Abstract
In the developing nervous system, synaptic connections are formed in excess and must remodel to achieve the precise synaptic connectivity characteristic of the mature organism. Synaptic pruning is a developmental process in which subsets of synapses are eliminated while the remaining synapses are preserved and strengthened. Recent findings have demonstrated unexpected roles for glial cells in this developmental process. These data demonstrate that phagocytic glia engulf synaptic and/or axonal elements in the developing nervous system and disruptions in this process result in sustained deficits in synaptic connectivity. These new findings highlight the importance of glia for nervous system development and function and may shed new light on mechanisms underlying nervous system disease.
INTRODUCTION
The synapse is a structure fundamental for the transmission of electrical and chemical signals between neurons. In the mature nervous system, synapses form exquisitely precise connections necessary for neural processing and function. In comparison, the developing nervous system is characterized by a crude synaptic wiring diagram that must undergo a significant degree of remodeling. In a process termed synaptic pruning, exuberant synaptic connections formed early in development are selectively eliminated while the remaining synapses are maintained and strengthened [1–5]. This may involve the elimination of axonal input that overshoots its target and/or the elimination of exuberant axonal collaterals innervating multiple targets [4] (Figure 1). Alternatively, pruning may involve the elimination of local, intact synapses (i.e., juxtaposed pre and postsynaptic elements) (Figures 1 and 2).
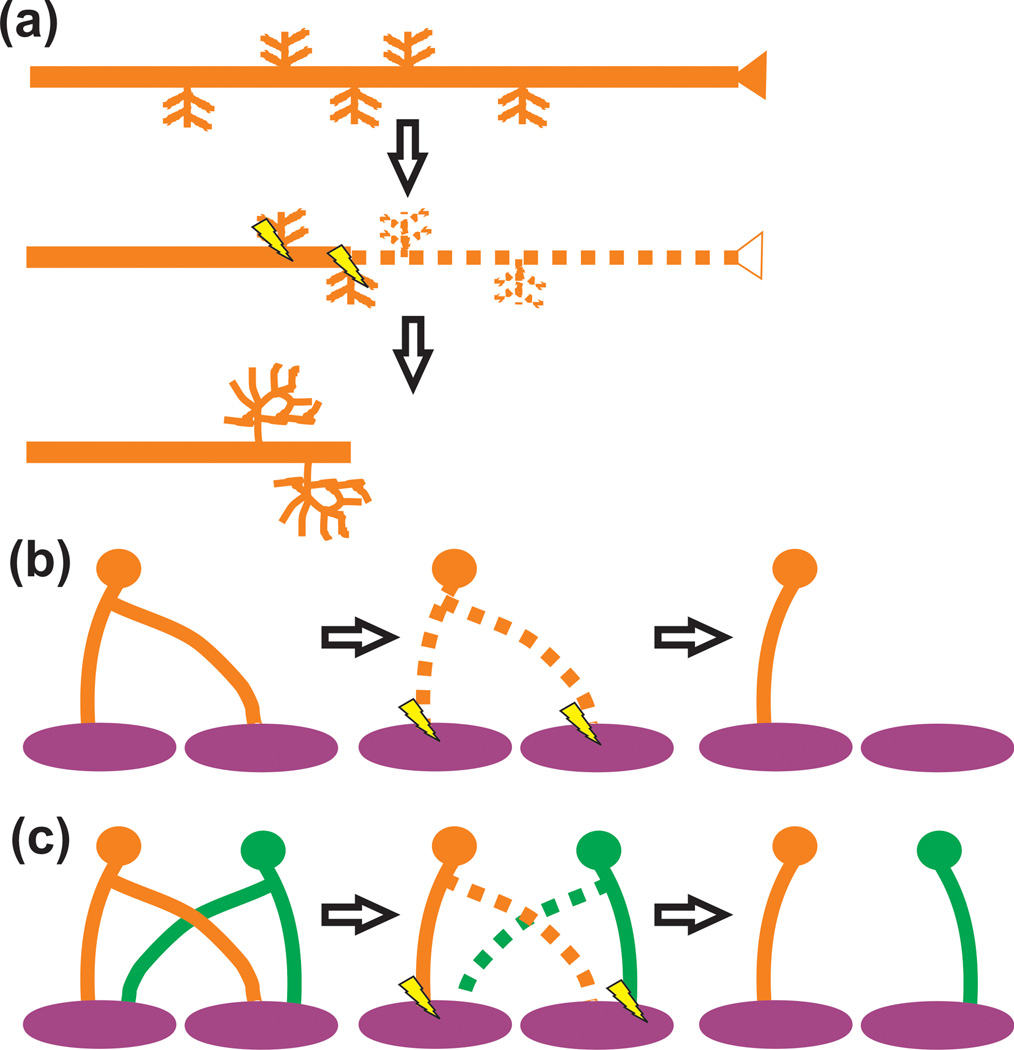
Figure 1. Axonal pruning in the nervous system.
(a) A presynaptic input (orange) with interstitial branches overshoots its target or is inappropriately targeted. Subsequently this input and small branches are pruned and remaining interstitial branches are elaborated (e.g., callosal projections). (b) A presynaptic input (orange) forms synapses on a postsynaptic target during early development (purple). These synaptic connections are subsequently eliminated and reform to form circuitry necessary for processing in the mature animal (e.g., Drosophila mushroom body). (c) Axon pruning involving the elimination of an axon collateral (orange and green dotted lines) from the postsynaptic target (purple) (e.g., NMJ). In all cases, axon pruning is driven by neural activity. Those synapses that are more active (lightning bolts) are maintained and strengthened.
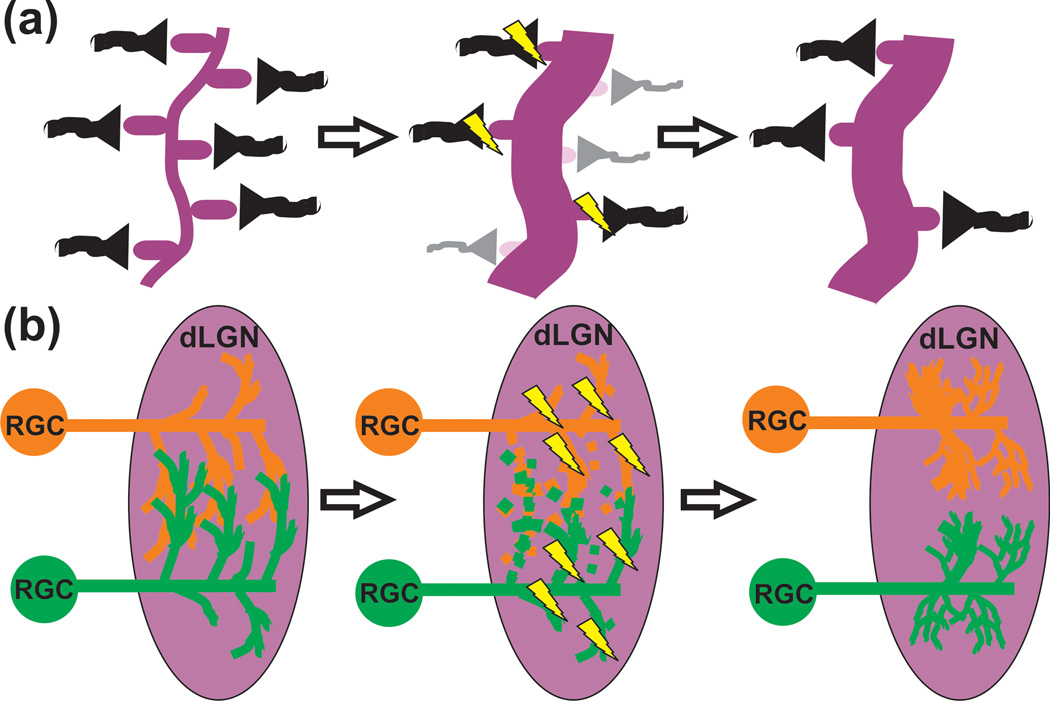
Figure 2. Pruning of local synaptic circuits in the CNS.
(a) Small scale pruning (e.g., visual cortex) can involve the local elimination of dendritic spines (light purple) and axon terminals (gray). Those presynaptic inputs (black) that are more active (lightning bolts) will be maintained and strengthened. (b) In the case of the retinogeniculate system, RGCs form transient, weak presynaptic inputs that innervate primarily the soma of postsynaptic dLGN relay neurons. Inputs from both eyes (red and green) are intermingled. During developmental pruning, smaller branches from both eyes that are less active (dotted lines) are removed. The remaining inputs that are more active (lightning bolts) are maintained, elaborated, and strengthened to form eye-specific territories within the dLGN.
Surprisingly, a flurry of recent studies have implicated glia in the remodeling of synaptic connections in the healthy, developing nervous system. In particular, a role for glia possessing high phagocytic capacity has emerged. These cells include microglia, astrocytes, and Schwann cells in mammals and their glial counterparts in Drosophila. Here, we review recent findings demonstrating critical roles for phagocytic glia in developmental synaptic pruning.
Glia and Developmental Synaptic Pruning: Axonal Pruning in the CNS
Some of the first evidence suggesting phagocytic glia were involved in synaptic pruning was a light and electron microscopy study in the developing cat corpus callosum undergoing large-scale axonal pruning (Figure 1) [6]. Within a developmental pruning window (E53-P39), there was an increase in callosal axon degeneration accompanied by the appearance of microglia and astrocytes containing degenerating axonal material within their cytoplasm.
Since this early work, little is still little known regarding the role of glia in large-scale axonal pruning in the mammalian CNS. However, elegant work in the developing Drosophila CNS demonstrated that glia play a key role in axonal pruning during metamorphosis from a larvae to a mature, adult insect [7–9]. During metamorphosis, γ neuron axons within the larval mushroom body are pruned away and new, adult-specific γ axons grow to their targets. While local axon degeneration mediated by the intrinsic ubiquitin-proteosome system (i.e., ecdysone) is an initiating step, two groups demonstrated that glia participate in this process by engulfing γ axons during the pruning period [7,8]. Furthermore, data suggest that these glial cells are not just passively scavenging leftover debris but rather active participants in the pruning process (Figure 3). First, glial cells accumulate within the mushroom body lobes prior to detectable degeneration and engulfed axonal varicosities, thought to be synaptic boutons, before these varicosities became fragmented [7]. In addition, blocking glial phagocytic function during development (i.e., glia-specific shibire mutant) resulted in a γ axon pruning deficit; however, it was not clear whether this effect was sustained into adulthood [8].
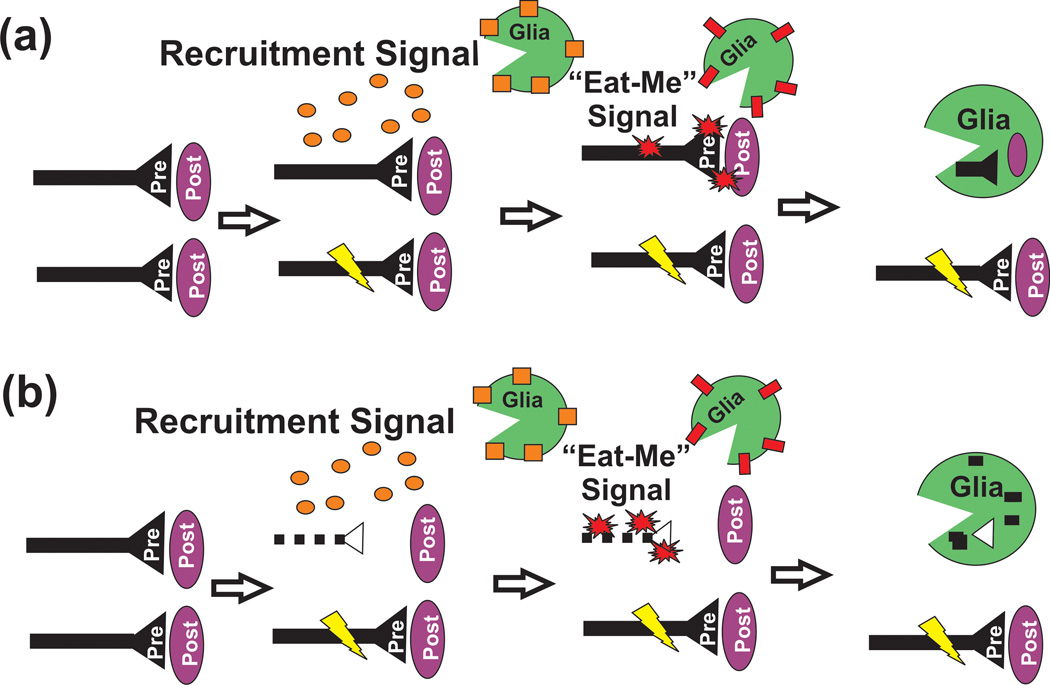
Figure 3. Models of glia-mediated synaptic pruning.
In both cases, glial cells may be attracted to sites of activity-dependent pruning by a local, soluble recruitment signal (orange circles; e.g., Cx3cl1) which binds receptors (orange squares; e.g., Cx3cr1) on the glial cell surface. Subsequently, glia engulf synaptic and/or axonal material through a local “eat-me” signal (red starbursts; e.g., C3) localized to the synapse and/or axon. This engulfment is mediated via a corresponding receptor (red rectangle; e.g., CR3, Draper, etc.) localized to the glial cell surface. The remaining, more active synapse (lightning bolt) is maintained and strengthened. This process could occur through local elimination of (a) intact, pre and postsynaptic components and/or (b) following intrinsic, degeneration (dotted line) of presynaptic components.
To more specifically assess the role of glia in pruning, recent work has genetically targeted phagocytic pathways in Drosophila glia [9–11]. By deleting the glial engulfment receptor Draper and downstream signaling molecule dCED-6, glial cell invasion into the larval mushroom body was blocked. Furthermore, while there is still evidence of γ axon degeneration, larval γ axon fibers persist in the adult mushroom body lobes in these mutants. These results demonstrate that, within the context of the developing Drosophila, glial cells have a significant and sustained impact on the elimination of axonal inputs. It remains to be determined if similar mechanisms apply to large-scale axonal pruning in the developing mammalian CNS.
Glia and Developmental Synaptic Pruning: Axonal Pruning in the PNS
Due to its simplicity, large size relative to its CNS counterpart, and accessibility for live imaging, the mammalian neuromuscular junction (NMJ) has served as an exquisite model system for studying pruning in the developing nervous system [1,12]. During early development, a single NMJ is innervated by multiple motor neuron axons (Figure 1). These multiple inputs are subsequently prune leaving a single remaining axon to be maintained and strengthened. One known regulator of this process is neural activity where, by Hebbian mechanisms, those synaptic inputs that are more active are more likely to retain postsynaptic territory while less active inputs are eliminated [13–16].
Using the postnatal mouse NMJ, a live imaging and serial electron microscopy (EM) revealed a role for perisynaptic Schwann cells, glial cells known to ensheath NMJ synapses, in motor axon pruning [17]. This study demonstrated that motor axons destined for elimination form large retraction bulbs containing degenerating presynaptic material, which shed small fragments of presynaptic material termed “axosomes.” During this process, Schwann cells were observed to enwrap retraction bulbs and engulf previously shed axosomes. While intriguing, several questions remain. For example, it was unclear whether Schwann cells were performing an active (i.e., initiating or facilitating retraction and pruning of axons) and/or passive (i.e., cleaning up debris) role during the pruning process (Figure 3). In addition, the molecular mechanisms underlying Schwann cell-presynaptic input interactions were unknown. Last, it remains unknown if Schwann cells are necessary for developmental pruning of motor axons.
To address whether Schwann cells were playing a more active role in the activity-dependent elimination of extranumerary presynaptic inputs at the mammalian NMJ, recent work used simultaneous calcium imaging in perisynaptic Schwann cells and recording from dually innervated neuromuscular junctions in the developing mouse [18]. The authors found that purinergic receptor-mediated intracellular calcium fluxes in Schwann cells reflected the relative synaptic strength of nerve terminals competing for postsynaptic ‘turf,’ suggesting that Schwann cells are able to detect relative strengths of synapses. However, it remains unknown whether these calcium fluxes influence physical associations (e.g., phagocytosis) between the Schwann cell and retracting presynaptic inputs.
Work in Drosophila has addressed more specific molecular mechanisms underlying activity-dependent glia-axon interactions at the developing NMJ [19]. While relative levels of activity between presynaptic inputs will result in a selective elimination of less active inputs and strengthening of more active inputs, globally blocking neural activity in all inputs will result in a reduced capacity to eliminate synapses and globally increasing activity results in an increased rate of synapse elimination [1,13–15]. Based on this principle, Fuentes-Medel et al. activated motor neurons with Channelrhodopsin in developing larvae and observed an increase in presynaptic debris and unattached presynaptic terminals at the mammalian NMJ [19]. Similar to the study in mice, this presynaptic material was engulfed by glial cells. Furthermore, the authors demonstrated that deficits in glia-specific Draper/dCED-6 phagocytic signaling resulted in a sustained accumulation of presynaptic debris. These data suggest that glia perform an active role in activity-dependent axonal pruning in the PNS by engulfing presynaptic machinery and axonal arbors destined for removal.
Glia and Developmental Synaptic Pruning: Pruning of local synaptic circuits
Recent work has revealed that glia also play a key role in pruning of local, intact synaptic circuits. In contrast to the axonal pruning described above, this more localized synapse elimination is thought to occur in the absence of significant degeneration and involves the elimination of exuberant, intact presynaptic inputs (terminals and small axon branches) and/or postsynaptic dendritic spines (Figure 2). Similar to axonal pruning, activity is known to drive this process [1–4].
An interesting example of phagocytic signaling regulating local synaptic circuitry is in the case of retinal pigmented epithelium (RPE), a glial-like cell, that has been shown to phagocytose photoreceptor outer segments which are chemosensory receptive structures reminiscent of structural, neuronal synapses [20]. Since this work, emerging evidence implicates another phagocytic glial cell, microglia, in activity-dependent pruning of local synaptic circuits throughout the CNS. These cells have long been recognized as resident phagocytes that clear debris and dying cells in the injured and disease brain. However, recent fate mapping studies have demonstrated that microglia, which are mesodermal in origin, enter the CNS during early embryonic development (~E9) [21,22]. Therefore, microglia are in the CNS at the right time to influence a wide range of developmental processes, including synaptic pruning.
Using two-photon in vivo live imaging in the mouse, two landmark studies demonstrated that microglial processes actively survey the extracellular environment (including surrounding synapses) in the healthy, intact brain [23,24]. Following this initial work, more recent live imaging in the anesthetized mouse and electron microscopy studies revealed that microglia were interacting with synaptic elements in their extracellular environment in a manner dependent upon spontaneous and experiencedriven neural activation [25,26].
To address how sensory experience regulates microglia-synapse interactions during development, authors utilized a developmental window of enhanced synaptic remodeling within the juvenile primary visual cortex (V1; i.e., the ‘critical period’) [26]. During this developmental window, microglia were observed by live imaging to more frequently contacted smaller dendritic spines. These smaller spines tended to increase in size upon microglial contact and were often no longer present during later imaging sessions. In addition, to test experience-dependent changes in microglia-synapse interactions, mice were placed in the dark during the V1 ‘critical period’ (P20-P28/P30) followed by re-exposure to light. Using this dark adaptation paradigm, microglia were observed to more frequently contact synaptic elements (i.e., dendritic spines, synaptic boutons, synaptic clefts) in response to changes in visual experience (dark adapted or dark adapted and then re-exposed to light) as compared to light-reared controls. Furthermore, this increase in synaptic contact was accompanied by an increase in phagocytic inclusions that resembled synaptic elements by ultrastructure. These data suggested that microglia may be actively participating in small-scale, activity-dependent pruning through a phagocytic mechanism. However, the functional consequences of these microglia-synapse interactions in developing V1 and the underlying molecular mechanisms are unknown.
Consistent with data from the visual cortex, another study in the postnatal mouse hippocampus has provided evidence that microglia phagocytose synaptic elements [27]. Immunohistochemistry revealed that vesicles within microglial processes contain immunoreactivity for pre and post-synaptic components (SNAP25 and PSD95, respectively). In addition, these authors addressed functional consequences of manipulating microglia function by assessing mice deficient in the fractalkine receptor (Cx3cr1KO). In the context of the healthy CNS, Cx3cr1 is specific to microglia and its ligand, fractalkine (Cx3cl1) is expressed by neurons. Postnatal Cx3cr1KO mice had a transient reduction in microglial density in the postnatal hippocampus that was temporally correlated with increases in structural dendritic spine density and immature synapses (measured by electrophysiology and seizure susceptibility) as compared to wild-type controls. Importantly, as microglial density returned to normal at later postnatal ages in Cx3cr1KO mice, spine density and synapse maturation returned to wild-type levels. While correlative at this point, these data offer a potential functional role for microglia in maturation of hippocampal synapses. It remains to be determined if this delayed synapse maturation is due to a deficit in the physical elimination of synapses or another mechanism. Furthermore, these data offer fractalkine signaling as a potential mechanism by which microglia may be interacting with synapses in the healthy, developing CNS. Given the transient decrease in microglia density, it may be that Cx3cr1 is working as a signal to recruit microglia to synaptic sites. However, because the effects were not sustained into adulthood, these data demonstrate that other molecular mechanisms must be involved.
Increasing evidence implicates other immune-related molecules in developmental synaptic pruning [28,29]. Included in these repertoire of immune molecules are the classical complement cascade components C1q and C3, which have been shown to mediate synapse elimination in the developing mouse visual system [30]. However, until recently, it was unclear precisely how these molecules traditionally associated with peripheral immune system function were mediating synaptic pruning. In the innate immune system, C1q and C3 work as opsins that “tag” unwanted cellular material (e.g. invading bacteria, cellular debris, etc.) for removal by either cell lysis or clearance by professional phagocytes (e.g., macrophages) [31]. It was hypothesized that C1q and/or C3 could be ‘tagging’ synapses for removal in the developing brain followed by elimination by microglia, the resident CNS phagocyte. This hypothesis was recently tested in the developing mouse retinogeniculate system, a classic system for studying synapse elimination in the CNS [32–35]. The retinogeniculate system is comprised of retinal ganglion cells (RGCs) that project to the dorsal lateral geniculate nucleus (dLGN) of the thalamus. During early postnatal development, RGC presynaptic inputs within the dLGN undergo activity-dependent synaptic pruning to achieve the precise connectivity characteristic of the adult system [33,34] (Figure 2). Using this system, high resolution confocal microscopy and EM showed that microglia engulf RGC presynaptic inputs during the peak of early postnatal retinogeniculate pruning in a complement-dependent manner [35]. Specifically, it was demonstrated that mice deficient in complement component C3, which was enriched at synapses, or its receptor complement receptor 3 (CR3), a surface receptor specific to microglia in the context of the healthy CNS, resulted in a ~50% deficit in the ability of microglia to engulf presynaptic inputs. Furthermore, consistent with microglial involvement in activity-dependent synaptic pruning, engulfment was regulated by neural activity. When activity was either pharmacologically blocked (TTX) or increased (forskolin) in one eye, microglia preferentially engulfed presynaptic inputs from the less active eye. Accompanying the engulfment phenotype, CR3 KO and C3 KO mice also had an increase in structural synapses that was sustained into adulthood. These data demonstrate that microglia engulf synaptic elements in the postnatal brain in an activity and complement-dependent manner and disrupting this phagocytic signaling results in sustained pruning deficits.
Several questions arose out of this work. One question was whether the upstream component, C1q, could regulate microglia-mediated engulfment. As a result, very recent data has now demonstrated that mice deficient in C1q and its upstream regulator TGF-beta have deficits in microglia-mediated engulfment of presynaptic inputs [36•]. Intriguing questions remain as to whether complement molecules are regulated by neural activity and whether complement-dependent and/or other phagocytic immune signaling regulates engulfment of synapses in other brain regions. Indeed CR3 and C3 KO mice have ∼50% phenotype in the retinogeniculate system, data which suggests other pathways are involved. Some interesting candidate pathways are fractalkine, which appears to be involved in synapse development in the hippocampus, and MHC class I molecules, which are immune molecules that have previously been shown to regulate synaptic pruning [27••, 37, 38, 39 and 40]. Last, it is unknown whether astrocytes, which also have phagocytic capacity, play a role in this system. Future work is necessary to address these important questions.
Conclusion
It is becoming increasingly clear that glia are active participants in synaptic pruning. Importantly, phagocytic signaling is emerging as a primary mechanism. In the developing Drosophila, glia engulf presynaptic terminals and axonal debris during developmental pruning in a Draper/dCED-6-dependent manner. At the developing mammalian NMJ, Schwann cells perform a similar function by an unknown mechanism. Finally, microglia have emerged as active participants in activity-dependent pruning of local synaptic circuits in the mammalian CNS through, in part, complement-dependent phagocytic signaling.
While these new findings offer significant insight into a cellular mechanism underlying activity-dependent synaptic pruning, several critical questions remain. First, engulfment of synaptic elements by phagocytic glia in the CNS has never been captured in real time. Therefore, it remains unclear whether glia are actively engulfing intact pre and postsynaptic components destined for elimination or rather more passively engulfing debris following cell-autonomous retraction and/or degeneration (Figure 3). Second, the molecular mechanisms underlying glia-mediated phagocytosis of synaptic elements are still relatively unknown. While it is clear in the Drosophila that the Draper pathway is involved, it still remains to be determined whether the homologous pathway is involved in pruning in the mammalian system. In addition, it is unclear whether complement-dependent phagocytosis or other, yet to be identified phagocytic pathways, are involved in pruning throughout the developing nervous system. Third, while disrupting microglial phagocytic function (CR3 and C3 KOs) results in a sustained deficit in synaptic pruning in the dLGN and a transient decrease in microglia density (Cx3cr1KO) results in a temporally correlated delay in synapse maturation in the hippocampus, the functional consequences of glia-synapse interactions throughout the rest of the CNS and PNS are unknown. Last, while very early evidence suggests that astrocytes may be engulfing axonal debris during large-scale pruning of CNS callosal projections, it remains to be determined if these cells are active contributors to pruning throughout the CNS. Given astrocytes express many phagocytic receptors [41], these glial cells are a highly provocative candidate to work along with microglia to remove excess synapses by phagocytosis.
Answers to the questions outlined above will offer significant insight into the basic biological mechanisms underlying activity-dependent synaptic pruning in the developing nervous system as well as synaptic plasticity associated with mature nervous system function (e.g., learning and memory). Furthermore, synapse loss and glial cell activation have been linked to several diseases ranging from neuropsychiatric disorders to neurodegenerative disease [28,31,42–50]. Thus, elucidating mechanisms underlying developmental synaptic pruning in the healthy nervous system will most certainly have far-reaching implications.
Glia play actives roles in activity-dependent synaptic pruning.
Phagocytosis is a common mechanism by which glia eliminate developing synapses.
Schwann cells are involved in axonal pruning at the developing mammalian NMJ.
Drosophila glial cells are involved in axonal pruning at the NMJ and mushroom body.
Microglia play key roles in local synaptic circuit pruning in the mammalian CNS.
Acknowledgements
This work was supported by grants from the Smith Family Foundation (B.S.), Dana Foundation (B.S.), John Merck Scholars Program (B.S.), National Institute of Neurological Disorders and Stroke (RO1-NS-07100801; B.S.), Ruth L. Kirschstein National Research Service Award, National Institute of Neurological Disorders and Stroke (F32-NS-066698; D.P.S.), Nancy Lurie Marks Foundation (D.P.S.).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annual Review of Neuroscience. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 2.Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- 3.Katz L, Shatz C. Synaptic activity and the constuction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 4.Luo L, O'Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 5.Kano M, Hashimoto K. Synapse elimination in the central nervous system. Curr Opin Neurobiol. 2009;19:154–161. doi: 10.1016/j.conb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 6. Berbel P, Innocenti GM. The development of the corpus callosum in cats: a light- and electron-microscopic study. J Comp Neurol. 1988;276:132–156. doi: 10.1002/cne.902760109. Using light and electron microscopy, the authors provide one of the first pieces of evidence that phagocytic glial cells (astrocytes and microglia) were engulfing xons undergoing pruning in the developing brain.
- 7. Watts RJ, Hoopfer ED, Luo L. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 2003;38:871–885. doi: 10.1016/s0896-6273(03)00295-2. This landmark study in the developing Drosophila mushroom body provided one of the first pieces of definitive evidence that glial cells play an active role in developmental axon pruning. When the authors disrupted glial-specific phagocytic function (shibire mutant), γ axon pruning was significantly impaired.
- 8. Awasaki T, Ito K. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol. 2004;14:668–677. doi: 10.1016/j.cub.2004.04.001. This elegant work in the developing Drosophila mushroom body provided one of the first pieces of definitive evidence that glial cells participate in axonal pruning by accumulating in the mushroom body lobes and engulfing γ axons prior to detectable axon fragmentation.
- 9.Logan MA, Freeman MR. The scoop on the fly brain: glial engulfment functions in Drosophila. Neuron Glia Biol. 2007;3:63–74. doi: 10.1017/S1740925X07000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, Ito K. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50:855–867. doi: 10.1016/j.neuron.2006.04.027. The authors identify Draper and the downstream signaling molecule dCed-6 as an underlying molecular mechanism mediating the clearance of pruning γ axons by phagocytic glial cells in the developing Drosophila mushroom body.
- 11. Hoopfer ED, McLaughlin T, Watts RJ, Schuldiner O, O'Leary DD, Luo L. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 2006;50:883–895. doi: 10.1016/j.neuron.2006.05.013. This paper demonstrates in the Drosophila mushroom body that, unlike axon degeneration following axotomy, axon degeneration during developmental pruning is independent of the Wlds pathway. In contrast, the authors show that the developmental and injury-induced axon pruning converge on a common mechanism of glial-mediated clearance of axons via the phagocytic receptor Draper.
- 12.Wyatt RM, Balice-Gordon RJ. Activity-dependent elimination of neuromuscular synapses. J Neurocytol. 2003;32:777–794. doi: 10.1023/B:NEUR.0000020623.62043.33. [DOI] [PubMed] [Google Scholar]
- 13.Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372:519–524. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- 14.Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- 15.Busetto G, Buffelli M, Tognana E, Bellico F, Cangiano A. Hebbian mechanisms revealed by electrical stimulation at developing rat neuromuscular junctions. J Neurosci. 2000;20:685–695. doi: 10.1523/JNEUROSCI.20-02-00685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stellwagen D, Shatz C. An Instructive Role for Retinal Waves in the Development of Retinogeniculate Connectivity. Neuron. 2002;33:357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- 17. Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44:651–661. doi: 10.1016/j.neuron.2004.10.026. In this paper at the developing NMJ, the authors were the first to definitively demonstrate by in vivo live imaging and serial EM that glial cells (Schwann cells) in the mammalian system participate in synaptic pruning by enwrapping and engulfing retracting presynaptic motor neuron inputs.
- 18. Darabid H, Arbour D, Robitaille R. Glial cells decipher synaptic competition at the mammalian neuromuscular junction. J Neurosci. 2013;33:1297–1313. doi: 10.1523/JNEUROSCI.2935-12.2013. The authors show by calcium imaging that Schwann cells can distinguish less active from more active presynaptic inputs undergoing pruning at the developing mammalian NMJ.
- 19. Fuentes-Medel Y, Logan MA, Ashley J, Ataman B, Budnik V, Freeman MR. Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biol. 2009;7:e1000184. doi: 10.1371/journal.pbio.1000184. This elegant work demonstrates that phagocytic glial cells at the developing Drosophila NMJ participate in activity-dependent synaptic pruning. Remarkably, disruptions in Draper/dCed-6-mediated phagocytosis in glia resulting in a sustained accumulation of presynaptic material.
- 20.Shaham S. Chemosensory organs as models of neuronal synapses. Nat Rev Neurosci. 2010;11:212–217. doi: 10.1038/nrn2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Holscher C, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 22.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 24.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 25.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. The authors demonstrate by 2-photon live imaging and serial EM that microglia contact synapses in the juvenile mouse visual cortex in a manner dependent upon visual experience. Increased phagocytic inclusions that resembled synaptic components were also observed within the microglia in response to changes in visual experience.
- 27. Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. This was one of the first papers to demonstrate that microglia affect the maturation of synaptic circuits and phagocytose synaptic components in the developing mouse hippocampus.During this developmental window, genetically disrupting microglia function (Cx3cr1KO) resulted in a transient reduction in microglial numbers and a concomittent, transient increase in hippocampal spine density and delay in synapse maturation.
- 28.Schafer DP, Stevens B. Synapse elimination during development and disease: immune molecules take centre stage. Biochem Soc Trans. 2010;38:476–481. doi: 10.1042/BST0380476. [DOI] [PubMed] [Google Scholar]
- 29.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 31.Alexander A, Barres B, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annual Review of Neuroscience. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 32.Hong YK, Chen C. Wiring and rewiring of the retinogeniculate synapse. Curr Opin Neurobiol. 2011;21:228–237. doi: 10.1016/j.conb.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huberman AD. Mechanisms of eye-specific visual circuit development. Curr Opin Neurobiol. 2007;17:73–80. doi: 10.1016/j.conb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. Using the postnatal mouse retinogeniculate system, this was the first paper to show that microglia specifically engulf presynaptic terminals undergoing synaptic pruning in a manner dependent upon spontaneous activity and C3/CR3 phagocytic signaling. Genetic or pharmacological disruptions in microglia-mediated phagocytosis resulted in a sustained deficit the pruning of local synaptic circuits.
- 36. Bialis AR, Stevens B. TGF-β Signaling Regulates Complement C1q Expression and Developmental Synaptic Refinement. Nat Neurosci. 2013 doi: 10.1038/nn.3560. in press. Using the postnatal mouse retinogeniculate system, this paper demonstrates that C1q and TGF-beta regulate microglia-mediated engulfment and pruning of developing synapses.
- 37.Datwani A, McConnell MJ, Kanold PO, Micheva KD, Busse B, Shamloo M, Smith SJ, Shatz CJ. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci U S A. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arnold SE. Neurodevelopmental abnormalities in schizophrenia: insights from neuropathology. Dev Psychopathol. 1999;11:439–456. doi: 10.1017/s095457949900214x. [DOI] [PubMed] [Google Scholar]
- 45.Waites CL, Garner CC. Presynaptic function in health and disease. Trends Neurosci. 2011;34:326–337. doi: 10.1016/j.tins.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Woo TU, Crowell AL. Targeting synapses and myelin in the prevention of schizophrenia. Schizophr Res. 2005;73:193–207. doi: 10.1016/j.schres.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 47.Melom JE, Littleton JT. Synapse development in health and disease. Curr Opin Genet Dev. 2011;21:256–261. doi: 10.1016/j.gde.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- 49.Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Derecki NC, Cronk JC, Kipnis J. The role of microglia in brain maintenance: implications for Rett syndrome. Trends Immunol. 2013;34:144–150. doi: 10.1016/j.it.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]