Abstract
Early B cell factor (EBF) is a transcription factor essential for specification and commitment to the B cell fate. In this study, we show downregulation of a developmentally regulated cluster of hoxa genes, notably hoxa9, coincides with induction of EBF at the Pro-B cell stage of B cell differentiation. Analysis of the hematopoietic progenitor compartment in Hoxa9−/− mice revealed significantly reduced frequencies and expression levels of Flt3, a cytokine receptor important for lymphoid priming and the generation of B cell precursors (BCPs). We show that Hoxa9 directly regulates the flt3 gene. Chromatin immunoprecipitation analysis revealed binding of Hoxa9 to the flt3 promoter in a lymphoid progenitor cell line. Knockdown of Hoxa9 significantly reduced Flt3 transcription and expression. Conversely, forced expression of Hoxa9 increased Flt3 transcription and expression in a Pro-B cell line that expressed low levels of Flt3. Hoxa9 inversely correlated with ebf1 in ex vivo-isolated bone marrow progenitors and BCPs, suggesting that EBF might function to silence a Hoxa9 transcriptional program. Restoration of EBF function in an EBF−/− cell line induced B lineage gene expression but did not directly suppress hoxa9 transcription, revealing alternate mechanisms of Hoxa9 regulation in BCPs. These data provide new insight into Hoxa9 function and regulation during lymphoid and B cell development. Furthermore, they suggest that failure to upregulate Flt3 provides a molecular basis for the lymphoid/early B cell deficiencies in Hoxa9−/− mice.
The generation of B cell precursors from hematopoietic stem cells (HSCs) is orchestrated through complex genetic networks that function to instruct lymphoid lineage and B cell fate specification, commitment, and differentiation into naive B cells. Much progress has been made in the identification of regulatory proteins that play key roles in these networks (1). However, a comprehensive understanding of the genetic circuits and their components that direct the generation of B cell precursors from hematopoietic stem cells is far from complete.
Immunophenotypic fractionation and functional analysis studies have identified various developmental intermediates between HSCs andcommitted Pro-Bcells.Well-characterized subsets include long-term repopulating cells (lineage negative/low [Lin−] c-kithi Sca-1+ [LSK+] CD34−CD27−Flt3−), short-term repopulating cells (LSK+CD34+FLT3−), multipotential progenitors (MPPs; LSK+ CD27+CD34+Flt3+), lymphoid-biased MPPs (LMPPs; LSK+CD27+ Flt3+hiVCAM1−), common lymphoid progenitors (CLPs; Lin−c-kitlo Sca-1+IL-7R+), Pre–Pro-B cells (CD45R/B220+CD43+Flt3+ CD19−), and Pro-B cells (CD45R/B220+CD43+ CD19+) (2). Flt3+hi LMPPs are the earliest subset to evidence lymphoid priming (3, 4). Expression of the IL-7R is concomitant with transition to the CLP stage wherein IL-7 signaling promotes B cell fate specification by upregulating the B cell fate specification factor early B cell factor (EBF) (5, 6).
The transcription factors E2A, EBF, and Pax5 play sequential, synergistic, and nonredundant roles in orchestrating lymphoid lineage and B cell differentiation. E2A is required for generation of LMPPs and regulates a subset of genes expressed in this population, including igh, rag1, tdt, and ebf (7–9). EBF and E2A synergize to induce the early program of B lineage gene expression, including the B lineage commitment factor Pax5 (10). Together, these factors coordinate critical early B lineage differentiation events and restrict alternative developmental programs. The importance of E2A, EBF, and Pax5 in regulation of B lymphopoiesis is underscored by the retention of developmental plasticity in cell lines derived from mice deficient in any of these B lineage regulators (11–13). Currently, a comprehensive understanding of the genetic networks these factors regulate that facilitate B cell fate specification and commitment is far from complete.
Cell lines derived from gene-targeted mice are valuable tools for the identification and characterization of genetic circuits that regulate cellular differentiation pathways (11–13). Importantly, they circumvent limitations imposed by molecular manipulation of rare populations and the developmental heterogeneity inherent to ex vivo isolated immunophenotypically defined subsets. Long-term expanded EBF−/− and Pax5−/− cell lines exhibit considerable developmental plasticity and retain many molecular and cellular features of their in vivo counterparts (12–14). The goal of this study was to identify novel genetic events that accompany B cell fate specification through comparative analysis of EBF−/− and Pax5−/− cell lines. We found differences in expression of a progenitor-associated gene program that tracked with expression of Hoxa9. Analysis of hematopoietic progenitors from Hoxa9−/− mice revealed significant reductions in surface expression, as well as frequencies and numbers, of Flt3+ cells. This observation prompted us to investigate if Hoxa9 regulates Flt3. Indeed, we determined Hoxa9 regulation of flt3 is direct. Chromatin immunoprecipitation (ChIP) analysis revealed binding of Hoxa9 to the flt3 promoter in vivo, and data obtained from knockdown and ectopic expression studies revealed that modulation of Hoxa9 levels altered Flt3 transcription and expression. Although EBF and Hoxa9 inversely correlate during B cell differentiation, EBF does not directly regulate hoxa9 transcription. These data provide new information regarding the role of Hoxa9 in regulation of lymphopoiesis and B cell development and address the role of EBF in silencing a Hoxa9-driven progenitor program.
Materials and Methods
Mice
C57Bl6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Hoxa9−/− and Hoxa7−/− have been described (15, 16). Animals were bred and maintained at the Mayo Clinic animal facility and analyzed at 6–10 wk of age. All experiments were carried out in accordance with Mayo Clinic Institutional Animal Care and Use Committee guidelines.
Cell lines
EBF−/−, Pax5−/−, RAG2−/−, and E2A−/− cell lines have been described (11, 13, 17). These cell lines were maintained on irradiated OP42 stromal cells in previously defined culture medium containing recombinant human Flt3-ligand (10 ng/ml) and recombinant murine IL-7 (10 ng/ml) (EBF−/− and E2A−/−), IL-7 alone (5 ng/ml) (RAG2−/−), or 10 ng/ml of IL-7 and 5 ng/ml Flt3-ligand (Pax5−/−) (18). All cytokines were purchased from PeproTech (Rocky Hill, NJ).
Flow cytometry and isolation of hematopoietic progenitor populations
Methods for flow cytometry and progenitor isolation have been described (19, 20). Flow cytometric analysis was performed on the FACSCalibur or Canto flow cytometers (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Tree Star, Ashland, OR). Abs used included: c-kit, CD27, CD34, CD45R/B220, Flt3, IL-7R, Sca-1, AA4.1, CD19, IgM, CD150, CD48, and Lineage mixture (CD45R/B220, Mac-1, Gr-1, CD3e, and Ter119) (eBioscience, San Diego, CA, or BD Pharmingen, San Diego, CA). Expression of biotin-labeled Abs was revealed with Streptavidin-PerCP (BD Pharmingen) or eFluor Streptavidin-780 (eBioscience). Bone marrow (BM) cells were blocked with normal rat serum for 10 min at room temperature preaddition of primary Abs. For isolation of HSCs, MPPs, myeloid precursors, and CLPs, BM cells were harvested and depleted of Lin− cells using magnetic bead depletion, followed by sorting on the FACSAria (BD Biosciences) using the following combinations of Abs: HSCs: Lin+PeCy7, c-kit APC, CD27 PE, Sca-1 PeCy5.5 (Lin−c-kithi Sca-1+CD27−); MPPs: Lin+PeCy7, c-kit APC, Sca-1 PeCy5.5, CD27 PE (Lin− c-kithi Sca-1+CD27+); myeloid precursors: Lin+PeCy7, c-kit APC, Sca-1 PeCy5.5 (Lin−c-kithi Sca-1−); and CLPs: Lin+PeCy7, c-kit APC, Sca-1 PeCy5.5, IL-7R PE (Lin−c-kitlo Sca-1+IL-7R+). B cell precursor (BCP) populations were sorted after magnetic bead depletion of ACK-lysed BM cells using Abs to Ter119, Gr-1, Mac-1, and CD3e, followed by staining with Abs to B220, CD43, and IgM. Pro-B cells were sorted as B220+CD43+IgM− and Pre-B cells as B220+CD43−IgM−. Flow cytometric analysis of hematopoietic progenitor and BCPs was performed as described (19, 21).
Quantitative PCR
RNA was extracted using RNA isolation kits (Qiagen, Valencia, CA, or Stratagene, La Jolla, CA) and treated with DNAse I (Invitrogen, Carlsbad, CA) to eliminate genomic DNA. Total RNA was reverse transcribed with random hexamers and Superscript III reverse transcriptase (Invitrogen). Real-time RT-PCR was performed using the Mx3005 system (Stratagene). The RT-PCR reactions consisted of first-strand cDNA, gene-specific primers, Rox reference dye, and Brilliant SYBR Green 2× reagent (Stratagene). PCR reactions were performed in triplicate. The primers used were: tcfe2a (F) 5′-CCAGTCTTTTGCATAACCAT-3′ and (R) 5′-AGGTCCTTCTTGTCCTCTTC-3′; pax5 (F) 5′-TCCTACCCTATTGTCACAGG-3′ and (R) 5′-GAATACTGAGGGTGGCTGTA-3′; rag1 (F) 5′-CTGCAGACATTCTAGCACTC-3′ and (R) 5′-AACTGAAGCTCAGGGTAGAC-3′; b29 (F) 5′-AAGGAGTTCTCTGGGGATAG-3′ and (R) 5′-AACCATGGTCCTCCTAGACT-3′; mb-1 (F) 5′-TATTAAAACGCTCCTGTGGT-3′ and (R) 5′-AGGCCCTCATAGAGATTTTC-3′; vpreb (F) 5′-GGCCTATCTCACAGGTTGT-3′ and (R) 5′-GGAAGAAGATGCTAATGGTG-3′; flt3 (F) 5′-CATCCAAGACAACATCTCCT-3′ and (R) 5′-CCCTGAAGTCAACGTAGAAG-3′; cd27 (F) 5′-TCTCTCCAGACTACCACACC-3′ and (R) 5′-CACACTCTGTACATTCCTGGT-3′; and cd34 (F) 5′-AAGGGAGAAATCAAATGCTCT-3′ and (R) 5′-CCTCCTCCTTTTCACACAGTA-3′. Sequences of the remaining primers used in this study have been published (20, 22–25). Relative mRNA expression levels were normalized to gapdh using the 2−ΔΔCT method.
RNA purification and microarray analysis
RNA was prepared from three independent flasks of RAG2−/−, Pax5−/−, and EBF−/− cells and integrity determined by Agilent testing (Palo Alto, CA). Total RNA was converted to cDNA and double-stranded cDNA purified by phase-lock gel with phenol-chloroform extraction. Purified cDNA was in vitro transcribed into biotinylated cRNA using the Affymetrix RNA transcript-labeling reagent (Affymetrix, Santa Clara, CA). Labeled cRNAs were fragmented and hybridized to individual Affymetrix Mouse Genome 430 2.0 Arrays and scanned using the Affymetrix GeneChip Scanner 3000 (Affymetrix). Robust multiarray coupled with the GC content of the probes was performed to normalize the data and generate background adjusted intensity values. ANOVA was performed on the normalized data from robust multiarray coupled with the GC content of the probes to identify statistically significant differentially expressed genes (parametric test, variances not assumed equal by Welch t test, p value cutoff 0.05). The microarray data were deposited in the Gene Expression Omnibus database under accession number GSE16002 (http://www.ncbi.nlm.nih.gov/geo) and the analysis used in the study provided in Supplemental Tables I and II.
ChIP assays
ChIP analysis was performed using the EZ-ChIP kit (Millipore, Bedford, MA) according to the manufacturer’s instructions. Briefly, 2 × 107 EBF−/− or RAG2−/− cells were collected and subjected to formaldehyde cross-linking followed by incubation with 0.125 M glycine to stop the reaction. The cell pellet was resuspended in lysis buffer and sonicated to shear DNA (400 1-kb fragments). Abs for ChIP (used at 1 µg/2 × 106 cell equivalents) included anti-HoxA9 (Upstate Biotechnology, Lake Placid, NY), anti-Meis1 (Abcam, Cambridge, MA), anti-Pbx1 (Cell Signaling Technology, Beverly, MA), rabbit IgG, anti-PU.1, and anti-Runx1 (all from Santa Cruz Biotechnology, Santa Cruz, CA). The ChIP primers used to amplify the Flt3 promoter or IL-7RS2 have been described (26, 27).
Retroviral transductions
Individual MISSION lentiviral short hairpin RNA (shRNA) (Sigma-Aldrich, St. Louis, MO) constructs encoding shRNA’s targeting Hoxa9 were cotransfected with the ViraPower packing mix (Sigma-Aldrich) into 293T cells per the manufacturer’s instructions to generate viral supernatants. Viral supernatants were diluted 1:3 and incubated overnight with EBF−/− cells in the presence of 8 µg polybrene. After overnight culture, viral supernatants were removed and the cells given fresh media and cytokines. Twenty-four hours later, puromycin selection was initiated (2 µg/ ml). The cultures were maintained for 10–14 d under puromycin selection, then harvested and processed for RNA and flow cytometric analysis. To determine if ectopic expression of Hoxa9 altered Flt3 transcription and expression, we transduced a Pax5−/− cell line expressing low levels of Flt3 with retroviral constructs encoding an empty vector (MigR1-GFP) or encoding a full-length Hoxa9 cDNA (Mig-Hoxa9-GFP, purchased from Addgene, Cambridge, MA). Retroviral supernatants obtained from Plat-E cells transfected with MigR1-GFP or Mig-HoxA9-GFP plasmid DNA were used to transduce Pax5−/− cells. Pax5−/− cells expressing MigR1-GFP or Hoxa9-GFP were isolated based on GFP expression by cell sorting and maintained as cell lines under identical conditions as nontransduced Pax5−/− cells. An EBF−/− cell line stably expressing a tamoxifen-inducible EBF–estrogen receptor (ER) fusion construct was established by retroviral transduction of an EBF−/− line with EBF–ER-GFP–containing viral supernatants as previously described and transduced cells sorted based on GFP expression (20, 28). EBF activity was induced in the EBF−/−–EBF–ER line by addition of 4-hydroxytamoxifen (4-HT) (Sigma-Aldrich) or diluent (95% ethanol). Cells were harvested at 24–72 h posttreatment and processed for molecular and cellular analysis.
Statistical analysis
Statistics were done using the Student t test.
Results
Comparative analysis of EBF−/− and Pax5−/− cell lines
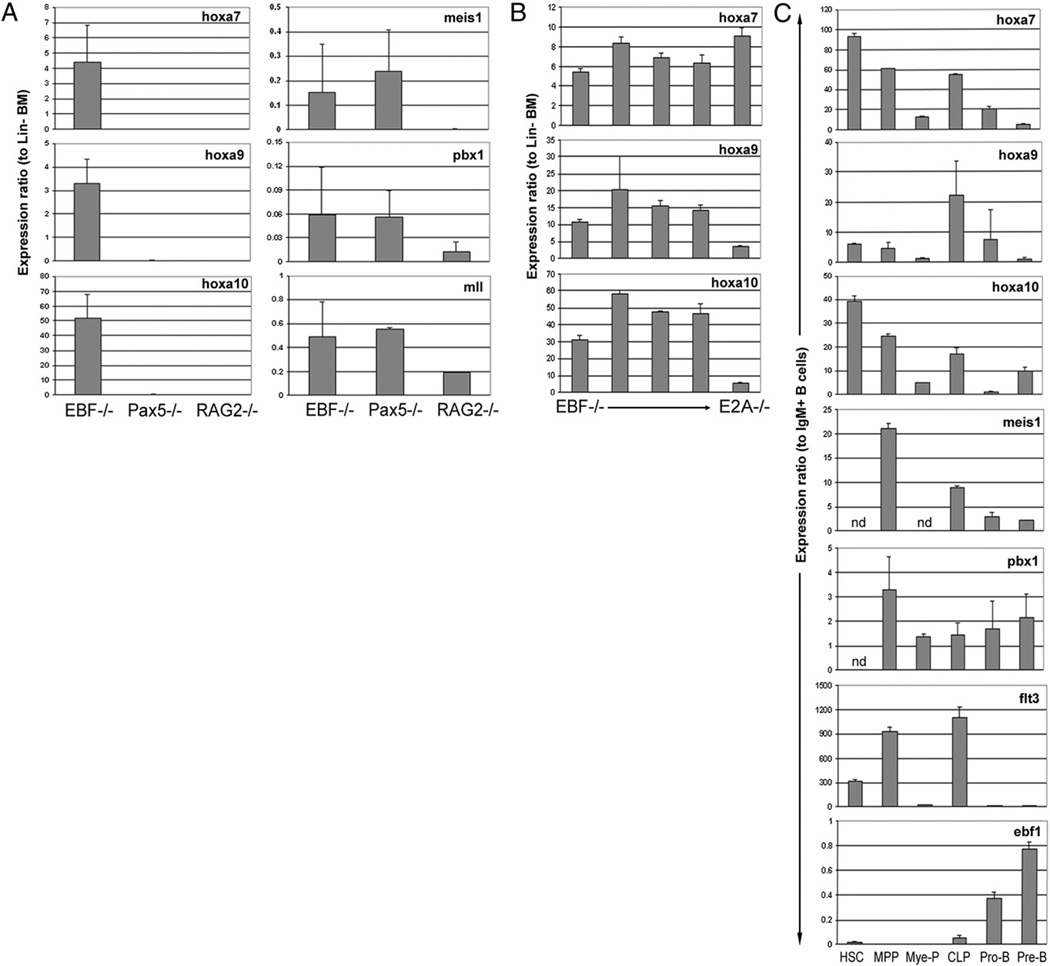
EBF is required for the early program of B lineage gene expression, including the B lineage commitment factor Pax5 (20). We previously showed that forced expression of Pax5 cannot bypass the requirement for EBF in B cell development, suggesting Pax5-independent roles for EBF (20). To identify novel events that accompany EBF expression and B cell fate specification, we performed a cellular and molecular comparison of EBF−/−, Pax5−/−, and RAG2−/− cell lines. First, we compared the expression patterns of the three essential B lineage transcription factors in the cell lines (Fig. 1A). All three lines cells showed relatively equivalent levels of E2A (tcfe2a) transcripts. Ebf1 transcripts are expressed in Pax5−/− cells. Consistent with the critical role of EBF in regulation of the pax5 gene and our previous findings, pax5 transcripts were very low to undetectable in EBF−/− cells (20). To determine if the differential expression of EBF and Pax5 in the EBF−/− and Pax5−/− cell lines resulted in quantitative differences in B lineage gene expression, we compared transcripts for several early lymphoid/B lineage genes, including rag-1, b29, vpreB1, and cd79a (mb-1) (Fig. 1A). All were significantly reduced or undetectable in EBF−/− cells compared with Pax5−/− cells, consistent with previous findings (20). Flt3 transcripts were high in EBF−/− cells, low in Pax5−/− cells, and below the level of detection in RAG2−/− cells. The varying levels of flt3 transcripts correlated well with surface expression of Flt3 in the cell lines (Fig. 1B).
FIGURE 1.
Comparative analysis of EBF−/−, Pax5−/−, and RAG2−/− cell lines. A, Real-time RT-PCR analysis of transcript abundance. All data were normalized to gapdh, and the bar graphs represent the mean and SE of data pooled from two to three independent analyses per cell line per transcript. B, Cell-surface phenotype of EBF−/−, Pax5−/−, and RAG2−/− cell lines. The filled histograms represent RAG2−/− cells, the open histograms Pax5−/− cells, and the dashed lines EBF−/− lines. The dotted line represents the fluorescent pattern of unstained cells. The data are representative of .3 analyses per cell line. C, Flow cytometric analysis of CD27 and CD34 expression on CLPs. CLPs were gated as Lin−IL-7R+c-kitloAA4.1+. D, Flow cytometric analysis of CD27 and CD34 during B cell differentiation. The contour plot on the left depicts the two major gated populations, B220+CD43+ and B220+CD43−. The contour plots on the right depict the expression patterns of CD34 or CD27 on either B220+CD43+ or B220+CD43− subsets (indicated by the label above the plots). Data are representative of three independent BM analyses and depict the staining patterns of 6-wk-old C57Bl6 mice.
Comparative analysis of the cell lines revealed significant differences in gene expression. Therefore, we hypothesized that there may be additional differences between the lines that could be informative with regard to differentiation-related events that accompany B cell fate specification. Lymphoid progenitors are enriched within hematopoietic progenitor subsets that express CD34 and CD27 (22, 29). Therefore, we compared surface expression of these markers on the cell lines. Flow cytometric analysis revealed that the EBF−/− cells expressed high levels of CD34, CD27, and Flt3, compared with Pax5−/− or RAG2−/− cells (Fig. 1B). All three lines expressed the lymphoid lineage markers AA4.1 and the IL-7R (Fig. 1B). Only the RAG2−/− cell line expressed the B lineage-restricted Ag CD19. Long-term expanded cell lines may not exactly align with their in vivo developmental counterparts. Therefore, we evaluated expression of the markers differentially expressed on EBF−/− cell lines in BM CLPs through mature B cells. In particular, we focused on CD34 and CD27, because regulated expression of Flt3 as a function of B cell differentiation has been reported by others (30). CLPs are enriched in the Lin−c-kitlo IL-7R+ fraction of BM (Fig. 1C) (31). Approximately 50% of Lin−c-kitloIL-7R+ defined CLPs express the lymphoid lineage-associated marker AA4.1, and the majority of Lin− c-kitloIL-7R+AA4.1+ coexpress CD34 or CD27 (Fig. 1C) (32). Next, we examined the expression patterns of CD34 and CD27 on BCPs. Pre–Pro-B and Pro-B cells were gated as B220+CD43+ and Pre-B/sIg+ cells as B220+CD43− (Fig. 1D, boxed regions). The top right panels in Fig. 1D represent the staining pattern of CD34 and CD27 within the Pre–Pro-B/Pro-B–enriched fraction and the bottom panels within the Pre-B/sIg+ subset. Expression of CD34 and CD27 is absent from the vast majority of BCPs. This in vivo analysis is consistent with our cell line data and shows that B cell differentiation is accompanied by downregulation of a cellular program preferentially expressed by primitive hematopoietic progenitors.
Downregulation of HoxA transcription accompanies B cell fate specification
To identify novel genetic events that accompany B cell fate specification, we took a functional genomics approach. The EBF−/− cell line exhibits a block at the lymphoid, but not B lineage-specified stage, the Pax5−/− cell line at the specified Pro-B cell stage, and the RAG2−/− cell line at the committed Pro-B cell stage. RAG2−/− cells represent a homogeneous population of IL-7–dependent Pro-B cells, as opposed to the variable mixture of Pro-B and Pre-B cells present in wild-type Pro-B cultures or ex vivo-isolated BCPs (33). We reasoned that a RAG2−/− transcriptome subtracted functional genomics comparison of EBF−/− and Pax5−/− cell lines would preferentially illuminate molecular events that accompany B cell fate specification. Consistent with our real-time RT-PCR analysis of the cell lines (Fig. 1A), gene expression profiling (GEP) of the cell lines revealed that a subset of genes, including pbef1, lef1, cd79b, pou2af1, and vpreb1, was underexpressed in EBF−/− cells compared with Pax5−/− cells (Table I). Not surprising, two of the differentially expressed genes, cd79b and vpreb1, are established EBF targets (34, 35).
Table I.
EBF−/− cells express a novel transcriptome
| Gene | Fold Change | Description |
|---|---|---|
| Underexpressed in EBF−/− compared with Pax5−/− cell lines | ||
| Pbef1 | 0.414 | Pre-B cell colony enhancing factor 1 |
| Lef1 | 0.0204 | Lymphoid enhancer binding factor 1 |
| CD79b | 0.00726 | B29, Ig b |
| Pou2af1 | 0.00533 | POU domain, class 2, associating factor 1 |
| Vpreb1 | 0.00453 | Pre-B lymphocyte gene 1, surrogate L chain |
| Overexpressed in EBF−/− compared with Pax5−/− cell lines | ||
| Hoxa9 | 1558 | Homeo box A9 |
| cd34 | 623.4 | CD34 Ag |
| Tyrobp | 464.3 | TYRO protein tyrosine kinase binding protein |
| Fcgr2b | 256.4 | FcR, IgG, low-affinity IIb |
| Gpr105 | 184.3 | G protein-coupled receptor 105 |
| Ankrd3 | 177.5 | Ankyrin repeat domain 3, protein amino acid phosphorylation |
| Hoxa10 | 105.8 | Homeo box A10 |
| Ncf1 | 96.69 | Neutrophil cytosolic factor 1 |
| Ramp1 | 88.96 | Receptor (calcitonin) activity modifying protein 1 |
| Il18rap | 81.36 | IL-18R accessory protein |
| Ccl3 | 50.65 | Chemokine (C-C motif) ligand 3 |
| Selenbp1 | 39.08 | Selenium binding protein 1 |
| Hoxa7 | 36.82 | Homeo box A7 |
| Prkar2a | 35.15 | Protein kinase, cAMP dependent regulatory, type II a |
| Cd7 | 30.79 | CD7 Ag, immune response |
| Selenbp2 | 30.31 | Selenium binding protein 2 |
| Foxf1a | 24.79 | Forkhead box F1a |
| Clnk | 18.79 | Cytokine-dependent hematopoietic cell linker |
| Igfbp4 | 17.32 | Insulin-like growth factor binding protein 4 |
| Emr1 | 16.96 | EGF-like module containing, mucin-like, hormone receptor-like sequence 1 |
| Ptpre | 12.38 | Protein tyrosine phosphatase, receptor type, E |
| Abcb10 | 12.2 | ATP-binding cassette, subfamily B (MDR/TAP), member 10 |
| Gpr34 | 11.5 | G protein-coupled receptor 34 |
Data reflect genes expressed >10-fold in EBF−/− but not Pax5−/− cell lines.
Next, we focused on transcripts differentially expressed in EBF−/− cells compared with Pax5−/− cells to distinguish genetic changes that accompany B cell fate specification. Interestingly, transcripts corresponding to a cluster of homeobox genes (hoxa9, hoxa10, and hoxa7) were highly expressed in EBF−/− cells (Table I). The levels of hoxa7, -9, and -10 transcripts in EBF−/− cells were striking (ranging from .37- to .1500-fold over levels expressed in Pax5−/− cells). In addition to hoxa transcripts, EBF−/− cells expressed transcripts for cd34, consistent with the surface expression of CD34 on this cell line (Fig. 1B). Other differentially expressed genes between EBF−/− and Pax5−/− cells included those involved in cell signaling (tyrobp, fcgr2b, ccl3, clnk, igfbp4, gpr105, gpr34), signal transduction (prkar2a, ptpre, selenbp1, selenbp2), transcriptional regulation (foxf1a, ankrd3), and immune responses (cd7).
Differential expression of hoxa7–10 transcripts in EBF−/− cells has not been reported. HoxA transcripts are enriched in primitive hematopoietic progenitors and downregulated during the course of cellular differentiation (16, 36). First, the differential expression of hoxa7, hoxa9, and hoxa10 transcripts was confirmed by real-time RT-PCR (Fig. 2A). Next, we determined if expression of the hoxa7–10 gene cluster was unique to the clonal line used in the GEP platform or a shared feature of ex vivo-expanded EBF−/− cell lines. Hoxa7–10 transcript abundance was evaluated by real-time RT-PCR in three nonclonal EBF−/− cell lines and the clonal line used in the GEP (Fig. 2B). Transcripts corresponding to the three hoxa genes were expressed in all EBF−/− cell lines analyzed. Thus, expression of hoxa transcripts is a shared feature of EBF−/− cell lines. The EBF−/− cell lines are fetal liver, as opposed to BM, derived. Therefore, the differences in expression of HoxA genes could reflect the tissue of origin of the EBF−/− cell lines. However, all three HoxA transcripts were detected in an E2A−/− BM-derived cell line (Fig. 2B) (11). We conclude, therefore, that expression of hoxa transcripts likely reflects the developmental stage represented by EBF−/− cell lines and is not a result of fetal liver derivation.
FIGURE 2.
HoxA gene expression in BCP and BM progenitors. Real-time RT-PCR analysis of transcript abundance corresponding to EBF−/−, Pax5−/−, and RAG2−/− cell lines (A), three parental (nonclonal) EBF−/− cell lines, one clonal (3-1) EBF−/− cell line, and one E2A−/− cell line (B), and sort-purified HSCs through Pre-B cells (C). The cycle threshold values for each transcript were normalized to gapdh. The bars for each transcript depict the mean and SD obtained from pooling normalized cycle threshold values from at least three independent analysis of each cell line or progenitor population. For visualization of changes in transcript abundance as a function of B cell differentiation, the data were plotted as expression ratios relative to Lin− BM (A, B) or IgM+ B cells (C).
Previous studies evaluated hoxa7 and hoxa9 transcripts in select subsets of hematopoietic progenitors (36, 37). However, a comparative quantitative analysis of hoxa7–10 transcripts in HSCs through Pre-B cells has not been performed. In addition, comparative analysis of expression patterns of hoxa versus ebf1 transcription in hematopoietic progenitors and BCPs has not been done and would be informative as to whether these events might be coordinately regulated during B cell differentiation. Real-time RT-PCR revealed high levels of hoxa7 and -10 transcripts in HSCs and diminished expression upon differentiation into the B and myeloid lineages (Fig. 2C). Hoxa9, in contrast, although expressed in HSCs and MPPs, was elevated in CLPs, then dramatically downregulated at the Pro-B cell stage. Interestingly, hoxa9 transcription followed a similar pattern as flt3. A dramatic down-regulation of the hoxa cluster and flt3 coincided with induction of ebf1 transcription. These data confirm and extend previous reports regarding hoxa expression patterns in hematopoietic progenitors and show that downregulation of hoxa transcription accompanies B cell fate specification (16, 36, 37).
Hoxa9−/− hematopoietic progenitors exhibit deficiencies in Flt3
Hoxa9 is important for hematopoiesis and the generation of BCP (15, 16). Hoxa9 has been implicated in regulation of flt3, and Flt3 signaling is critical for the maintenance of lymphoid progenitors from which BCPs are derived (26, 38). To determine if Hoxa9 is essential for expression of Flt3 in vivo, we examined Flt3 expression in the hematopoietic progenitor compartment of Hoxa9−/− mice compared with strain- and aged-matched controls. Analysis of Hoxa7−/− BM was included to control for genomic effects due to gene targeting, as Hoxa7 is a nearby gene. Flt3+ cells are enriched within the Lin− hematopoietic progenitor compartment (39). Previous studies reported that Hoxa9−/− mice had normal numbers and frequencies of HSCs and MPPs (16, 40). The gating strategy used in the previous phenotypic analysis excluded Flt3, precluding analysis of differential expression of Flt3 within the hematopoietic progenitor compartment. Differential expression of Flt3 and CD34 discriminates HSC and MPP subsets (41). Therefore, we also included an Ab to CD34 in the analysis as a further marker of hematopoietic differentiation.
Hematopoietic progenitors are enriched within the Lin− compartment (Fig. 3A, left panels, gated region). LSK+ cells (Fig. 3A, middle panels, boxed region) are a subset of Lin− cells enriched for HSCs and MPPs (Fig. 3A, left panels, gated region). As previously reported, Hoxa9−/− mice have a reduction in numbers of BM nucleated cells (17.1 ± 3.2 × 106 versus 23.2 ± 3.2 × 106, Hoxa9−/− [n = 4] versus control [n = 6], respectively) (15). Also, consistent with previous findings, we did not find any significant differences in frequencies (3.9 ± 0.61% versus 4.3 ± 0.96%, Hoxa9−/− [n = 4] versus control [n = 6], respectively) or numbers of LSK+ cells (4.4 ± 1.7 × 104 versus 3.3 ± 1.2 × 104, Hoxa9−/− versus control, respectively) (40). Next, we evaluated whether Hoxa9 deficiency altered percentages of LSK+ cells that expressed Flt3. As shown in Fig. 3A (flow cytometry, right panels, and summarized in Fig. 3B), the percentages of LSK+ cells expressing Flt3 is dramatically reduced in Hoxa9−/− BM compared with wild-type BM (37.3 ± 4.1% versus 69.2 ± 3.2%, Hoxa9−/− versus wild-type, respectively), as well as the mean fluorescence intensity of Flt3 levels (525 versus 2208, Hoxa9−/− versus wildtype, respectively).
FIGURE 3.
Reduction in Flt3+ hematopoietic progenitors in Hoxa9−/− mice. A, Wild-type and Hoxa9−/− BM cells were analyzed by multiparameter flow cytometry. BM hematopoietic progenitors were first gated as Lin−c-kit+/− cells (left panels). LSK+ cells are a subset of Lin−c-kit+/− cells. The LSK+ gate is shown as a boxed region in the middle panels. LSK+ cells were analyzed for differential expression of Flt3 and CD34 (right panels). B, Mean ± SD of percentages of LSK+Flt3+ cells in wild-type (n = 6) and Hoxa9−/− (n = 4). C, Fractionation of LSK+ cells by differential expression of CD150 and CD48. D, Diminished expression of Flt3 in CLPs. Lin− cells were analyzed for differential expression of c-kit and IL-7R. CLPs are enriched in the Lin−c-kitlo IL-7R+ fraction (left panels, boxed region). Lin−c-kitlo IL-7R+ CLPs were further analyzed based on differential expression of Sca-1 and Flt3 (right panels). E, Mean ± SD of percentages of Flt3+ CLP in wild-type (n = 7) and Hoxa9−/− (n = 5). F, B lineage precursor subsets were analyzed using differential expression of CD45R/B220, CD43, and IgM. The boxed region in the left panels indicates BCP broadly defined as B220+CD43+ cells. The right panels show the percentages of B220+CD43+IgM− subsets identified by differential expression of Flt3 and CD19. The data reflect analyses of four to six mice per genotype. A minimum of one million events within the BM mononuclear cell gate was collected for analysis of LSK+, 750,000 for CLP, and 250,000 for BCP subsets.
CD34 is expressed prior to Flt3 in hematopoietic progenitors (41). The vast majority of LSK+ cells express CD34. Hoxa9 has also been implicated in regulation of cd34 (42, 43). Interestingly, we found a statistically significant reduction in percentages of LSK+ cells expressing CD34 (81.8 ± 3.8% versus 93.9 ± 0.8%, Hoxa9−/− versus control, respectively; p = 0.0008). We note that although there are reductions in percentages of CD34+ cells in the LSK+ compartment, surface expression of CD34 is not compromised by Hoxa9 deficiency, in stark contrast to Flt3.
The expression patterns of Flt3 and CD34 are altered in Hoxa9−/− mice. However, from this analysis, it is difficult to conclude if these alterations indicate a requirement for Hoxa9 in regulation of these genes or reflect a functional requirement for Hoxa9 in HSCs/MPPs that alters the composition of the compartments defined by these markers. To address the latter, we analyzed the HSC/MPP compartments using the SLAM markers CD150 and CD48 (21). As shown in Fig. 3C, percentages of LSK+ HSC/MPP subsets discriminated by differential expression of CD150 and CD48 is unchanged between Hoxa9−/− and wild-type mice (n = 4 mice of each genotype). Because Hoxa9 deficiency does not appear to directly alter the composition of the hematopoietic progenitor compartment, the decreased frequency of CD34+ and Flt3+ cells we found could be due to a molecular requirement for Hoxa9 in regulation of the cd34 and/or flt3 genes. A molecular requirement for Hoxa9 in regulation of flt3 is supported by a statistically significant reduction in numbers of LSK+CD150−CD48+Flt3+ (p = 0.0069), but not LSK+CD150−CD48+Flt3− cells in Hoxa9−/− mice compared with wild-type (Table II). In contrast to flt3, although we observed a statistically significant reduction in percentages of LSK+ CD150+CD48− that express CD34+ (24.5 ± 8.2% versus 63.6 ± 12.3%, Hoxa9−/− versus wild-type, respectively), the absolute numbers of this immunophenotypically defined subset were not significantly reduced by Hoxa9 deficiency (1276 ± 518 versus 881 ± 336, Hoxa9−/− versus wild-type, respectively; p = 0.13). These findings support a more stringent requirement for Hoxa9 in transcriptional regulation of flt3 than cd34.
Table II.
Absolute numbers of hematopoietic progenitor and BCP subsets per long bone
| LSK+CD150− CD48+FLT3− (× 104) |
LSK+CD150− CD48+FLT3+ (× 103) |
LSK+FLT3+ (× 104) |
CLP (× 104) | B220+CD43+ FLT3−CD19− (× 104) |
B220+CD43+ FLT3+CD19− (× 104) |
B220+CD43+ FLT3+CD19+ (× 104) |
B220+CD43+ FLT3−CD19+ (× 104) |
|
|---|---|---|---|---|---|---|---|---|
| Wild-type | 1.6 ± 0.6 | 0.71 ± 0.3 | 2.3 ± 0.8 | 1.4 ± 0.5 | 13.9 ± 4.7 | 38.1 ± 21.5 | 10.9 ± 4.7 | 51.1 ± 17.6 |
| Hoxa9 −/− | 2.9 ± 1.3 | 0.16 ± 0.1* | 1.6 ± 0.7 | 0.4 ± 0.3** | 35.7 ± 6.8*** | 31.5 ± 12.5 | 1.9 ± 1.3**** | 15.5 ± 7.6***** |
CLPs are Lin−c-kitloIL-7R+Sca-1+ Flt3+.
p = 0.0069;
p = 0.0021;
p = 0.0019;
p = 0.01;
p = 0.0099.
Flt3 is critical for the maintenance of lymphoid progenitors from which BCPs are derived (38, 44). Hoxa9−/− mice have reductions in CLPs, similar to mice defective in Flt3-ligand (16, 38). We showed that Hoxa9−/− mice have significant reductions in LSK+ Flt3+ cells. Next, we examined if the defect in Flt3 expression extended into the CLP and B lineage compartments. CLPs are enriched in the Lin−c-kitloIL-7R+ subset (Fig. 3D), and cells expressing this combination of markers were reduced ~2-fold in Hoxa9−/− mice compared with wild-type mice, consistent with previous findings (31). Approximately 30% of Lin−c-kitloIL-7R+ cells express Sca-1 and Flt3 (Fig. 3D). Cell-surface levels of Flt3 did not change appreciably between the LSK+ and CLP compartments in wild-type mice (Fig. 3A, 3D). However, we observed decreased surface expression of Flt3 on Lin−c-kitloIL-7R+ cells from Hoxa9−/− mice as well as decreased percentages of Flt3+ CLPs (Fig. 3D and summarized in Fig. 3E). As Flt3+ CLPs are the primary precursors of BCPs, these data are consistent with the B cell deficiency in Hoxa9−/− animals (32).
In differentiating BCPs, Flt3+ cells are restricted to the Pre–Pro-B and Pro-B subsets in BM (30). Consistent with previous findings, we did not finding a significant reduction in percentages or absolute numbers of B220+CD43+IgM− BCPs (Fig. 3F and data not shown) (16). Pre–Pro-B and Pro-B cells are enriched in the B220+CD43+ IgM− fraction of BM and can be distinguished, in part, by differential expression of CD19 (Fig. 3F). We found a statistically significant increase in percentages and numbers of B220+CD43+Flt3−CD19− cells in Hoxa9−/− mice compared with controls (Fig. 3F, Table II). Further flow cytometric analysis of this population revealed that these cells unanimously expressed the NK marker, NK1.1 and thus were not BCPs (data not shown). Unexpectedly, percentages and numbers of B220+CD43+Flt3+ CD19− Pre–Pro-B cells were not altered in Hoxa9−/− mice (Fig. 3F, Table II) (45). However, both B220+CD43+CD19+Flt3+ and B220+CD43+CD19+Flt3− BCPs were significantly reduced in Hoxa9−/− mice (Fig. 3F, Table II). These data indicate a specific reduction in BCPs acquiring expression of CD19 as a consequence of Hoxa9 deficiency. No significant alterations in LSK+, CLP, or BCP subsets were observed in Hoxa7−/− mice, consistent with previous findings (data not shown) (16). Taken together, these data indicate a crucial role for Hoxa9 in regulation of Flt3 in hematopoiesis.
Hoxa9 directly regulates flt3 in vivo
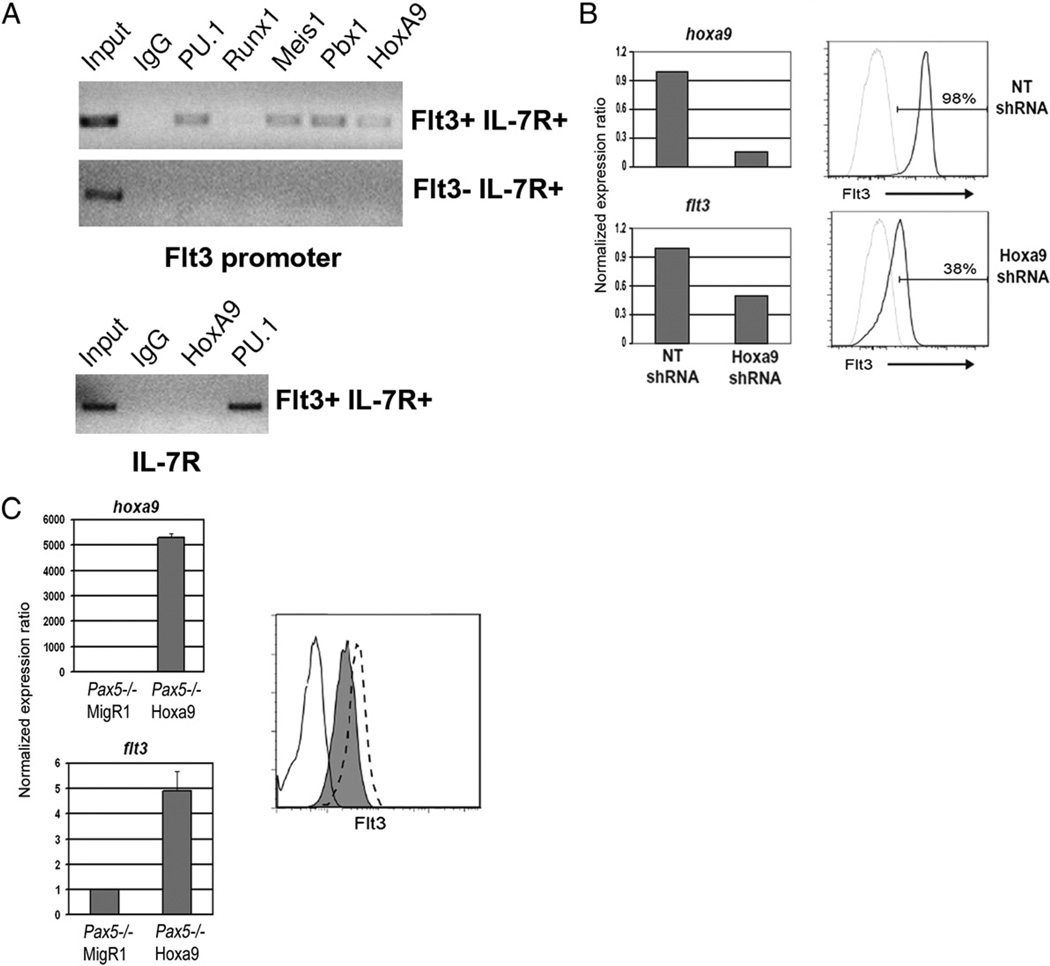
Flow cytometric analysis of Hoxa9−/− mice suggested a role for Hoxa9 in regulation of flt3. A previous study showed Hoxa9 and Meis1 bound to the Flt3 promoter in vivo in myeloid cell lines (26). The high levels of hoxa9 and flt3 transcripts we documented in CLPs (Fig. 2C) suggested that Hoxa9 may similarly regulate flt3 in lymphoid progenitors. EBF−/− cell lines express high levels of Flt3, as well as transcripts corresponding to hoxa9, meis1, and pbx1 (Figs. 1B, 2A). To determine if Hoxa9, alone or in combination with Meis1 and/or Pbx1, directly regulates flt3 in EBF−/− cells, we performed ChIP assays. As shown in Fig. 4A, Hoxa9, Meis1, and Pbx1 are bound to the Flt3 promoter in EBF−/− cells. PU.1, which has also been implicated in regulation of flt3, was also bound to the Flt3 promoter (46). A previous study showed that flt3 transcripts are reduced by AML-1 deficiency (47). However, we did not observe direct binding of AML-1 binding to the flt3 promoter in EBF−/− cells. The binding of Hoxa9, Meis1, and Pbx1 to the Flt3 promoter was specific. We did not observe binding of Hoxa9 to the Flt3 promoter in RAG2−/− cells that do not express Flt3 or binding of Hoxa9 to a regulatory element in the il-7ra locus in EBF−/− cells (Fig. 4A). These data suggest a direct molecular connection between Hoxa9 and Flt3 in vivo.
FIGURE 4.
Requirement for Hoxa9 in regulation of flt3. A, In vivo cross-linking of Hoxa9, Meis1, and Pbx1 to the Flt3 promoter (top panel) in Flt3+IL-7R+ EBF−/− cells but not or Flt3−IL-7R+ RAG2−/− Pro-B cells. PU.1 also regulates Flt3 and was included as a positive control for the assay. The formaldehyde cross-linked chromatin was analyzed using PCR primers that span the murine Flt3 promoter or an irrelevant site in the il-7ra gene. Control rabbit IgG and an Ab to Runx1 were included as controls for nonspecific binding of the Abs. Data are representative of three independent experiments. B, shRNA knockdown of hoxa9 reduces flt3 transcription and expression. EBF−/− cells were transduced with lentiviral supernatants containing either a no-template control (NT shRNA) or Hoxa9-targeted shRNA (Hoxa9 shRNA) vector. Puromycin-resistant cells were harvested and analyzed by real-time RT-PCR for the indicated transcripts or for expression of Flt3 by flow cytometry (the black line represents Flt3 staining, and the gray line indicates unstained cells). Data depict a representative analysis from three independent experiments. C, Ectopic expression of Hoxa9 in Pax5−/− cells upregulates flt3 transcription and cell surface expression. Left panels indicate hoxa9 and flt3 transcript levels in the empty vector (MigR1) or Hoxa9-transduced Pax5−/− cell lines. The right panel indicates surface expression of Flt3 on the MigR1 (gray filled histogram), Hoxa9-transduced (hatched line) versus unstained cells (open histogram). Data are representative of three independent analyses of MigR1 and Mig-Hoxa9–transduced Pax5−/− cells.
Next, we sought to determine if Hoxa9 was required for maintenance of Flt3 expression. Lentiviral supernatants encoding shRNAs specific to Hoxa9 were generated and used to transduce the EBF−/− cell line used in the ChIP assay. EBF−/− transduced cells were selected by puromycin resistance conferred by a puromycin-resistance cassette in the lentiviral vector. Ten to 14 d post-initiation of puromycin selection, the cells were harvested and subject to FACS for evaluation of surface expression of Flt3 and quantitative RT-PCR (qRT-PCR) for analysis of hoxa9 and flt3 transcripts. As shown in Fig. 4B, an shRNA was identified that reproducibly reduced Hoxa9 transcripts to ~20% of normal levels. qRT-PCR revealed reduced flt3 transcripts, and flow cytometry showed a significant reduction in surface expression of Flt3.
Pax5−/− cells lack Hoxa9 transcripts and express low levels of Flt3. The decreased expression of Flt3 could be the result of silencing of Hoxa9. Therefore, we determined if ectopic expression of Hoxa9 in Pax5−/− cells would increase transcription and expression of Flt3. Pax5−/− cells were transduced with the empty vector MigR1-GFP or Mig-Hoxa9-GFP retroviral supernatants. GFP+ cells were isolated by cell sorting and expanded in vitro. qRT-PCR confirmed Hoxa9 transcripts in the Hoxa9-transduced cells (Fig. 4C). Importantly, we documented a 3–5-fold increase in flt3 transcripts in Pax5−/− cells expressing Hoxa9 (Fig. 4C). The increase in flt3 transcripts corresponded to an increase in surface expression of Flt3 (Fig. 4C, right panel, hatched line). These data, combined with the BM analysis of Hoxa9−/− mice, ChIP data, and shRNA knockdown studies, provide compelling molecular evidence that Hoxa9 directly regulates flt3.
EBF does not directly regulate hoxa transcription
Downregulation of HoxA transcription accompanies B cell fate specification. Hoxa7–10 transcription inversely correlates with ebf and is sustained in lymphoid progenitor cell lines deficient in EBF. Together, these data support the hypothesis that EBF might function to limit HoxA expression and/or function in developing BCP. To determine if EBF is a direct negative regulator of hoxa7– 10 transcription, we established an EBF−/− cell line expressing a 4-HT–inducible EBF–ER fusion construct (28). Twenty-four hours post-exposure to 1 µM 4-HT, the cells were harvested and analyzed for induction of EBF target genes, including b29, mb-1, and pax5. As shown in Fig. 5A, EBF induced high levels of expression of all three genes within 24 h of 4-HT induction. Flt3 transcription in BCPs is silenced by Pax5 (30). Although Pax5 transcripts were expressed at high levels in 4-HT–treated EBF−/− cells within 24 h, we did not observe appreciable changes in Flt3 transcription over a 24–48 h time period (Fig. 5A and data not shown). Consistent with the failure to downregulate flt3 transcription, we documented no change in transcript abundance for hoxa7, hoxa9, hoxa10, or meis1 upon induction of EBF. The failure of Pax5 to silence flt3 transcription in EBF−/− cells is in contrast to that previously documented in Pax5−/− cells and likely reflects the primitive developmental state of EBF−/− cells (30). Consistent with that possibility, Flt3 expression was diminished within 72 h of 4-HT treatment (Fig. 5B). In contrast to flt3, we observed downregulation of cd34 and cd27 within 24 h of 4-HT administration and diminished surface expression with 72 h of 4-HT treatment (Fig. 5B). Taken together, these findings suggest that induction of EBF initiates a series of events that culminate in downregulation of a Hoxa9-driven regulatory network.
FIGURE 5.
EBF does not directly inhibit hoxa transcription. A, An EBF−/− cell line stably expressing a tamoxifen-inducible EBF–ER fusion protein were treated with 1 µM 4-HT for 24 h to induced nuclear localization of EBF. After 24 h, the cells were harvested, then analyzed by real-time RT-PCR for transcripts representing established EBF target genes (b29, mb1, pax5), transcripts corresponding to cell surface markers differentially expressed on EBF−/− cells and downregulated as a function of B cell differentiation (flt3, cd34, cd27), or hoxa and meis1 transcripts. The labels indicate Ctr (media control), diluent (95% ethanol), and 4-HT. Data are plotted as the mean ± SD of normalized expression ratios obtained from three independent experiments. B, Downregulation of hematopoietic progenitor markers on EBF−/− cells treated with 4-HT. Three days posttreatment with 4-HT (hatched lines), the EBF−/− cells were harvested and analyzed by flow cytometry for the indicated cell-surface markers. The black line represents EBF−/− cells without 4-HT and the gray filled histogram unstained cells.
Discussion
In this study, we sought to identify novel genetic events that accompany B cell fate specification and investigate the role of EBF in their regulation. Gene expression profiling of EBF−/− and Pax5−/− cell lines revealed downregulation of hoxa transcription accompanies B cell fate specification. Hoxa9 has been implicated in regulation of flt3 (26). We show reduced frequencies and numbers of Flt3+ cells in Hoxa9−/− mice, suggesting that Hoxa9 may be a key component of the regulatory circuitry that regulates the flt3 gene. Using a variety of experimental approaches, we determined that Hoxa9 regulation of flt3 is direct. Experimental manipulation of Hoxa9 levels directly impacted flt3 transcription and expression. Hoxa9 transcription inversely correlated with EBF. However, EBF does not directly suppress hoxa9 or meis1 transcription, suggesting alternate mechanisms of HoxA regulation in BCPs. Taken together, these data provide new insight into the role of Hoxa9 in lymphoid/B cell development and reveal that suppression of a Hoxa9-driven transcriptional program in BCPs is not directly regulated by EBF.
Two different groups have described B lineage defects in Hoxa9−/− mice (15, 16). However, the molecular basis of these deficiencies has not been determined. In this study, we show dramatic deficiencies in frequencies, absolute numbers, and expression levels of Flt3 in Hoxa9−/− hematopoietic progenitors. Flt3 tracks with Hoxa9 in MPPs through the Pre-B stages of B cell differentiation. Hoxa9 is bound to the flt3 promoter in vivo, and knockdown of Hoxa9 reduced flt3 transcription and expression. Conversely, forced expression of Hoxa9 increased flt3 transcription and surface expression in a cell line expressing low levels of Flt3. Taken together, these data provide compelling molecular evidence that Hoxa9 is a critical component of the genetic circuitry that regulates the flt3 gene.
Flt3 is a receptor tyrosine kinase enriched in primitive hematopoietic progenitors, but not murine HSCs (39, 48). Defective expression of Flt3 or deficiencies in Flt3-ligand result in significant reductions in Flt3+ MPPs, CLPs, and BCPs (38, 44). Previous studies demonstrated reductions in CLPs and BCPs in Hoxa9−/− mice, but did not investigate if these reductions correlated with alteration in Flt3 (16). In this study, we show that Hoxa9−/− mice exhibit significant deficiencies in percentages and absolute numbers of Flt3+ cells from the earliest onset of Flt3 expression. Flt3 signaling has been shown to activate the serine-threonine kinase Pim-1 (49). Hoxa9−/− mice are deficient in Flt3 and Pim-1. Hu et al. (50) have shown that the hematopoietic progenitor defect in Hoxa9−/− mice could be restored, in part, by ectopic expression of Pim-1. Alterations in Pim-1 levels have been shown to influence the size of the B lineage progenitor compartment (51). Pim-1 stimulates c-Myb activity (52). c-Myb is required for normal expression of IL-7Rα as well as IL-7 signaling (53). We did not observe diminished surface expression of IL-7Rα in Hoxa9−/− CLPs or Pro-B cells. However, Hoxa9−/− mice have defective responses to hemopoietic cytokines (40). Importantly, Pim-1 is a limiting factor for IL-7 responsiveness (51). IL-7–dependent Flt3-independent proliferation is concomitant with expression of CD19, and we documented selective reductions in CD19+ Pro-B cells. Taken together, our current findings, together with published works by others, suggest that Hoxa9 is a key component of the regulatory circuitry that initiates lymphoid priming through transcriptional activation of flt3. In a subset of wild-type LSK+ cells, Hoxa9, in combination with PU.1, induces expression of Flt3 (46). We suggest that signaling via Flt3 induces Pim-1, which is important for c-Myb activation. c-Myb, in turn, is required for lymphoid progenitors and BCP to respond to IL-7. Thus, failure to upregulate Flt3 to initiate this cascade of events provides a molecular explanation for decreased lymphoid priming and numbers of CLPs and BCPs in Hoxa9 mice.
Dysregulated expression of Hoxa9 or Hoxa10 inhibits B lymphopoiesis in some developmental contexts (54, 55). Forced expression of Hoxa9 expands MPPs but impairs the generation of IL-7 responsive BCPs. Interestingly, the consequence of forced expression of Hoxa9 is strikingly similar to aberrant Flt3 signaling, reinforcing the necessity for strict control of Hoxa9 and Flt3 for B cell differentiation beyond the Pro-B cell stage (30, 54). Holmes et al. (30) have shown that Pax5 silences Flt3 transcription in BCPs. However, regulatory circuits that limit Hoxa9 transcription and/or function during B lymphopoiesis have not been determined. Our observation that EBF deficiency correlates with sustained HoxA expression suggested that EBF might function in silencing HoxA transcription during B cell differentiation. There is experimental precedent for lineage determining factors in silencing HoxA gene expression. GATA-1 has been shown to regulate Hoxa10 during megakaryocyte differentiation and is a primary cell fate determinant for that lineage (56). Gfi-1 directly represses Hoxa9, Pbx1, and Meis1 during granulopoiesis (57). Our gain-of-function data revealed that EBF does not directly suppress hoxa7–10 or meis1 transcription, suggesting alternate mechanisms of regulation in the B lineage. HoxA proteins are also regulated by microRNAs (58). It is possible that EBF indirectly regulates HoxA function by inducing a microRNA that impairs HoxA, Meis1, or Pbx1 functions. Future experiments utilizing genome-wide microRNA expression profiling will determine if EBF limits a HoxA-driven transcriptional program by controlling expression of microRNAs that regulate HoxA proteins.
In summary, this study provides new insight into molecular and cellular events that accompany B cell fate specification. Importantly, they set the foundation for future studies addressing mechanisms of HoxA regulation in BCPs as well as other EBF-regulated events that accompany B cell fate specification.
Supplementary Material
Acknowledgments
We thank L. Grimes for providing the Hoxa9−/− and Hoxa7−/− mice, M. Kondo for the EBF–ER plasmid, Unnikrishnan Gopinathan and the Mayo Advanced Genomics Technology Core Microarray Facility for processing and analysis of the microarray platform, Erin Maetzold and Meibo Chen for expert technical assistance, and Ginny Shapiro for critical review of the manuscript.
This work was supported by the Concern Foundation, the Fraternal Order of Eagles Cancer Research Fund, and Grant RO1HL096108 from the National Heart, Lung, and Blood Institute to K.L.M.
Abbreviations used in this paper
- BCP
B cell precursor
- BM
bone marrow
- ChIP
chromatin immunoprecipitation
- CLP
common lymphoid progenitor
- Ctr
media control
- EBF
early B cell factor
- ER
estrogen receptor
- GEP
gene expression profiling
- HSC
hematopoietic stem cell
- 4-HT
4-hydroxytamoxifen
- Lin−
lineage negative/low
- LMPP
lymphoid-biased multipotential progenitor
- MPP
multipotential progenitor
- NT
no-template control
- qRT-PCR
quantitative RT-PCR
- shRNA
short hairpin RNA
Footnotes
The microarray data presented in this article have been submitted to the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo) under accession number GSE16002.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Medina KL, Singh H. Genetic networks that regulate B lymphopoiesis. Curr. Opin. Hematol. 2005;12:203–209. doi: 10.1097/01.moh.0000160735.67596.a0. [DOI] [PubMed] [Google Scholar]
- 2.Ramírez J, Lukin K, Hagman J. From hematopoietic progenitors to B cells: mechanisms of lineage restriction and commitment. Curr. Opin. Immunol. 2010;22:177–184. doi: 10.1016/j.coi.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai AY, Lin SM, Kondo M. Heterogeneity of Flt3-expressing multipotent progenitors in mouse bone marrow. J. Immunol. 2005;175:5016–5023. doi: 10.4049/jimmunol.175.8.5016. [DOI] [PubMed] [Google Scholar]
- 4.Sitnicka E, Buza-Vidas N, Ahlenius H, Cilio CM, Gekas C, Nygren JM, Månsson R, Cheng M, Jensen CT, Svensson M, et al. Critical role of FLT3 ligand in IL-7 receptor independent T lymphopoiesis and regulation of lymphoid-primed multipotent progenitors. Blood. 2007;110:2955–2964. doi: 10.1182/blood-2006-10-054726. [DOI] [PubMed] [Google Scholar]
- 5.Dias S, Silva H, Jr, Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J. Exp. Med. 2005;201:971–979. doi: 10.1084/jem.20042393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kikuchi K, Lai AY, Hsu CL, Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J. Exp. Med. 2005;201:1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dias S, Månsson R, Gurbuxani S, Sigvardsson M, Kee BL. E2A proteins promote development of lymphoid-primed multipotent progenitors. Immunity. 2008;29:217–227. doi: 10.1016/j.immuni.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romanow WJ, Langerak AW, Goebel P, Wolvers-Tettero IL, van Dongen JJ, Feeney AJ, Murre C. E2A and EBF act in synergy with the V(D)J recombinase to generate a diverse immunoglobulin repertoire in nonlymphoid cells. Mol. Cell. 2000;5:343–353. doi: 10.1016/s1097-2765(00)80429-3. [DOI] [PubMed] [Google Scholar]
- 9.Yang Q, Kardava L, St Leger A, Martincic K, Varnum-Finney B, Bernstein ID, Milcarek C, Borghesi L. E47 controls the developmental integrity and cell cycle quiescence of multipotential hematopoietic progenitors. J. Immunol. 2008;181:5885–5894. doi: 10.4049/jimmunol.181.9.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Riordan M, Grosschedl R. Coordinate regulation of B cell differentiation by the transcription factors EBF and E2A. Immunity. 1999;11:21–31. doi: 10.1016/s1074-7613(00)80078-3. [DOI] [PubMed] [Google Scholar]
- 11.Ikawa T, Kawamoto H, Wright LY, Murre C. Long-termculturedE2Adeficient hematopoietic progenitor cells are pluripotent. Immunity. 2004;20:349–360. doi: 10.1016/s1074-7613(04)00049-4. [DOI] [PubMed] [Google Scholar]
- 12.Nutt SL, Urbánek P, Rolink A, Busslinger M. Essential functions of Pax5 (BSAP) in pro-B cell development: difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgH locus. Genes Dev. 1997;11:476–491. doi: 10.1101/gad.11.4.476. [DOI] [PubMed] [Google Scholar]
- 13.Pongubala JM, Northrup DL, Lancki DW, Medina KL, Treiber T, Bertolino E, Thomas M, Grosschedl R, Allman D, Singh H. Transcription factor EBF restricts alternative lineage options and promotes B cell fate commitment independently of Pax5. Nat. Immunol. 2008;9:203–215. doi: 10.1038/ni1555. [DOI] [PubMed] [Google Scholar]
- 14.Bertolino E, Reddy K, Medina KL, Parganas E, Ihle J, Singh H. Regulation of interleukin 7-dependent immunoglobulin heavy-chain variable gene rearrangements by transcription factor STAT5. Nat. Immunol. 2005;6:836–843. doi: 10.1038/ni1226. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence HJ, Helgason CD, Sauvageau G, Fong S, Izon DJ, Humphries RK, Largman C. Mice bearing a targeted interruption of the homeobox gene HOXA9 have defects in myeloid, erythroid, and lymphoid hematopoiesis. Blood. 1997;89:1922–1930. [PubMed] [Google Scholar]
- 16.So CW, Karsunky H, Wong P, Weissman IL, Cleary ML. Leukemic transformation of hematopoietic progenitors by MLL-GAS7 in the absence of Hoxa7 or Hoxa9. Blood. 2004;103:3192–3199. doi: 10.1182/blood-2003-10-3722. [DOI] [PubMed] [Google Scholar]
- 17.Nutt SL, Morrison AM, Dörfler P, Rolink A, Busslinger M. Identification of BSAP (Pax-5) target genes in early B-cell development by lossand gain-of-function experiments. EMBO J. 1998;17:2319–2333. doi: 10.1093/emboj/17.8.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ceredig R, Andersson J, Melchers F, Rolink A. Effect of deregulated IL-7 transgene expression on B lymphocyte development in mice expressing mutated pre-B cell receptors. Eur. J. Immunol. 1999;29:2797–2807. doi: 10.1002/(SICI)1521-4141(199909)29:09<2797::AID-IMMU2797>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat. Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- 20.Medina KL, Pongubala JM, Reddy KL, Lancki DW, Dekoter R, Kieslinger M, Grosschedl R, Singh H. Assembling a gene regulatory network for specification of the B cell fate. Dev. Cell. 2004;7:607–617. doi: 10.1016/j.devcel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 22.Lai AY, Kondo M. Asymmetrical lymphoid and myeloid lineage commitment inmultipotent hematopoietic progenitors. J. Exp.Med. 2006;203:1867–1873. doi: 10.1084/jem.20060697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizusawa N, Hasegawa T, Ohigashi I, Tanaka-Kosugi C, Harada N, Itakura M, Yoshimoto K. Differentiation phenotypes of pancreatic islet beta- and alpha-cells are closely related with homeotic genes and a group of differentially expressed genes. Gene. 2004;331:53–63. doi: 10.1016/j.gene.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Sanyal M, Tung JW, Karsunky H, Zeng H, Selleri L, Weissman IL, Herzenberg LA, Cleary ML. B-cell development fails in the absence of the Pbx1 proto-oncogene. Blood. 2007;109:4191–4199. doi: 10.1182/blood-2006-10-054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeisig BB, Milne T, García-Cuéllar MP, Schreiner S, Martin ME, Fuchs U, Borkhardt A, Chanda SK, Walker J, Soden R, et al. Hoxa9 and Meis1 are key targets for MLL-ENL-mediated cellular immortalization. Mol. Cell. Biol. 2004;24:617–628. doi: 10.1128/MCB.24.2.617-628.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang GG, Pasillas MP, Kamps MP. Persistent transactivation by meis1 replaces hox function in myeloid leukemogenesis models: evidence for co-occupancy of meis1-pbx and hox-pbx complexes on promoters of leukemiaassociated genes. Mol. Cell. Biol. 2006;26:3902–3916. doi: 10.1128/MCB.26.10.3902-3916.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeKoter RP, Lee HJ, Singh H. PU.1 regulates expression of the interleukin-7 receptor in lymphoid progenitors. Immunity. 2002;16:297–309. doi: 10.1016/s1074-7613(02)00269-8. [DOI] [PubMed] [Google Scholar]
- 28.Kikuchi K, Kasai H, Watanabe A, Lai AY, Kondo M. IL-7 specifies B cell fate at the common lymphoid progenitor to pre-proB transition stage by maintaining early B cell factor expression. J. Immunol. 2008;181:383–392. doi: 10.4049/jimmunol.181.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 30.Holmes ML, Carotta S, Corcoran LM, Nutt SL. Repression of Flt3 by Pax5 is crucial for B-cell lineage commitment. Genes Dev. 2006;20:933–938. doi: 10.1101/gad.1396206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 32.Harman BC, Northrup DL, Allman D. Resolution of unique Sca-1highc-Kit-lymphoid-biased progenitors in adult bone marrow. J. Immunol. 2008;181:7514–7524. doi: 10.4049/jimmunol.181.11.7514. [DOI] [PubMed] [Google Scholar]
- 33.Lee G, Medina K, Kincade PW. Growth requirements of B lineage lymphocytes from scid and normal mice. Curr. Top. Microbiol. Immunol. 1989;152:33–37. doi: 10.1007/978-3-642-74974-2_5. [DOI] [PubMed] [Google Scholar]
- 34.Akerblad P, Rosberg M, Leanderson T, Sigvardsson M. The B29 (immunoglobulin beta-chain) gene is a genetic target for early B-cell factor. Mol. Cell. Biol. 1999;19:392–401. doi: 10.1128/mcb.19.1.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sigvardsson M, O’Riordan M, Grosschedl R. EBF and E47 collaborate to induce expression of the endogenous immunoglobulin surrogate light chain genes. Immunity. 1997;7:25–36. doi: 10.1016/s1074-7613(00)80507-5. [DOI] [PubMed] [Google Scholar]
- 36.Pineault N, Helgason CD, Lawrence HJ, Humphries RK. Differential expression of Hox, Meis1, and Pbx1 genes in primitive cells throughout murine hematopoietic ontogeny. Exp. Hematol. 2002;30:49–57. doi: 10.1016/s0301-472x(01)00757-3. [DOI] [PubMed] [Google Scholar]
- 37.Sauvageau G, Lansdorp PM, Eaves CJ, Hogge DE, Dragowska WH, Reid DS, Largman C, Lawrence HJ, Humphries RK. Differential expression of homeobox genes in functionally distinct CD34+ subpopulations of human bone marrow cells. Proc. Natl. Acad. Sci. USA. 1994;91:12223–12227. doi: 10.1073/pnas.91.25.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sitnicka E, Bryder D, Theilgaard-Mönch K, Buza-Vidas N, Adolfsson J, Jacobsen SE. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 2002;17:463–472. doi: 10.1016/s1074-7613(02)00419-3. [DOI] [PubMed] [Google Scholar]
- 39.Matthews W, Jordan CT, Wiegand GW, Pardoll D, Lemischka IR. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell. 1991;65:1143–1152. doi: 10.1016/0092-8674(91)90010-v. [DOI] [PubMed] [Google Scholar]
- 40.Lawrence HJ, Christensen J, Fong S, Hu YL, Weissman I, Sauvageau G, Humphries RK, Largman C. Loss of expression of the Hoxa-9 homeobox gene impairs the proliferation and repopulating ability of hematopoietic stem cells. Blood. 2005;106:3988–3994. doi: 10.1182/blood-2005-05-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adolfsson J, Borge OJ, Bryder D, Theilgaard-Mönch K, Astrand-Grundström I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 42.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 43.Wang GG, Pasillas MP, Kamps MP. Meis1 programs transcription of FLT3 and cancer stem cell character, using a mechanism that requires interaction with Pbx and a novel function of the Meis1 C-terminus. Blood. 2005;106:254–264. doi: 10.1182/blood-2004-12-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3:147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 45.Mansson R, Zandi S, Anderson K, Martensson IL, Jacobsen SE, Bryder D, Sigvardsson M. B-lineage commitment prior to surface expression of B220 and CD19 on hematopoietic progenitor cells. Blood. 2008;112:1048–1055. doi: 10.1182/blood-2007-11-125385. [DOI] [PubMed] [Google Scholar]
- 46.Carotta S, Dakic A, D’Amico A, Pang SH, Greig KT, Nutt SL, Wu L. The transcription factor PU.1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner. Immunity. 2010;32:628–641. doi: 10.1016/j.immuni.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Okada H, Watanabe T, Niki M, Takano H, Chiba N, Yanai N, Tani K, Hibino H, Asano S, Mucenski ML, et al. AML1(−/−) embryos do not express certain hematopoiesis-related gene transcripts including those of the PU.1 gene. Oncogene. 1998;17:2287–2293. doi: 10.1038/sj.onc.1202151. [DOI] [PubMed] [Google Scholar]
- 48.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc. Natl. Acad. Sci. USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim KT, Baird K, Ahn JY, Meltzer P, Lilly M, Levis M, Small D. Pim-1 is up-regulated by constitutively activated FLT3 and plays a role in FLT3-mediated cell survival. Blood. 2005;105:1759–1767. doi: 10.1182/blood-2004-05-2006. [DOI] [PubMed] [Google Scholar]
- 50.Hu YL, Passegué E, Fong S, Largman C, Lawrence HJ. Evidence that the Pim1 kinase gene is a direct target of HOXA9. Blood. 2007;109:4732–4738. doi: 10.1182/blood-2006-08-043356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Domen J, van der Lugt NM, Acton D, Laird PW, Linders K, Berns A. Pim-1 levels determine the size of early B lymphoid compartments in bone marrow. J. Exp. Med. 1993;178:1665–1673. doi: 10.1084/jem.178.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leverson JD, Koskinen PJ, Orrico FC, Rainio EM, Jalkanen KJ, Dash AB, Eisenman RN, Ness SA. Pim-1 kinase and p100 cooperate to enhance c-Myb activity. Mol. Cell. 1998;2:417–425. doi: 10.1016/s1097-2765(00)80141-0. [DOI] [PubMed] [Google Scholar]
- 53.Greig KT, de Graaf CA, Murphy JM, Carpinelli MR, Pang SH, Frampton J, Kile BT, Hilton DJ, Nutt SL. Critical roles for c-Myb in lymphoid priming and early B-cell development. Blood. 2010;115:2796–2805. doi: 10.1182/blood-2009-08-239210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thorsteinsdottir U, Mamo A, Kroon E, Jerome L, Bijl J, Lawrence HJ, Humphries K, Sauvageau G. Overexpression of the myeloid leukemia-associated Hoxa9 gene in bone marrow cells induces stem cell expansion. Blood. 2002;99:121–129. doi: 10.1182/blood.v99.1.121. [DOI] [PubMed] [Google Scholar]
- 55.Thorsteinsdottir U, Sauvageau G, Hough MR, Dragowska W, Lansdorp PM, Lawrence HJ, Largman C, Humphries RK. Overexpression of HOXA10 in murine hematopoietic cells perturbs both myeloid and lymphoid differentiation and leads to acute myeloid leukemia. Mol. Cell. Biol. 1997;17:495–505. doi: 10.1128/mcb.17.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gosiengfiao Y, Horvat R, Thompson A. Transcription factors GATA-1 and Fli-1 regulate human HOXA10 expression in megakaryocytic cells. DNA Cell Biol. 2007;26:577–587. doi: 10.1089/dna.2007.0575. [DOI] [PubMed] [Google Scholar]
- 57.Horman SR, Velu CS, Chaubey A, Bourdeau T, Zhu J, Paul WE, Gebelein B, Grimes HL. Gfi1 integrates progenitor versus granulocytic transcriptional programming. Blood. 2009;113:5466–5475. doi: 10.1182/blood-2008-09-179747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen WF, Hu YL, Uttarwar L, Passegue E, Largman C. MicroRNA-126 regulates HOXA9 by binding to the homeobox. Mol. Cell. Biol. 2008;28:4609–4619. doi: 10.1128/MCB.01652-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.