Abstract
Background:
Study of the pathophysiology and treatment of anemia of prematurity is facilitated by direct measurement of red cell volume (RCV) utilizing microliter quantities of blood samples. Our objective was to compare concurrent measurements of multiple direct RCV methods in infants.
Methods:
Eighteen preterm infants receiving clinically-indicated transfusions had concurrent flow cytometric determinations of RCV and 24 h red blood cell (RBC) recovery based on donor-recipient differences of biotin–labeled RBCs (BioRBCs), Kidd antigen mismatched RBCs, and HbF positive (HbF+) RBCs. HPLC was also used to measure HbF and HbA protein concentrations for RCV determination.
Results:
Concurrent RCV measurements using BioRBCs (18 and 54 μg/ml), Kidd antigen, and HbF flow cytometry were not statistically different compared to RCVs measured using the reference BioRBC density (6 μg/ml). In contrast, the HbF HPLC method over estimated RCV by 45% compared to the reference method. All methods demonstrated 100% 24 h post-transfusion RBC recovery (PTR24).
Conclusions:
Because BioRBC, Kidd antigen, and HbF flow cytometry are safe and accurate methods requiring <10 μl of patient blood to determine RCV and PTR24 in preterm infants, they can be useful in clinical and research studies of anemia and other conditions.
INTRODUCTION
Anemia is a common and serious clinical problem among critically ill premature infants. Important contributors to anemia of prematurity include low Hb at birth, blood loss due to phlebotomy for laboratory testing, and inability of the infant to produce sufficient red blood cells (RBCs) to overcome blood loss and postnatal expansion of blood volume with rapid growth. Currently, the two most common treatments for anemia in premature infants are allogeneic RBC transfusion and recombinant erythropoietin (rEPO) (1). To assess the effectiveness of these and other therapies, safe, accurate, and versatile methods for determining circulating red cell volume (RCV), blood volume (BV), and 24 h post-transfusion recovery (PTR24) of transfused blood are needed. Among ill premature infants, RCV is deemed a better indicator of the need for RBC transfusion than either whole blood Hb or hematocrit (HCT) levels (2, 3).
Flow cytometric enumeration of RBCs can be used in determining RCV and BV and requires only a few microliters of blood. This method relies on its ability to discriminate transfused from endogenously produced RBC populations. Flow cytometry has been used to detect fetomaternal hemorrhage (4, 5), determine RBC phenotype following bone marrow transplant (6), measure RCV (7-9), detect illicit blood transfusions in athletes (10), and determine RBC survival (11, 12). Recently, our group has for the first time demonstrated that RCV can be accurately determined in adult humans and sheep using multiple distinct populations of biotin–labeled RBCs (BioRBCs) enumerated by flow cytometry (8, 13).
In the present study, we extend this previous work with the objective of comparing concurrent RCV determinations using four different methods in very low birth weight (VLBW) premature infants weighing < 1.5 kg at birth: flow cytometric methods, 1) multi-density BioRBC; 2) Kidd antigen (Jka and Jkb) mismatches between adult donor and infant RBCs; 3) dilution of infant RBCs containing primarily HbF by donor RBCs containing primarily HbA; and 4) a non-flow cytometric method, change in proportion of HbA and HbF proteins measured by HPLC. None of these three methods requires labeling of donor RBCs prior to transfusion. Because of extremely limited recovery data for stored donor blood in infants, we also determined the PTR24 of transfused RBCs using the same methods.
Based on our prior findings in adults (8, 11), we hypothesized that 1) RCV determined using RBCs biotinylated at three high biotin densities (18, 54 and 162 μg of biotinylating reagent per ml RBC), Kidd antigen mismatch, and Hb type differences methods would not differ significantly from RCV determined using a previously validated low BioRBC density (6 μg/ml) as the reference method; 2) allogeneic RBCs would completely equilibrate within first 20 min post-transfusion (i.e., there would be no mixing or spleen effect); and 3) PTR24 assessed by all the methods would not be significantly different than 100%.
RESULTS
Eighteen premature infants with gestational ages at birth between 26 and 30 wks were studied (Table 1). Mean (± SD) birth weight was 0.96 ± 0.24 kg (range 0.39 to 1.40 kg). On the day the study transfusion was administrated, infants were 18 ± 14 d old (range 1 to 45 d) with body weights of 1.21 ± 0.45 kg (range 0.37 to 2.21 kg).
Table 1.
Study subject demographics
| Subject number |
Gestational age at birth (wk) |
Birth weight (kg) |
Study age (d) |
Study weight (kg) |
|---|---|---|---|---|
| 1a,b,c | 27.9 | 0.83 | 22 | 1.25 |
| 2a,c | 28.7 | 1.31 | 40 | 2.21 |
| 3a,c | 28.0 | 0.88 | 23 | 1.10 |
| 4a,b,d | 28.0 | 1.09 | 8 | 0.96 |
| 5a,c,e | 28.7 | 1.37 | 10 | 1.32 |
| 6a,d,e | 28.0 | 0.93 | 1 | 0.93 |
| 7a,c | 29.9 | 0.73 | 3 | 0.76 |
| 8a,c,e,f | 30.1 | 1.40 | 33 | 1.96 |
| 9a,c,e,f | 27.4 | 0.91 | 45 | 1.76 |
| 10a,c,f | 26.7 | 0.86 | 33 | 1.55 |
| 11a,c,f,g | 27.4 | 0.83 | 15 | 0.98 |
| 12a,d,f | 26.3 | 0.39 | 1 | 0.37 |
| 13a,c,e,f,g,h | 27.4 | 1.08 | 15 | 1.20 |
| 14a,c,e,f,g | 28.9 | 0.92 | 34 | 1.52 |
| 15a,b,c,e,f,g | 27.0 | 1.00 | 9 | 0.87 |
| 16a,b,c,e,f,g | 27.0 | 0.93 | 13 | 1.06 |
| 17a,b,d,e,f,g | 26.3 | 0.95 | 5 | 0.92 |
| 18a,c,e,f,g | 27.4 | 0.95 | 13 | 0.98 |
|
| ||||
| Mean | 27.8 | 0.96 | 18 | 1.21 |
| SD | 1.06 | 0.24 | 14 | 0.45 |
BioRBC, n=18;
Kidd Jka mismatch, n=5;
peripheral capillary post-transfusion whole blood sample, n=14;
central arterial post-transfusion whole blood sample, n=4;
Kidd Jkb mismatch, n=10;
HbF flow cytometry, n=11;
HbF HPLC, n=7;
outlier
Flow Cytometric Identification of RBC Populations
The four discrete BioRBC densities and the unlabeled RBCs demonstrated complete separation, permitting accurate enumeration of each of the four BioRBC populations (Figure 1a). Pre- and post-transfusion histograms based on Kidd Jka and Jkb antigens also exhibited no peak overlap (Figure 1b and c). Because the study transfusion was the first transfusion for this specific infant, there was only a single peak (Jkb-RBCs) originating from the infant in the pre-transfusion sample (Figure 1b). After transfusion of Jkb mismatched RBCs, the expected two peaks were present. In contrast, both pre- and post-transfusion samples from an infant who had already received Kidd antigen mismatched blood prior to the study showed two distinct peaks (Figure 1c). After the study transfusion, the proportion of Jka− donor RBCs increased while the proportion of Jka+ infant RBCs decreased. The histograms for HbF+RBCs in subjects without and with previous transfusions demonstrated similar peak patterns to the two Kidd antigens (Figure 1d and e).
Figure 1.
Flow cytometry histograms of pre- and post-transfusion samples showing number of RBCs enumerated (y-axis) to log of florescent intensity (x-axis) showing distinct RBC populations detected by different methods. Dashed lines indicate pre-transfusion samples and solid lines indicate post-transfusion sample results. (a) Subject 15, BioRBCs detected using Alexa-streptavidin; (b) Subject 14 with no previous transfusion, Jkb+ RBCs detected using anti-Jkb (infant RBCs are Jkb− and donor RBCs are Jkb+); (c) Subject 4 with a previous transfusion, Jka+ cells detected using anti-Jka (Infant RBCs are Jka+ and donor RBCs are Jka−); (d) Subject 12 with no previous transfusion, HbF+RBCs detected using anti-HbF; and (e) Subject 15 with a previous transfusion, HbF+RBCs detected using anti-HbF.
Comparison of RCV Measurement Methods by Regression Analysis
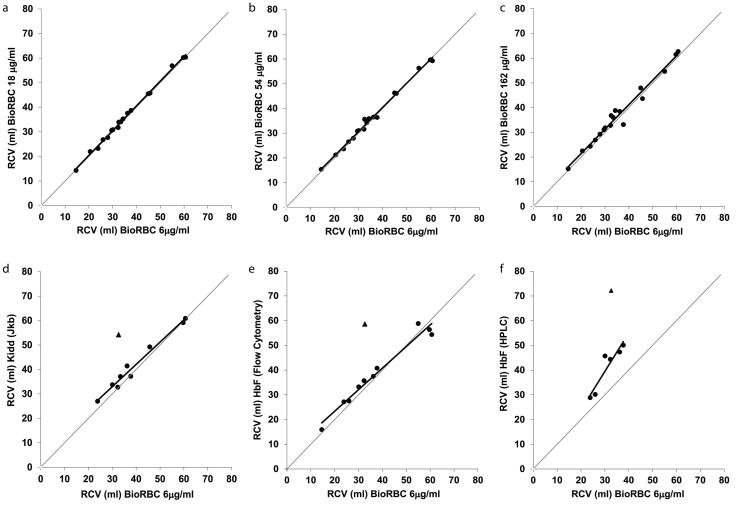
RCV measured at 20 min post-transfusion using the reference BioRBC density agreed well with RCV measured using the higher BioRBC densities, Kidd antigen, and HbF flow cytometry methods, but not with the HbF HPLC method (Figure 2). The agreement of the reference BioRBC density and densities 18, 54, 162 μg/ml were greater than for the Kidd antigen and HbF flow cytometry methods as judged by range of the 95% CI for both the slope and y intercept (Figure 2a-c; Table 2). Among all the methods, the HbF HPLC method showed the weakest correlation with the reference BioRBC density (R2 = 0.869 and the widest 95% CIs) (Figure 2f; Table 2).
Figure 2.
Agreement of RCV measurements determined at 20 min using BioRBC densities (a) 18 μg/ml, (b) 54 μg/ml, (c) 162 μg/ml, (d) Kidd antigen (Jkb), (e) HbF flow cytometry methods plotted versus RCV measured from the reference BioRBC density, (f) RCVs determined by HbF HPLC method were overestimated compare to the reference BioRBC density. The line of identity is indicated by the thin diagonal gray line. The triangles in panels (e)-(f) represent the outlier data point.
Table 2.
Parameters and 95% CIs of the linear regressions for each method relative to the reference BioRBC density
| Assay method | Slope | 95% CI of slope |
Intercept | 95% CI of intercept |
R2 |
|---|---|---|---|---|---|
| BioRBC 18 μg/ml | 1.01 | 0.98 to 1.04 | 0.3266 | −0.83 to 1.48 | 0.997 |
| BioRBC 54 μg/ml | 0.98 | 0.94 to 1.02 | 1.1777 | −0.45 to 2.81 | 0.993 |
| BioRBC 162 μg/ml | 0.99 | 0.90 to 1.08 | 1.6459 | −1.65 to 4.95 | 0.973 |
| Kidd Jkb | 0.91 | 0.78 to 1.04 | 5.6351 | 0.28 to 10.99 | 0.976 |
| HbF flow cytometry | 0.87 | 0.74 to 1.01 | 5.9154 | −0.40 to 11.43 | 0.965 |
| HbF HPLC | 1.55 | −0.72 to 2.39 | −7.1417 | −33.44 to 19.17 | 0.869 |
BioRBC densities, n=18; Kidd Jkb, n=9; HbF flow cytometry, n=10; HbF HPLC, n=6. Kidd Jka not included because of the small number of available study subjects (n = 5).
There was a single notable outlier RCV determined using the Kidd antigen and both HbF methods (Subject 13 shown as a triangle in Figure 2d-f). RCV values for this subject were greater than mean ± 2 SD relative to the other RCV values. We have no explanation for this outlier. This point was excluded from the regression analyses.
Comparison of 20- and 90-Min Posttransfusion RCV Determinations
In our previous adult sheep studies, we observed a slowly equilibrating pool of intravascular RBCs that accounted for about one-third of the total circulating RBCs (13). This was likely due to pool of RBCs sequestered in the ovine spleen that equilibrates slowly with the circulating RBCs and BioRBCs. To determine whether a similar phenomenon was present in VLBW infants, we compared paired RCV values at 20 and 90 min post-transfusion using a linear mixed model analysis for repeated measures (Table 3). Differences between mean RCV values at 20 and 90 min were not significant, thus indicating no reservoir of slowly mixing RBCs in the spleen of VLBW infants.
Table 3.
Comparison of mean ± SEM RCV results for different methods at 20 and 90 min
| RCV Mean (SEM) (ml) |
% Difference (95% CI) (90 min − 20 min) ÷ 20 min |
|||
|---|---|---|---|---|
| Assay method | 20 min | 90 min | P-value | |
| BioRBC 6 μg/ml | 33.7 (0.30) | 33.4 (0.30) | −0.7% (−2.8%, 1.4%) | 0.283 |
| BioRBC 18 μg/ml | 34.2 (0.30) | 34.2 (0.30) | −0.2% (−2.6%, 2.3%) | 0.821 |
| BioRBC 54 μg/ml | 34.3 (0.30) | 34.1 (0.30) | −0.6% (−2.9%, 1.8%) | 0.465 |
| BioRBC 162 μg/ml | 35.0 (0.31) | 35.1 (0.31) | 0.5% (−3.1%, 4.2%) | 0.683 |
| Kidd Jka | 35.6 (0.32) | 36.1 (0.31) | 1.5% (−20.0%, 28.8%) | 0.520 |
| Kidd Jkb | 37.9 (0.35) | 37.7 (0.35) | −0.4% (−2.8%, 2.0%) | 0.545 |
| HbF flow cytometry | 37.4 (0.37) | 36.7 (0.36) | −2.0% (−9.4%, 6.1%) | 0.388 |
| HbF HPLC | 47.6 (0.45) | 49.7 (0.46) | 4.5% (−6.0%, 16.3%) | 0.120 |
Effect of Method on RCV
Two statistical comparisons of the RCV results from the individual methods were performed: linear mixed model analysis with repeated measures and Bland-Altman plot analysis. In both comparisons RCV determined by individual methods was expressed as a ratio relative to the reference BioRBC density RCV. Because mean post-transfusion RCV values at 20 and 90 min were not significantly different, the linear mixed model analysis of the methods was based on the RCV values averaged for these two post-transfusion times. In this analysis results were deemed not different if the ± 95% CI ratio included 1.0. Mean RCV ratios were not significantly different among the methods with two exceptions: 1) 162 μg/ml BioRBC density overestimated the RCV by 4.4%, a difference likely to be unimportant for most clinical and research applications; and 2) HbF HPLC overestimated RCV by 44.9%, a large difference (Table 4).
Table 4.
Mean RCV method ratio results relative to 6 μg/ml reference BioRBC density
| Average of 20 min and 90 min Ratios |
||||
|---|---|---|---|---|
| Assay method | Mean Ratio | 95% CI Mean Ratio |
P-value | |
| BioRBC 18 μ/ml | 1.018 | 0.995 | 1.042 | 0.14 |
| BioRBC 54 μg/ml | 1.019 | 0.995 | 1.043 | 0.15 |
| BioRBC 162 μ/ml | 1.044 | 1.007 | 1.082 | 0.02 |
| Kidd Jka | 1.067 | 0.995 | 1.145 | 0.07 |
| Kidd Jkb | 1.126 | 0.966 | 1.312 | 0.15 |
| HbF flow cytometry | 1.104 | 0.920 | 1.326 | 0.44 |
| HbF HPLC | 1.449 | 1.103 | 1.903 | <0.01 |
In the second statistical comparison, agreement among the methods for measuring RCV at 20 min post-transfusion was assessed by Bland-Altman plots (Figure 3). Using the reference BioRBC density as the denominator, the ratios of the RCV for each method demonstrated agreement among all methods except for HbF HPLC. For the three higher BioRBC densities, the 95% limits of agreement with the reference method were as follows: −3.3% to +6.5% for 18 μg/ml; −4.4% to +7.9% for 54 μg/ml; and −7.6% to +15.6% for 162 μg/ml. Agreement with the reference BioRBC method was not as close for the Kidd antigen (Jkb) or the HbF flow cytometry methods. The limits of agreement were −6.4% to +19.2% for Jkb, and −9.6% to +20.1% for HbF flow cytometry. The HbF HPLC method significantly overestimated RCV; the limits of agreement were +6.8% to +56.5%. As with the regression analyses, Subject 13’s outlier data point was excluded from the Kidd antigen and both HbF analyses. Due to the small sample number (n = 4), comparison of RCV values by the Kidd Jka method against the reference BioRBC density was not performed.
Figure 3.
Bland-Altman plots of RCV relative to the reference BioRBC density compared to: (a) BioRBC 18 μg/ml, (b) BioRBC 54 μg/ml, (c) BioRBC 162 μg/ml, (d) Kidd-Jkb, (e) HbF flow cytometry, and (f) HbF HPLC. The mean ratio of RCVs (solid line), 95% CI (dashed line), and ratio = 1.0 (dotted line) are shown. With the exception of (f) in which HbF HPLC significantly overestimated the limits of agreement for RCV (i.e., the area between the two dashed horizontal lines), all other plots demonstrated good agreement relative to the reference BioRBC density. The triangle data points in panels (d)-(f) indicate the single outlier data point.
Short-term Recovery of Transfused RBCs
To determine PTR24, we assessed the percentage of transfused RBCs remaining 24 h post transfusion using the four BioRBC densities, Kidd antigen, and both HbF methods. Differences in means between 20 min and 24 h for each method were tested for significance by mixed model analysis for repeated measures. Mean PTR24 measured by BioRBC for the 14 study subjects were not different between 20 min and 24 h (Table 5). Indeed, PTR24was not significantly different from 100% for any of the methods (Bonferroni adjusted P-values: 0.14 to >0.99), thus providing evidence that the reticuloendothelial system of VLBW infants does not remove any of the transfused RBCs — whether labeled with biotin or differentiated from recipient RBCs by minor antigen or HbF — within the first 24 h post-transfusion. The mean percent change from 20 min ranged from a decrease of –3.7% (95% CI: –9.8%, 2.8%) for HbF flow cytometry, to an increase of 3.7% (95% CI: –2.4%, 10.2%) for Kidd Jkb. Due to the small sample number, Kidd Jka method (2 data points) data were not analyzed. Because four subjects did not have an appropriate 24 h post-transfusion sample, they were also excluded.
Table 5.
Short-term PTR24 survival of transfused-RBCs
| Assay method | PTR24
Mean ± SD (%) |
Difference (95% CI) |
Range of CI | P-value |
|---|---|---|---|---|
| BioRBC 6 μg/ml | 102.5 ± 3.5 | 2.50% | −0.5% to 5.5% | 0.142 |
| BioRBC 18 μg/ml | 101.0 ± 3.4 | 0.90% | −2.0% to 3.9% | >0.99 |
| BioRBC 54 μg/ml | 101.1 ± 3.1 | 1.10% | −1.5% to 3.8% | >0.99 |
| BioRBC 162 μg/ml | 100.4 ± 5.1 | 0.40% | −4.7% to 5.8% | >0.99 |
| Kidd Jkb | 104.2 ± 4.5 | 3.70% | −2.4% to 10.2% | 0.377 |
| HbF flow cytometry | 96.4 ± 4.2 | −3.70% | −9.8% to 2.8% | 0.424 |
| HbF HPLC | 99.8 ± 6.9 | −0.30% | −20.6% to 25.2% | >0.99 |
BioRBC densities 6, 18 and 54 μg/ml, n=14; BioRBC 162 μg/ml, n=11; Kidd Jka, n=2; Kidd Jkb, n=7; HbF flow cytometry, n=7; HbF HPLC, n =4.
RCV and BV per kg
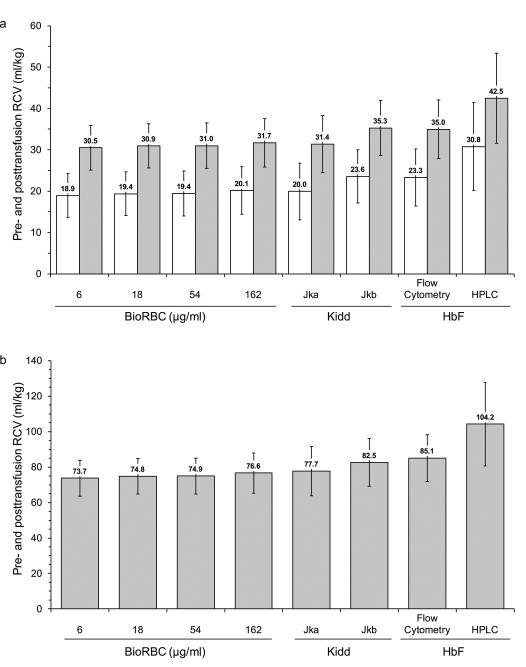
Because RCV and BV values at 20 min post-transfusion normalized by body weight are of clinical interest, we calculated the mean pre- and post-transfusion RCV (ml/kg) and BV (ml/kg) using each of the four BioRBC densities, Kidd antigen, and both HbF methods. Mean pre-transfusion RCV ranged between 18.9 and 23.6 ml/kg for the flow cytometric methods, but was 30.8 ml/kg for HbF HPLC method (Figure 4a). Following RBC transfusion, infant mean RCV ml/kg increased about 50% for all methods except for RCV determined by HbF HPLC method where the mean increase was only 37%. Mean post-transfusion BV ranged between 73.7 and 85.1 ml/kg when measured using the flow cytometric methods. In contrast, mean post-transfusion BV measured by the HbF HPLC method was 104.2 ml/kg (Figure 4b).
Figure 4.
Results of mean (± SD) RCV and BV determinations by each method. (a) Pretransfusion RCV in ml/kg (white bars) and posttransfusion RCV in ml/kg (gray bars); (b) posttransfusion BV in ml/kg.
DISCUSSION
The present study determined concurrent RCV and PTR24 measured in VLBW infants undergoing clinically indicated allogeneic RBC transfusions using multiple methods of analysis. These methods included flow cytometric analysis of multi-density-labeled BioRBCs, Kidd antigen and HbF positive RBCs, and HbF protein by HPLC. Our study provide evidence that RCV measured using lowest density reference BioRBC (6 μg/ml) agreed well with RCV measured using BioRBC densities 18 and 54 μg/ml, Kidd antigen, and HbF flow cytometry. PTR24 also agreed well among all methods. All methods required only microliter volumes of blood and none were associated with identifiable safety issues.
Since the 1970s, accurate measurement of RCV in infants has not been reported, primarily for ethical concerns related to unnecessary radiation exposure of infants enrolled in research studies. Simultaneous determinations of multiple distinct RBC populations are not possible with 51Cr or with most other RBC labeling methods. Accordingly, we contend that the multi-density BioRBC method utilizing <10 μl sample volumes offers substantial potential for advancing our understanding of hematological and cardiorespiratory conditions and disease processes in the smallest, least mature anemic premature infants. Although a large majority of such infants develop significant anemia requiring RBC transfusion, RBC kinetic studies have never before been performed in the smallest, most fragile, high risk patients being cared for today.
Like the multi-density BioRBC method, the Kidd antigen and both HbF methods utilize microliter volumes of blood. Although the latter methods can only be performed at the time an allogeneic RBC transfusion is administrated, neither requires ex vivo pre-transfusion RBC manipulation (i.e., labeling). This feature of the Kidd antigen and HbF methods negates all safety issues other than those inherently associated with clinically ordered RBC transfusions.
In the present study, we first demonstrated the agreement among RCV measurements by the methods mentioned above using regression analysis (Figure 2; Table 2). For these comparisons the lowest BioRBC density was selected as the reference method. This was based of our prior data-supported hypothesis that labeling with the lowest biotin density would perturb RBCs the least (11, 12). RCV determined by the four BioRBC densities demonstrated a very close agreement with one another (R2 = 0.973 to 0.997). This observation is similar to our previous RCV results in adults using the same four BioRBC densities (8). Similar agreement was also observed in our adult sheep multi-density BioRBC RCV study with the exception that the highest BioRBC density studied (96 μg/ml for sheep) slightly overestimated RCV (7.6%) compared to the lowest BioRBC density (12 μg/ml) (13). Although in the present study, the non-BioRBC RCV determinations also agreed with the reference BioRBC density, level of agreement tended not to be as strong. When the inexplicable outlier was excluded from the Kidd antigen and HbF flow cytometry analyses, RCV results of these two methods demonstrated stronger agreement with the reference BioRBC density. The HPLC method of HbF determination demonstrated the weakest correlation with the reference BioRBC density, but HbF HPLC method also had the fewest number of usable data pairs (n = 6).
In addition, we demonstrated that 20 min was sufficiently long for complete equilibration of transfused RBCs with the infant’s RBCs. RCV determinations can be perturbed by changes in the enrichment of labeled or mismatched RBCs in the immediate (minutes) post-transfusion period as a result of incomplete mixing or equilibration with body distribution pools. As an example of the latter, in sheep we reported an apparent 37% increase in RCV during the first 60 min post-transfusion as the circulating transfused RBCs equilibrated with the non-circulating pool of RBCs in the ovine (13). In the present study, we observed no significant change in RCV determinations for any of the RCV methods between 20 and 90 min post-transfusion (Table 3). These RCV observations are consistent with our prior observations of no change in RCV after 20 min in adult humans (8). They are also consistent with results reported by Bratteby in term infants in which 51Cr-labeled autologous RBCs reached steady-state levels after an initial 10 min mixing phase (14).
We also showed that different BioRBC densities, Kidd antigen mismatch and HbF flow cytometric methods can be interchangeably used to determine RCV in infants undergoing RBC transfusions. Although statistical analyses indicated a significant (P = 0.02), but clinically unimportant, 4.4% over estimate of mean RCV (95% CI 0.7%to 8.2%) for the 162 μg/ml BioRBC density (Table 4), we concluded that 162 μg/ml BioRBC density can be used for RCV determination. In contrast, the HbF HPLC method overestimated RCV by 45%, clinically important discrepancy makes the HPLC method unsuitable for RCV determination.
As hypothesized based on our previous report in infants (15), PTR24 of stored allogeneic RBCs with all methods was not significantly different from 100%, i.e., relative to the 20 min post-transfusion sample. In addition, PTR24 values of 100% as determined by methods that do not require ex vivo RBC modification, i.e., the Kidd antigen and both HbF methods, supports our hypothesis that biotinylation of RBCs does not cause a change in PTR24 in infants. These findings stand in contrast to stored RBC PTR24 data in adults in which PTR24 data show a significant decline relative to immediate post-transfusion enrichment (16).
Using the RCV data determined in this study BV per kg was derived by dividing RCV by simultaneously determined hematocrit measurements. Mean post-transfusion BV derived in this manner ranged from 73.7 to 85.1 ml/kg for the three flow cytometric methods (Figure 4). Because accurate hematocrit values immediately prior to transfusion were not available, it was not possible to determine pre-transfusion BV. The close agreement among the post-transfusion BV determined among the flow cytometry methods and the lack of close agreement in the measurement of RCV using HbF protein by HPLC method (104.2 ml/kg) indicates that the latter method is associated with one or more unidentified systematic errors. While the derived BV values observed are within the ranges previously reported (17-20), reports of BV are highly variable because some — as in the present study — are derived from only RCV and HCT, while others are based on direct measurement of both RCV and plasma volume, or plasma volume alone. Studies using the latter approach report larger BVs because of the larger distribution space of the plasma (18, 21). In considering infant BV measurements derived using only RCV and HCT values must also be recognized that HCT determinations in infants are not as reproducible as in older children and adults. This is because of discrepancies in HCT with capillary versus venous sampling (22).
In conclusion, the present study demonstrates that RCV can be safely measured in premature VLBW infants using <10 μl of blood without exposure to radioactivity. Evidence of strong agreement in the concurrent RCV determinations among the flow cytometric methods suggests that these methods provide true and accurate direct measurement of infant RCV. Because of the high sensitivity, safety, and reproducibility of the multi-density BioRBC method and because BioRBC studies can be performed using either autologous or allogeneic RBCs (or both simultaneously), we contend that RCV measurements using biotin-labeled RBCs is the most useful and versatile in infants. Kidd antigen and HbF flow cytometry methods also perform well for evaluating the kinetics of allogeneic donor blood and have the advantage of not requiring ex-vivo labeling. The application of these newer flow cytometric methods in measuring RCV in the smallest, least mature, and critically ill infants are useful tools in advancing our understanding of their hematological and cardiorespiratory conditions and disease processes.
METHODS
Institutional Review Boards at the University of Iowa (performance site) and the University of Arkansas for Medical Sciences (analysis site) approved the study. This included approval for administering Kidd mismatched RBCs. Informed written parental consent was obtained.
Study Subjects
A prospective, convenience sample of 18 newborn infants eligible for study included those being treated with the expectation of survival who were <31 wks gestation and expected to receive a clinically indicated RBC transfusion. Infants with congenital anomalies were excluded. Infants who had received prior RBC transfusions were not excluded. Eight of 18 subjects had received one or more previous allogeneic RBC transfusions.
BioRBCs
When a clinical RBC transfusion (15 ml/kg packed RBCs with HCT of ~75%) was ordered, equal aliquots of allogeneic RBC (1.0 ml/kg packed RBCs for each density) were labeled at four discrete biotin densities as described below. Each BioRBC population transfused was calculated to produce a final enrichment of approximately 2% of the total number of circulating RBCs. Immediately following the infusion of the 11 ml/kg of the unlabeled clinical transfusion, the remaining 4 ml/kg containing the four BioRBC densities were infused over 10 min.
Kidd Antigen Mismatch
Potential donor blood units were screened to identify Kidd antigen mismatches with infant study subjects when possible. Donor blood units identified as mismatches were then used when a clinical transfusion was ordered. Some infants received mismatched RBCs by chance i.e. the donor was not pre-screened and selected for that infant.
Blood Sampling
Pre- and post-RBC transfusion blood samples were collected. Post-transfusion infant samples included 150 μl volumes drawn within 20 to 90 min following the completion of the BioRBC transfusion. Two of the 18 infants did not have 20 min post-transfusion samples, so we used 70 and 90 min samples for those two infants as earliest post-transfusion samples. A complete blood count analysis was performed using an automated hematology analyzer (Sysmex XE-2100, Sysmex Corp., Kobe, Japan) to determine RBC count and HCT on the first post-transfusion sample.
Biotinylation of RBCs and Analysis of BioRBCs by Flow Cytometry
The method for biotin labeling of RBCs at distinct densities in adult humans was used and aliquots of post-transfusion blood samples were processed for flow cytometric analysis of BioRBCs as described previously (8). Each distinct BioRBC population was detected as a separate peak on the post-transfusion histogram and the number of events counted under each peak used to determine the enrichment of each BioRBC population.
Analysis of Kidd Antigens by Flow Cytometry
For Jka+ or Jkb+ RBC flow cytometry analysis, triplicate 3 μl aliquots of the pre- and post-transfusion samples were washed to remove plasma proteins following the same procedures as for BioRBCs (13, 23). The RBCs were resuspended in 0.3 ml of wash buffer with 2% BSA (A30075, RPI Corp., Mount Prospect, IL) and incubated with 5 μl of anti-Jka or anti-Jkb primary antibody (Immucor Inc., Norcross, GA) overnight at room temperature with continuous mixing on a rotating wheel. These RBCs were washed twice and were incubated at 37 °C for 1 h with 5 μl of Alexa Fluor 488 conjugated secondary antibody (H10120, Invitrogen, Carlsbad, CA). RBCs were then washed three times and resuspended in wash buffer with 2% BSA (RPI Corp.) that had been filtered through a 0.2 micron filter to a final volume of 0.5 ml. Jka+ or Jkb+ RBCs were enumerated as described above for BioRBCs.
Analysis of HbF+RBCs by Flow Cytometry
The method for flow cytometric analysis of HbF+RBCs used to detect HbF+ cells following feto-maternal hemorrhage (4) was adapted to determine the RCV in premature infants undergoing adult donor RBC transfusion. The percent HbF+RBCs was determined using Invitrogen Fetal Hemoglobin Test (Invitrogen) with FITC conjugated monoclonal antibody directed against HbF. The manufacturer’s manual was followed with the following minor modifications. Duplicate 1.5 μl whole blood samples (~ 5×106 cells) were fixed in 0.25 ml of 0.05% glutaraldehyde. RBCs were permeabilized in 0.2 ml of 0.1% Triton X-100. Washed and pelleted RBCs were resuspended in 80 μl of PBS/BSA (Invitrogen/RPI Corp.) and incubated with FITC conjugated HbF monoclonal antibody for 30 min at room temperature. RBCs were washed and resuspended in 0.4 ml of 2% paraformaldehyde prior to flow cytometry analysis. HbF+RBCs were enumerated as described above for BioRBCs.
Analysis of HbF and HbA protein by HPLC
Analysis of HbA and HbF proteins by HPLC was performed using the method described by Witzhandler et al. (24). Briefly, analysis of stored blood samples maintained at −80°C was performed using a Dionex ProPac SCX-10 column (Thermo Scientific, Sunnyvale, CA) interfaced with a Waters 600E pump controller (GenTech Scientific, Arcade, NY), a SPD-10AV UV detector (Shimadzu Scientific Instruments, Columbia, MD), and an auto sampler (Perkin Elmer Inc., San Jose, CA). Replicate hemolysate samples were prepared by mixing 5 μl of blood in 1 ml of eluent solution (50 mM sodium phosphate and 2 mM potassium cyanide, pH 6.0). Ten μl of sample hemolysate was injected and the relative percentages of HbF and HbA proteins derived by using area under the peak analysis using Shimadzu data management software. With this method, acetylated HbF elutes as a separate peak that is added to the larger non-acetylated HbF to obtain the total percentage of HbF peak protein in the sample.
RCV and BV Calculations
Post-transfusion RCV was calculated for all methods using the dilution principle as follows:
where the total number of RBC (Tx)= Σ [volume of individual infusate × RBC concentration]. The volume of infusate for individual BioRBC densities was determined gravimetrically (11) using a specific gravity for transfused RBCs of 1.05 (g/ml); %A = percentage of individual BioRBC densities (6, 18, 54, or 162 μg/ml), Jka+, Jkb+, or HbF+RBCs (for HPLC, %HbF protein); Tx = transfusion; pre-Tx = pre-transfusion sample; post-Tx = post-transfusion sample; and MCV = mean corpuscular volume.
Although all study subjects had RCV measurements by BioRBC performed at all four densities, only 14 had Kidd antigen mismatches. For two infants, technical difficulties precluded inclusion of the Kidd antigen results, leaving 12 RCV determinations. For the HbF flow cytometry and HbF HPLC determinations, these methods had not been fully developed in our laboratory at the time the study was initiated: only 11 had RCV determined by HbF flow cytometry, and only 7 had RCV determined by HbF HPLC.
The post-transfusion BV ml/kg of each study subject was calculated using the following formula:
BV (ml/kg) = RCV(ml/kg)/ post-transfusion HCT.
Because pre-transfusion HCT was not measured, pre-transfusion RCV was determined by subtracting the total volume of RBCs transfused from post-transfusion RCV.
Statistical Analysis
Test for differences in mean RCV among the methods was performed by linear mixed model analysis for repeated measures. The natural log transformation of RCV was used to normalize the data distribution. The mixed model included method, time, and method by time interactions. In addition to estimating the fixed effects in the mixed model, this method of analysis allows for selection of the covariance structure that best fits the variance-covariance of the RCVs for the different methods and times in the same subject. From the linear mixed model analysis, the test for method by time interaction and the test for the post-transfusion time effect were examined. To report mean RCV in the original scale, the method ln(RCV) means from the fitted linear mixed model were back transformed, and the standard error computed using the delta method The differences in ln(RCV) between the BioRBC densities were also back-transformed. This provided estimates for the mean RCV ratio relative to reference BioRBC density.
Bland-Altman plots of the observed RCV ratio for a given density, Kidd antigen (Jkb), or both HbF methods relative to reference BioRBC method were included to display the distribution of ratios. The plots also show the mean ratio and the 95% limits of agreement for the mean ratio (i.e., mean ± 1.96 SD).
Descriptive data were expressed as the mean ± SD. Bonferroni correction for multiple comparisons was used. All the statistical analyses were performed with computer software (SAS, Version 9.3, 2002-2008, SAS Institute, Inc., Cary, NC). A P-value <0.05 was considered significant.
ACKNOWLEDGMENTS
We acknowledge helpful discussions with Robert S. Franco and Peter Veng-Pedersen, regarding technical and theoretical aspects of the present study. We appreciate the many contributions of the clinical laboratory staff led by Mitchell J. Owen and overseen by Matthew D. Krasowski, M.D., Ph.D., without which this work would not have been possible. We acknowledge the many outstanding clinical research contributions of Iowa’s neonatal nurse research team (Karen Johnson, Laura Knosp, Nancy Krutzfield, Ruthann Schrock Sara Scott, and Jin Zhou) and the research laboratory team (Earl Gingerich and Jessica Goehring). We thank S. Isil Cevik for her assistance with preparation of figures and Mark Hart for valuable secretarial assistance. The Sysmex XE-2100 automatic hematology analyzer used in this study was generously provided on an on-loan basis from Sysmex Corporation, Kobe, Japan. Last, we appreciate the willingness of study subject families in allowing their infants to participate.
STATEMENT OF FINANCIAL SUPPORT: This publication was supported in part by U.S. National Institutes of Health (NIH) grant P01 HL046925, by the Thrasher Research Fund 0285-3 (Salt Lake City, UT), and by grant UL1RR024979 from the National Center for Research Resources (NCRR). The paper’s contents are solely the responsibility of the authors and do not represent the official views of the NIH, the Thrasher Research Fund, or the NCRR.
Footnotes
Note: This trial has been registered at www.clinicaltrials.gov (identifier NCT 00731588).
DISCLOSURE: The authors have nothing to disclose.
REFERENCES
- 1.Strauss RG. Anaemia of prematurity: Pathophysiology and treatment. Blood Rev. 2010;24:221–5. doi: 10.1016/j.blre.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones JG, Holland BM, Hudson IR, Wardrop CA. Total circulating red cells versus haematocrit as the primary descriptor of oxygen transport by the blood. Br J Haematol. 1990;76:288–94. doi: 10.1111/j.1365-2141.1990.tb07886.x. [DOI] [PubMed] [Google Scholar]
- 3.Mock DM, Bell EF, Lankford GL, Widness JA. Hematocrit correlates well with circulating red blood cell volume in very low birth weight infants. Pediatr Res. 2001;50:525–31. doi: 10.1203/00006450-200110000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Davis BH, Olsen S, Bigelow NC, Chen JC. Detection of fetal red cells in fetomaternal hemorrhage using a fetal hemoglobin monoclonal antibody by flow cytometry. Transfusion. 1998;38:749–56. doi: 10.1046/j.1537-2995.1998.38898375514.x. [DOI] [PubMed] [Google Scholar]
- 5.Dziegiel MH, Nielsen LK, Berkowicz A. Detecting fetomaternal hemorrhage by flow cytometry. Curr Opin Hematol. 2006;13:490–5. doi: 10.1097/01.moh.0000245687.09215.c4. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard D, Bruneau V, Bernard D, et al. Flow cytometry analysis of dual red blood cell populations after bone marrow transplantation. Br J Haematol. 1995;89:741–7. doi: 10.1111/j.1365-2141.1995.tb08410.x. [DOI] [PubMed] [Google Scholar]
- 7.Hudson IR, Cavill IA, Cooke A, et al. Biotin labeling of red cells in the measurement of red cell volume in preterm infants. Pediatr Res. 1990;28:199–202. doi: 10.1203/00006450-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Mock DM, Matthews NI, Zhu S, et al. Red blood cell (RBC) volume can be independently determined in vivo in humans using RBCs labeled at different densities of biotin. Transfusion. 2011;51:148–57. doi: 10.1111/j.1537-2995.2010.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wynn R, Dixon S, al-Ismail SA, et al. Flow cytometric determination of pre-transfusion red cell volume in fetuses and neonates requiring transfusion based on RhD+ dilution by transfused D- red cells. Br J Haematol. 1995;89:620–2. doi: 10.1111/j.1365-2141.1995.tb08372.x. [DOI] [PubMed] [Google Scholar]
- 10.Arndt PA, Kumpel BM. Blood doping in athletes - detection of allogeneic blood transfusions by flow cytofluorometry. Am J Hematol. 2008;83:657–67. doi: 10.1002/ajh.21196. [DOI] [PubMed] [Google Scholar]
- 11.Mock DM, Matthews NI, Zhu S, et al. Red blood cell (RBC) survival determined in humans using RBCs labeled at multiple biotin densities. Transfusion. 2011;51:1047–57. doi: 10.1111/j.1537-2995.2010.02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mock DM, Matthews NI, Zhu S, et al. Comparison of red blood cell survival in sheep determined using red blood cells labeled with either biotin at multiple densities or [14C]cyanate: validation of a model to study human physiology and disease. Transfusion. 2012;52:963–73. doi: 10.1111/j.1537-2995.2011.03512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mock DM, Matthews NI, Zhu S, et al. Red blood cell (RBC) volume can be independently determined in vivo in the sheep using ovine RBCs labeled at different densities of biotin. Transfusion. 2010;50:2553–64. doi: 10.1111/j.1537-2995.2010.02744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bratteby LE. Studies on erythro-kinetics in infancy. VIII. Mixing, disappearance rates and distribution volume of labelled erythrocytes and plasma proteins in early infancy. Acta Soc Med Ups. 1967;72:249–71. [PubMed] [Google Scholar]
- 15.Strauss RG, Mock DM, Widness JA, Johnson K, Cress G, Schmidt RL. Posttransfusion 24-hour recovery and subsequent survival of allogeneic red blood cells in the bloodstream of newborn infants. Transfusion. 2004;44:871–6. doi: 10.1111/j.1537-2995.2004.03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess JR, Hill HR, Oliver CK, et al. Twelve-week RBC storage. Transfusion. 2003;43:867–72. doi: 10.1046/j.1537-2995.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 17.Aladangady N, Aitchison TC, Beckett C, Holland BM, Kyle BM, Wardrop CA. Is it possible to predict the blood volume of a sick preterm infant? Arch Dis Child Fetal Neonatal Ed. 2004;89:F344–7. doi: 10.1136/adc.2003.039008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bratteby LE. Studies on erythro-kinetics in infancy. XI. The change in circulating red cell volume during the first five months of life. Acta Paediatr Scand. 1968;57:215–24. doi: 10.1111/j.1651-2227.1968.tb04681.x. [DOI] [PubMed] [Google Scholar]
- 19.Phillips HM, Abdelmoiz A, Jones JG, et al. Determination of red-cell mass in assessment and management of anemia in babies needing blood-transfusion. Lancet. 1986;1:882–4. doi: 10.1016/s0140-6736(86)90988-8. [DOI] [PubMed] [Google Scholar]
- 20.Riley AA, Arakawa Y, Worley S, Duncan BW, Fukamachi K. Circulating blood volumes: a review of measurement techniques and a meta-analysis in children. ASAIO J. 2010;56:260–4. doi: 10.1097/MAT.0b013e3181d0c28d. [DOI] [PubMed] [Google Scholar]
- 21.Mollison PL, Veall N, Cutbush M. Red cell and plasma volume in newborn infants. Arch Dis Child. 1950;25:242–53. doi: 10.1136/adc.25.123.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivera LM, Rudolph N. Postnatal persistence of capillary-venous differences in hematocrit and hemoglobin values in low-birth-weight and term infants. Pediatrics. 1982;70:956–7. [PubMed] [Google Scholar]
- 23.Mock DM, Matthews NI, Strauss RG, Burmeister LF, Schmidt R, Widness JA. Red blood cell volume can be independently determined in vitro using sheep and human red blood cells labeled at different densities of biotin. Transfusion. 2009;49:1178–85. doi: 10.1111/j.1537-2995.2009.02095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weitzhandler M, Farnan D, Horvath J, et al. Protein variant separations by cation-exchange chromatography on tentacle-type polymeric stationary phases. J Chromatogr A. 1998;828:365–72. doi: 10.1016/s0021-9673(98)00521-4. [DOI] [PubMed] [Google Scholar]