Abstract
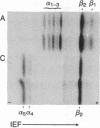
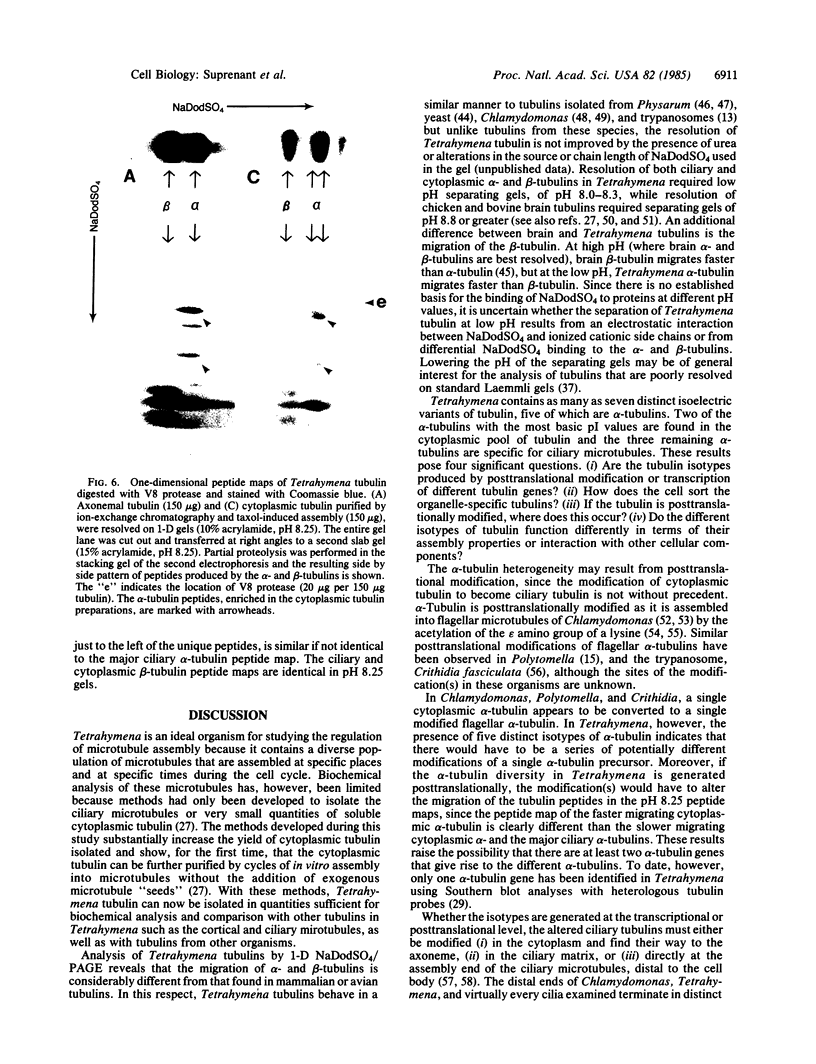
Most higher eukaryotic tubulins are separated into alpha- and beta-tubulin when electrophoresed in NaDodSO4- denaturing gels, while many lower eukaryotic tubulins are poorly resolved under these conditions, which include a stacking gel (pH 6.80) and a separating gel (pH 8.80). By lowering the pH of the separating gel to 8.25, we have found that tubulin isolated from the protozoan Tetrahymena thermophila is resolved by one-dimensional polyacrylamide gel electrophoresis into two alpha-tubulins and one beta-tubulin. Moreover, at least five alpha- and two beta-tubulin isotypes are identified in Tetrahymena by isoelectric focusing and two-dimensional polyacrylamide gel electrophoresis. Three of these alpha isotypes and one beta isotype are found specifically in ciliary microtubules, while the other two isotypes are found only in the cytoplasmic tubulin pool that was isolated and induced to self-assemble into microtubules in vitro. Peptide mapping by limited proteolytic digestion indicates that the tubulins are closely related. Possible mechanisms for the generation and selection of these tubulin isotypes are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibring T., Baxandall J., Denslow S., Walker B. Heterogeneity of the alpha subunit of tubulin and the variability of tubulin within a single organism. J Cell Biol. 1976 May;69(2):301–312. doi: 10.1083/jcb.69.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C., Crettol-Järvinen A. Peptide mapping of heterogeneous protein samples. J Biol Chem. 1979 Apr 25;254(8):2565–2567. [PubMed] [Google Scholar]
- Borisy G. G., Marcum J. M., Olmsted J. B., Murphy D. B., Johnson K. A. Purification of tubulin and associated high molecular weight proteins from porcine brain and characterization of microtubule assembly in vitro. Ann N Y Acad Sci. 1975 Jun 30;253:107–132. doi: 10.1111/j.1749-6632.1975.tb19196.x. [DOI] [PubMed] [Google Scholar]
- Bryan J. Biochemical properties of microtubules. Fed Proc. 1974 Feb;33(2):152–157. [PubMed] [Google Scholar]
- Bryan J., Wilson L. Are cytoplasmic microtubules heteropolymers? Proc Natl Acad Sci U S A. 1971 Aug;68(8):1762–1766. doi: 10.1073/pnas.68.8.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burland T. G., Gull K., Schedl T., Boston R. S., Dove W. F. Cell type-dependent expression of tubulins in Physarum. J Cell Biol. 1983 Dec;97(6):1852–1859. doi: 10.1083/jcb.97.6.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A., Binder L. I., Payne M. R., Bender P., Rebhun L., Steward O. Differential subcellular localization of tubulin and the microtubule-associated protein MAP2 in brain tissue as revealed by immunocytochemistry with monoclonal hybridoma antibodies. J Neurosci. 1984 Feb;4(2):394–410. doi: 10.1523/JNEUROSCI.04-02-00394.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R. C., Shalke G., Gorovsky M. A. Developmental rearrangements associated with a single type of expressed alpha-tubulin gene in Tetrahymena. Cell. 1984 Feb;36(2):441–445. doi: 10.1016/0092-8674(84)90237-x. [DOI] [PubMed] [Google Scholar]
- Clayton L., Quinlan R. A., Roobol A., Pogson C. I., Gull K. A comparison of tubulins from mammalian brain and Physarum polycephalum using SDS-polyacrylamide gel electrophorsis and peptide mapping. FEBS Lett. 1980 Jun 30;115(2):301–305. doi: 10.1016/0014-5793(80)81192-6. [DOI] [PubMed] [Google Scholar]
- Crowle A. J., Cline L. J. An improved stain for immunodiffusion tests. J Immunol Methods. 1977;17(3-4):379–381. doi: 10.1016/0022-1759(77)90122-3. [DOI] [PubMed] [Google Scholar]
- Cumming R., Burgoyne R. D., Lytton N. A. Immunocytochemical demonstration of alpha-tubulin modification during axonal maturation in the cerebellar cortex. J Cell Biol. 1984 Jan;98(1):347–351. doi: 10.1083/jcb.98.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentler W. L. Attachment of the cap to the central microtubules of Tetrahymena cilia. J Cell Sci. 1984 Mar;66:167–173. doi: 10.1242/jcs.66.1.167. [DOI] [PubMed] [Google Scholar]
- Dentler W. L., Granett S., Witman G. B., Rosenbaum J. L. Directionality of brain microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1974 May;71(5):1710–1714. doi: 10.1073/pnas.71.5.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentler W. L. Microtubule-membrane interactions in cilia and flagella. Int Rev Cytol. 1981;72:1–47. doi: 10.1016/s0074-7696(08)61193-6. [DOI] [PubMed] [Google Scholar]
- Dentler W. L., Rosenbaum J. L. Flagellar elongation and shortening in Chlamydomonas. III. structures attached to the tips of flagellar microtubules and their relationship to the directionality of flagellar microtubule assembly. J Cell Biol. 1977 Sep;74(3):747–759. doi: 10.1083/jcb.74.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Field D. J., Collins R. A., Lee J. C. Heterogeneity of vertebrate brain tubulins. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4041–4045. doi: 10.1073/pnas.81.13.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I., Barnstable C. J. Monoclonal antibodies that recognize discrete forms of tubulin. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2579–2583. doi: 10.1073/pnas.79.8.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I., Sweadner K. J. Multiple tubulin forms are expressed by a single neurone. Nature. 1981 Dec 3;294(5840):477–480. doi: 10.1038/294477a0. [DOI] [PubMed] [Google Scholar]
- Gundersen G. G., Kalnoski M. H., Bulinski J. C. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984 Oct;38(3):779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- Hall J. L., Dudley L., Dobner P. R., Lewis S. A., Cowan N. J. Identification of two human beta-tubulin isotypes. Mol Cell Biol. 1983 May;3(5):854–862. doi: 10.1128/mcb.3.5.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvik J. W., Rosenbaum J. L. Oversized flagellar membrane protein in paralyzed mutants of Chlamydomonas reinhardrii. J Cell Biol. 1980 May;85(2):258–272. doi: 10.1083/jcb.85.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues K. J., Kaufman T. C., Raff R. A., Raff E. C. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell. 1982 Dec;31(3 Pt 2):655–670. doi: 10.1016/0092-8674(82)90321-x. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V. Purification of yeast tubulin by self-assembly in vitro. Biochemistry. 1981 Jun 9;20(12):3629–3633. doi: 10.1021/bi00515a050. [DOI] [PubMed] [Google Scholar]
- Krauhs E., Little M., Kempf T., Hofer-Warbinek R., Ade W., Ponstingl H. Complete amino acid sequence of beta-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4156–4160. doi: 10.1073/pnas.78.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Hernault S. W., Rosenbaum J. L. Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry. 1985 Jan 15;24(2):473–478. doi: 10.1021/bi00323a034. [DOI] [PubMed] [Google Scholar]
- L'Hernault S. W., Rosenbaum J. L. Chlamydomonas alpha-tubulin is posttranslationally modified in the flagella during flagellar assembly. J Cell Biol. 1983 Jul;97(1):258–263. doi: 10.1083/jcb.97.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Hernault S. W., Rosenbaum J. L. Reversal of the posttranslational modification on Chlamydomonas flagellar alpha-tubulin occurs during flagellar resorption. J Cell Biol. 1985 Feb;100(2):457–462. doi: 10.1083/jcb.100.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy M. R., Sisskin E. E., McConkey C. L. A protease that increases during a period of enzymic and metabolic adjustment in Tetrahymena. Arch Biochem Biophys. 1976 Feb;172(2):634–647. doi: 10.1016/0003-9861(76)90118-1. [DOI] [PubMed] [Google Scholar]
- Little M., Ludueña R. F., Langford G. M., Asnes C. F., Farrell K. Comparison of proteolytic cleavage patterns of alpha-tubulins and beta-tubulins from taxonomically distant species. J Mol Biol. 1981 Jun 15;149(1):95–107. doi: 10.1016/0022-2836(81)90262-x. [DOI] [PubMed] [Google Scholar]
- Ludueńa R. F., Shooter E. M., Wilson L. Structure of the tubulin dimer. J Biol Chem. 1977 Oct 25;252(20):7006–7014. [PubMed] [Google Scholar]
- Maekawa S., Sakai H. Characterization and in vitro polymerization of Tetrahymena tubulin. J Biochem. 1978 Apr;83(4):1065–1075. doi: 10.1093/oxfordjournals.jbchem.a131995. [DOI] [PubMed] [Google Scholar]
- McKeithan T. W., Lefebvre P. A., Silflow C. D., Rosenbaum J. L. Multiple forms of tubulin in Polytomella and Chlamydomonas: evidence for a precursor of flagellar alpha-tubulin. J Cell Biol. 1983 Apr;96(4):1056–1063. doi: 10.1083/jcb.96.4.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell E. J., Zimmerman A. M. Biochemical evidence for the presence of an actin protein in Tetrahymena pyriformis. J Cell Sci. 1985 Feb;73:279–297. doi: 10.1242/jcs.73.1.279. [DOI] [PubMed] [Google Scholar]
- Nelles L. P., Bamburg J. R. Comparative peptide mapping and isoelectric focusing of isolated subunits from chick embryo brain tubulin. J Neurochem. 1979 Feb;32(2):477–489. doi: 10.1111/j.1471-4159.1979.tb00374.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Morris N. R. A beta-tubulin mutation in Aspergillus nidulans that blocks microtubule function without blocking assembly. Cell. 1981 Jun;24(3):837–845. doi: 10.1016/0092-8674(81)90109-4. [DOI] [PubMed] [Google Scholar]
- Orias E., Bruns P. J. Induction and isolation of mutants in Tetrahymena. Methods Cell Biol. 1976;13:247–282. [PubMed] [Google Scholar]
- Orias E., Flacks M., Satir B. H. Isolation and ultrastructural characterization of secretory mutants of Tetrahymena thermophila. J Cell Sci. 1983 Nov;64:49–67. doi: 10.1242/jcs.64.1.49. [DOI] [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M., Kempf T. Complete amino acid sequence of alpha-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 May;78(5):2757–2761. doi: 10.1073/pnas.78.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannestad J., Williams N. E. The synthesis of microtubule and other proteins of the oral apparatus in Tetrahymena pyriformis. J Cell Biol. 1971 Sep;50(3):709–720. doi: 10.1083/jcb.50.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remillard S. P., Witman G. B. Synthesis, transport, and utilization of specific flagellar proteins during flagellar regeneration in Chlamydomonas. J Cell Biol. 1982 Jun;93(3):615–631. doi: 10.1083/jcb.93.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roobol A., Pogson C. I., Gull K. In vitro assembly of microtubule proteins from myxamoebae of Physarum polycephalum. Exp Cell Res. 1980 Nov;130(1):203–215. doi: 10.1016/0014-4827(80)90057-9. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J. L., Moulder J. E., Ringo D. L. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol. 1969 May;41(2):600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G., Gull K. Flagellar regeneration of the trypanosome Crithidia fasciculata involves post-translational modification of cytoplasmic alpha tubulin. Mol Cell Biol. 1984 Jun;4(6):1182–1185. doi: 10.1128/mcb.4.6.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G., Miller D., Gull K. Tubulin heterogeneity in the trypanosome Crithidia fasciculata. Mol Cell Biol. 1984 Apr;4(4):779–790. doi: 10.1128/mcb.4.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff P. B., Fant J., Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979 Feb 22;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Stephens R. E. Primary structural differences among tubulin subunits from flagella, cilia, and the cytoplasm. Biochemistry. 1978 Jul 11;17(14):2882–2891. doi: 10.1021/bi00607a029. [DOI] [PubMed] [Google Scholar]
- Sullivan K. F., Cleveland D. W. Sequence of a highly divergent beta tubulin gene reveals regional heterogeneity in the beta tubulin polypeptide. J Cell Biol. 1984 Nov;99(5):1754–1760. doi: 10.1083/jcb.99.5.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. C., Asai D. J., Carney D. H. Heterogeneity among microtubules of the cytoplasmic microtubule complex detected by a monoclonal antibody to alpha tubulin. J Cell Biol. 1984 Mar;98(3):1017–1025. doi: 10.1083/jcb.98.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa H. Structures and activities of protease inhibitors of microbial origin. Methods Enzymol. 1976;45:678–695. doi: 10.1016/s0076-6879(76)45058-9. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]
- Williams N. E. Regulation of microtubules in Tetrahymena. Int Rev Cytol. 1975;41:59–86. doi: 10.1016/s0074-7696(08)60966-3. [DOI] [PubMed] [Google Scholar]
- Williams N. E., Vaudaux P. E., Skriver L. Cytoskeletal proteins of the cell surface in Tetrahymena I. Identification and localization of major proteins. Exp Cell Res. 1979 Oct 15;123(2):311–320. doi: 10.1016/0014-4827(79)90473-7. [DOI] [PubMed] [Google Scholar]
- Witman G. B. The site of in vivo assembly of flagellar microtubules. Ann N Y Acad Sci. 1975 Jun 30;253:178–191. doi: 10.1111/j.1749-6632.1975.tb19199.x. [DOI] [PubMed] [Google Scholar]
- Wolfe J. Structural analysis of basal bodies of the isolated oral apparatus of Tetrahymena pyriformis. J Cell Sci. 1970 May;6(3):679–700. doi: 10.1242/jcs.6.3.679. [DOI] [PubMed] [Google Scholar]