conjugate, but is still evident when a relative avidity is calculated per peptide, by multiplying the Kd of the polymer by the average number of fMLF moieties that it carries. The ratio of avidity (1/Kd) to cellular activation (“toxicity”) activity (1/EC50), measured by EC50/Kd, is 0.168 for free fMLF and increases from 5.05 to 27.8 for conjugates as number of fMLF moieties increases from 3 tof 8. Although the effect of molecular shape was not systematically studied, note that both avidity and cellular activation activity were nearly the same for branched and linear polymers harboring four copies of fMLF.
Targeting Drugs to Cells
To directly show the accumulation of PEG/A–drug–ligand conjugates, PEG/A–fMLF4–DIG4 (333 nM) was incubated with differentiated HL-60 cells. The DIG ELISA demonstrated that 18% of this conjugate had been internalized by these cells (Figure 5). When the negative control conjugate, PEG/A–DIG8, was incubated with HL-60 cells, 4.7% uptake was observed. This much lower accumulation (P < 0.006) in the absence of fMLF confirmed the importance of this ligand in the interaction of conjugates with differentiated HL-60 cells. Lymphocytic Jurkat cells, which do not express the fMLF receptor, accumulated both PEG/A–DIG8 (1.5%) and PEG/A–fMLF4–DIG4 (1.3%) to a much lesser extent than did differentiated HL-60 cells (Figure 5). The difference between uptake of the nontargeted conjugate by differentiated HL-60 cells and Jurkat cells was significant (P < 0.003), as was the difference between these two cell types for uptake of the targeted conjugate (P < 0.004). Note that for the Jurkat cells, there was no significant difference between the uptake of the two conjugates (P = 0.80). These values represent nonspecific cellular binding of polymers. These results indicate the specificity of binding of PEG/A–fMLF4–DIG4 to differentiated HL-60 cells. The 4.7% accumulation of PEG/A–DIG8 by differentiated HL-60 cells might reflect the tendency of these cells to bind and phagocytose polymeric PEG/A molecules nonspecifically.
Figure 5.
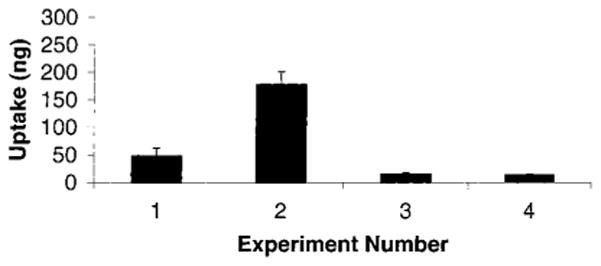
Uptake of PEG/A–fMLF4–DIG4 and PEG/A–DIG8 by phagocytic cells. Uptake of the indicated conjugate by cells was measured by ELISA for DIG, as described in Experimental Procedures. Uptake of PEG/A–fMLF4–DIG4 (experiments 2 and 4) and PEG/A–DIG8 (experiments 1 and 3) to DMSO-differentiated HL-60 cells (experiments 1 and 2) and Jurkat T-cells (experiments 3 and 4) were measured (n = 3). The total conjugate added to each culture was 1 μg.
Injection of PEG/A–fMLF4–DIG4 into the peritoneal cavity of BALB/c mice was used to determine the in vivo properties of this conjugate, in contrast to using the differentiated HL-60 cell line. The mice in this study were not injected with thioglycol, and, therefore, the macrophages in the peritoneal cavity were not activated. These nonactivated peritoneal cells accumulated DIG to about a 5-fold greater extent (P < 0.0003) from the targeted conjugate, PEG/A–fMLF4–DIG4, than from the nontargeted conjugate, PEG/A–DIG8 (Figure 6). In fact, 17% of the administered PEG/A–fMLF4–DIG4 was taken up by peritoneal cells, whereas they accumulated only 3.3% of the nontargeted PEG/A–DIG8. This low level of accumulation of nontargeted DIG is not surprising, since we have shown that in culture there is some nonspecific binding of PEG/A to promyelocytic HL-60 cells (Figure 5). Peritoneal incubation for less than 1 h (15 or 30 min) gave similar results (data not shown). It should be noted that the DIG that was accumulated in cells is expected to remain conjugated to its PEG/A carrier, but this was not confirmed.
Figure 6.
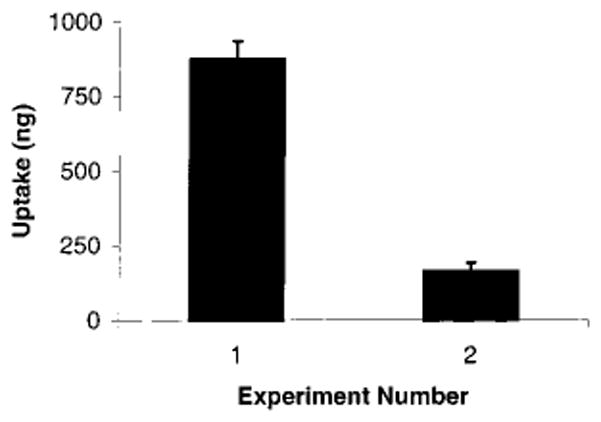
Specific targeting of peritoneal macrophages by fMLF. Each BALB/C mouse was injected intraperitoneally with 5 μg of PEG/A–fMLF4–DIG4 (experiment 1, n = 3) or PEG/A–DIG8 (experiment 2, n = 4). After 1 h, the peritoneal cavity was flushed, and cells were collected and lysed as described in Experimental Procedures. ELISA was performed to determine intracellular digoxigenin; a standard curve for digoxigenin was included on every assay plate. The results are normalized to the amount of protein in each lysate, to correct for variations in cell number and efficiency of lysis.
DISCUSSION
In this study, we have demonstrated the ability of multiple fMLF moieties to target a polymeric drug delivery molecule (Huang et al., 1998) to phagocytic cells. Such phagocytic cells populate the reticuloendothelial system and play a key role in host defense against bacteria (Seifert and Schultz, 1991). Macrophages recognize bacteria through a specific receptor for peptides containing N-formyl-methionine, which is the initiating amino acid in prokaryote protein synthesis. The high affinity formyl methionine receptor (referred to here as the fMLF receptor) is a member of the G-protein coupled receptor family (Gierschik et al., 1991; Prossnitz and Ye, 1997; Le et al., 2001). The binding of fMLF to this receptor initiates a series of cascades, resulting in chemotaxis (Martinet et al., 1994) and cellular activation (Montero et al., 1994). Since this receptor is present on phagocytic cells, such as macrophages and neutrophils (Niedel et al., 1980), it could serve as a targeting ligand for delivering drugs to these cells. Although binding of fMLF initiates the activation process, activation is not required for cellular uptake of fMLF (Prossnitz et al., 1999).
Our approach to drug delivery is based on the use a PEG-based carrier with multiple attachment sites to which drugs or targeting moieties can be linked. By appending drugs via the bioreversible disulfide bond, which can be designed to varying degrees of susceptibility to cleavage by reducing agents (Huang et al., 1998), the circulating time of the carrier can be adjusted, allowing it to reach the targeted cells, before releasing its drug cargo. The multiple attachment sites on the PEG-based carrier can not only provide a high payload of drug, but also can be used for appending one or more moieties for targeting specific cellular receptors or promoting cell uptake of the conjugate. In this study, the enhancement in avidity for the targeted receptor on phagocytic cells by multivalent binding of the fMLF ligand is demonstrated. Thus, a concentration of only 0.18 nM B8–PEG–fMLF8 is needed to get 50% occupancy of the differentiated HL-60 cell surface fMLF receptors, as opposed to 190 nM for B8–PEG–fMLF1.1, with only one appended ligand, an increase in avidity of more than 1000-fold (Table 1).
The molecular basis of enhanced avidity by multivalency of ligand attachment to a single polymeric carrier molecule is not obvious. It is possible that such multimeric ligands show their enhanced binding by interacting with multiple cellular receptors. Since a single fMLF moiety bound to a branched PEG polymer shows less avidity for differentiated HL-60 cells than does the free peptide, it seems likely that the bulky carrier group sterically interferes with ligand interaction with its cellular receptor, but this interference is overcome by peptide multivalency on the polymer. This behavior of fMLF conjugated to PEG-based polymers resembles that described for α-sialoside groups, the ligands for the hemagglutinin protein of influenza virus. When a single α-sialoside is linked to a polyacrylamide polymer, the conjugate binds less avidly to the hemagglutinin receptor on the virus than does a low molecular weight form of the ligand (Lees et al., 1994). However polyacrylamides bearing many copies of the ligand show vastly greater binding avidity than the free ligand (Lees et al., 1994; Sigal et al., 1996).
After binding of the free fMLF chemotactic peptide to a phagocytic cell, a rapid receptor-mediated internalization takes place (Niedel et al., 1979). Our results suggest that polymers with multiple fMLF moieties are capable of binding to and/or entering cells in culture and in vivo. In the present study we have not fully defined the possible mechanism of internalization and have only used the model indicator compound digoxigenin stably linked by an amide bond to the carrier polymer rather than an actual therapeutic agent. We are currently studying the subcellular localization of delivered compounds and determining the kinetics of release of a disulfide-linked model drug from the targeted conjugate upon its uptake by phagocytic cells.
A potential cause of adverse effects of targeting fMLF receptors on phagocytes is cellular activation caused by this interaction. Cellular activation is mediated by a complex chain of signal transduction events (reviewed by Prossnitz and Ye, 1997) and can be monitored by the mobilization of calcium from intracellular stores (Camps et al., 1992). This transient increase in intracellular free calcium correlates with acidification and stimulation of superoxide (O2−) release. The level of free fMLF required for half-maximal effect on Ca2+ release has been reported to be 2–4 nM (Wenzel-Seifert and Seifert, 1990), in approximate agreement with our determination of 5 nM, while the EC50 for superoxide production has been reported to be 15–30 nM fMLF (Klinker et al., 1996). In this study all conjugates with four or more copies of fMLF and free fMLF displayed an EC50 value of about 5 nM for calcium release (Table 1 and Figure 4). Therefore, the cooperative effect that was observed in binding is less pronounced for cellular activation. This difference indicates that polymers targeted to phagocytic cells with multiple fMLF moieties should be much more potent as targeting agents than as cellular activators. At a concentration of 1 nM of B8–PEG–fMLF8, nearly 100% of the fMLF receptors were occupied by the targeted drug carrier, whereas little or no cellular activation occurred.
PEG-based copolymers have been found to be biocompatible and are currently being developed for multiple in vivo applications (Poiani et al., 1994). These include the use of biotin targeting moieties to promote sodium-dependent multivitamin transporter (SMVT) mediated transport of molecules across the intestinal epithelium (Ramanathan et al., 2001). In the present study, the high molecular weight (>20 KDa) and inertness of PEG-based conjugate would be expected to confer on appended drugs an extended in vivo half-life and protection from degradative processes and immune system surveillance (Davis et al., 1981). Therefore, we propose that a PEG-based carrier containing multiple copies of fMLF can be used to target drugs to macrophages and other phagocytic cells without the adverse effects of activation of these cells. Furthermore, the lack of enhancement of cellular activation by polymers carrying multiple targeting fMLF groups might indicate that drugs appended to such polymers would be less likely to be inactivated by the many degradative mechanisms induced by phagocyte activation.
Acknowledgments
This study was funded by grants from the Campbell Foundation (M.J.L.) and Award R01 AI45555-01 from the NIH (M.M.C.). S.P. was supported by IMSD Award R25 GM55145-05 from the NIH (M.J.L.). We thank L. Covey and J.A. Langer for critical comments on this manuscript.
Footnotes
Abbreviations: DCM, dichloromethane; DIG, digoxigenin; DIPC, diisopropylcarbodiimide; 4-DMAP, 4-(dimethylamino)-pyridine; DMF, N,N-dimethylformamide; fMLF, formyl-methionine-leucine-phenylalanine; HBSS, Hanks’s Balanced Salt Solution; NHS, N-hydroxysuccinimide; PBS, phosphate-buffered saline; PEG, poly(ethylene glycol); PEG/A, linear PEG-aspartate copolymer; PTSA, p-toluenethiosulfonic acid; tBoc, tert-butyl-oxycarbonyl; TFA, trifluoroacetic acid.
LITERATURE CITED
- 1.Anderson R, Mahomed Goolam A. Calcium efflux and influx in f-met-leu-phe (fMLP)-activated human neutrophils are chronologically distinct events. Clin Exp Immunol. 1997;110:132–138. doi: 10.1046/j.1365-2249.1997.5051403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azuma EK, Yuo A, Matsushima K, Kasahara T, Mizoguchi H, Saito M, Takaku F, Kitagawa S. Activation and priming of human monocytes by monocyte chemotactic and activating factor: Cooperation with other inflammatory cytokines and close association between an increase in cytoplasmic free Ca2+ and intracellular acidification. Exp Hematol. 1996;24:169–175. [PubMed] [Google Scholar]
- 3.Benelli R, Mortarini R, Anichini A, Giunciuglio D, Noonan DM, Monatalti S, Tacchetti C, Albini A. Monocyte-derived dendritic cells and monocytes migrate to HIV–Tat RGD and basic peptides. AIDS. 1998;12:261–268. doi: 10.1097/00002030-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Camps M, Carozzi A, Schnabel P, Scheer A, Parker PJ, Gierschik P. Lysosome-selective stimulation of phospholipaseC-b2 by G-protein-bg-subunits. Nature. 1992;369:684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- 5.Collins SJ. The HL-60 promyelocytic leukemia cell line: Proliferation, differentiation, and cellular oncogene expression. Blood. 1987;70:1233–1244. [PubMed] [Google Scholar]
- 6.Dahlgren C, Anderson T, Stendahl O. Chemotactic factor binding and functional capacity: A comparison between human granulocytes and differentiated HL-60 cells. J Leukocyte Biol. 1987;42:245–252. doi: 10.1002/jlb.42.3.245. [DOI] [PubMed] [Google Scholar]
- 7.Davis S, Abuchowiski A, Park YK, Davis FE. Alteration of the circulating half-life and antigenic properties of bovine adenosine deaminase in mice by attachment of poly(ethylene glycol) Clin Exp Immunol. 1981;46:649–652. [PMC free article] [PubMed] [Google Scholar]
- 8.Derian CK, Solomon HF, Higgins JD, III, Beblavy MJ, Santulli RJ, Bridger GJ, Pike MC, Kroon DJ, Fischman AJ. Selective inhibition of N-formylpeptide-induced neutrophil activation by carbamate-modified peptide analogues. Biochemistry. 1996;35:1265–1269. doi: 10.1021/bi952087k. [DOI] [PubMed] [Google Scholar]
- 9.Gierschik P, Moghtader R, Straub C, Dietrich K, Jacobs KH. Signal amplification in HL-60 granulocytes. Evidence that the chemotactic peptide receptor catalytically activates guanine-nucleotide-binding regulatory proteins in native plasma membranes. Eur J Biochem. 1991;197:725–732. doi: 10.1111/j.1432-1033.1991.tb15964.x. [DOI] [PubMed] [Google Scholar]
- 10.Huang SY, Pooyan S, Wang J, Choudhury I, Leibowitz MJ, Stein S. A poly(ethylene glycol) copolymer for carrying and releasing multiple copies of cysteine-containing peptides. Bioconjug Chem. 1998;9:612–617. doi: 10.1021/bc980038p. [DOI] [PubMed] [Google Scholar]
- 11.Klinker JF, Wenzel-Seifert K, Seifert R. G-Protein-coupled receptors in HL-60 human leukemia cells. Gen Pharmacol. 1996;27:33–54. doi: 10.1016/0306-3623(95)00107-7. [DOI] [PubMed] [Google Scholar]
- 12.Le Y, Oppenheim JJ, Wang JM. Pleiotropic roles of formyl peptide receptors. Cytokine Growth Factor Rev. 2001;12:91–105. doi: 10.1016/s1359-6101(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 13.Lees WJ, Spaltenstein A, Kingery-Wood JE, Whitesides GM. Polyacrylamides bearing pendant alpha-sialoside groups strongly inhibit agglutination of erythrocytes by influenza A virus: Multivalency and steric stabilization of particulate biological systems. J Med Chem. 1994;37:3419–3433. doi: 10.1021/jm00046a027. [DOI] [PubMed] [Google Scholar]
- 14.Lobenberg R, Kreuter J. Macrophage targeting of azidothymidine: a promising strategy for AIDS therapy. AIDS Res Human Retrovirol. 1996;12:1709–1715. doi: 10.1089/aid.1996.12.1709. [DOI] [PubMed] [Google Scholar]
- 15.Mankertz J, Matthes E, Rokos K, von Baeyer H, Pauli G, Riedel E. Selective endocytosis of fluorothymidine and azidothymidine coupled to LDL into HIV infected mononuclear cells. Biochim Biophys Acta. 1996;1317:233–237. doi: 10.1016/s0925-4439(96)00059-2. [DOI] [PubMed] [Google Scholar]
- 16.Martinet Y, Martinet N, Vignaud JD, Plenat F. Blood monocytes chemotaxis. J Immunol Methods. 1994;174:209–214. doi: 10.1016/0022-1759(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 17.Medda S, Das N, Mahato SB, Mahadaven PR, Basu MK. Glycoside-bearing liposome delivery systems against macrophage-associated disorders involving Mycobacterium leprea and Mycobacterium tuberculosis. Indian J Biochem Biophys. 1995;32:147–151. [PubMed] [Google Scholar]
- 18.Mistry PK, Wraight EP, Cox TM. Therapeutic delivery of proteins to macrophages: implications for treatment of Gaucher’s disease. Lancet. 1996;348:1555–1559. doi: 10.1016/S0140-6736(96)04451-0. [DOI] [PubMed] [Google Scholar]
- 19.Montero M, Garcia-Sancho J, Alvarez J. Activation by chemotactic peptide of a receptor-operated Ca2+ entry pathway in differentiated HL60 cells. J Biol Chem. 1994;269:29451–29456. [PubMed] [Google Scholar]
- 20.Nathan A, Zalipsky S, Ertel SI, Agathos SN, Yarmush ML, Kohn J. Copolymers of lysine and poly(ethylene glycol): a new family of functionalized drug carriers. Bioconjug Chem. 1993;4:54–62. 410. doi: 10.1021/bc00019a008. [DOI] [PubMed] [Google Scholar]
- 21.Niedel JE, Kahane I, Cuatrecasas P. Receptor-mediated internalization of fluorescent chemotactic peptide by human neutrophils. Science. 1979;205:1412–1414. doi: 10.1126/science.472759. [DOI] [PubMed] [Google Scholar]
- 22.Niedel J, Kahane I, Lachman L, Cuatrecasas P. A subpopulation of cultured human promyelocytic leukemia cells (HL-60) displays the formyl peptide chemotactic receptor. Proc Natl Acad Sci USA. 1980;77:1000–1004. doi: 10.1073/pnas.77.2.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poiani GJ, Riley DJ, Fox JD, Kemnitzer JE, Gean KF, Kohn J. Conjugation of cis-4-hydroxy-L-proline and poly(PEG-Lys), a water soluble poly(ether urethane): Synthesis and evaluation of antifibrotic effect in vitro and in vivo. Bioconjugate Chem. 1994;5:621–630. doi: 10.1021/bc00030a018. [DOI] [PubMed] [Google Scholar]
- 24.Prossnitz ER, Gilbert TL, Chiang S, Campbell JJ, Qin S, Newman W, Sklar LA, Ye RD. Multiple activation steps of the N-formyl peptide receptor. Biochemistry. 1999;38:2240–2247. doi: 10.1021/bi982274t. [DOI] [PubMed] [Google Scholar]
- 25.Prossnitz ER, Quechenberger O, Cochrane CG, Ye RD. Signal transducing properties of the N-formyl peptide receptor expressed in undifferentiated HL60 cells. J Immunol. 1993;151:5704–5715. [PubMed] [Google Scholar]
- 26.Prossnitz ER, Ye RD. The N-formyl peptide receptor: A model for the study of chemoattractant receptor structure and function. Pharmacol Ther. 1997;74:73–102. doi: 10.1016/s0163-7258(96)00203-3. [DOI] [PubMed] [Google Scholar]
- 27.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 28.Ramanathan S, Pooyan S, Stein S, Prasad PD, Wang J, Leibowitz MJ, Ganapathy V, Sinko PJ. Targeting the sodium-dependent multivitamin transporter (SMVT) for improving the oral absorption properties of a retro-inverso Tat nonapeptide. Pharm Res. 2001;18:950–956. doi: 10.1023/a:1010932126662. [DOI] [PubMed] [Google Scholar]
- 29.Rossi L, Brandi G, Schiavano GF, Balestra E, Millo E, Scarfi S, Damonte G, Gasparini A, Magnani M, Perno CF, Benatti U, De Flora A. Macrophage protection against human immunodeficiency virus or herpes simplex virus by red blood cell-mediated delivery of a heterodinucleotide of aziothymidine and acyclovir. AIDS Res Human Retrovirol. 1998;14:435–444. doi: 10.1089/aid.1998.14.435. [DOI] [PubMed] [Google Scholar]
- 30.Seifert R, Schultz G. The superoxide-forming NADPH oxidase of phagocytes: an enzyme regulated by multiple mechanisms. Rev Physiol Biochem Pharmacol. 1991;117:1–338. [PubMed] [Google Scholar]
- 31.Seifert R, Serke S, Huhn D, Bessler WG, Hauschildt S, Metzegae J, Wiesmuller K-H, Jung G. Incomplete functional differentiation of HL-60 leukemic cells by synthetic lipopropeptides. Partial inhibition by pertussis toxin of enhanced superoxide formation. Eur J Biochem. 1992;203:143–151. doi: 10.1111/j.1432-1033.1992.tb19839.x. [DOI] [PubMed] [Google Scholar]
- 32.Sigal GB, Mammen M, Dahmann G, Whitesides GM. Polyacrylamides bearing pendant α-sialoside groups strongly inhibit agglutination of erythrocytes by influenza virus: The strong inhibition reflects enhanced binding through cooperative polyvalent interactions. J Am Chem Soc. 1996;116:3789–3800. [Google Scholar]
- 33.Udenfriend S, Stein S, Bohlen P, Dairman W. Fluorescamine, a new reagent for assay of amino acids, peptides, proteins and other primary amines in the picomole range. Science. 1972;178:871. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- 34.Venier-Julienne MC, Vouldoukis I, Monjour L, Benoit JP. In vitro study of the anti-leishmanial activity of biodegradable nanoparticles. J Drug Targeting. 1995;3:23–29. doi: 10.3109/10611869509015929. [DOI] [PubMed] [Google Scholar]
- 35.Wenzel-Seifert K, Seifert R. Nucleotide-, chemotactic peptide- and phorbal ester induced exocytosis in HL-60 leukemic cells. Immunobiol. 1990;181:298–316. doi: 10.1016/S0171-2985(11)80499-7. [DOI] [PubMed] [Google Scholar]


