Abstract
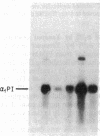
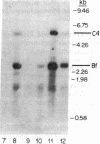
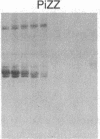
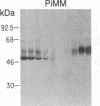
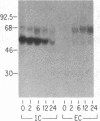
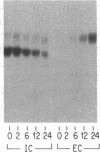
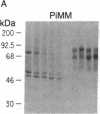
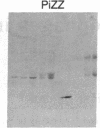
To determine the basis for low serum concentrations of alpha 1-proteinase inhibitor (alpha 1PI) in individuals with homozygous alpha 1PI deficiency (hereafter referred to as PiZZ), biosynthesis and secretion of alpha 1PI were studied in Xenopus oocytes microinjected with hepatic mRNA and in blood monocytes (an extrahepatic site of alpha 1PI gene expression). Although both the usual alpha 1PI (hereafter referred to as PiMM) and PiZZ alpha 1PI were secreted in functionally active form, the rate of secretion of alpha 1PI was significantly and selectively decreased in Xenopus oocytes injected with PiZZ liver mRNA and in monocytes from PiZZ individuals. The apparent size of alpha 1PI in the intracellular compartment of Xenopus oocytes injected with PiZZ liver mRNA was different from the corresponding intracellular PiMM alpha 1PI in oocytes injected with PiMM liver mRNA. There were also differences in the relative ratio of native and complexed alpha 1PI secreted by monocytes from individuals with PiMM and PiZZ phenotypes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathurst I. C., Stenflo J., Errington D. M., Carrell R. W. Translation and processing of normal (PiMM) and abnormal (PiZZ) human alpha 1-antitrypsin. FEBS Lett. 1983 Mar 21;153(2):270–274. doi: 10.1016/0014-5793(83)80622-x. [DOI] [PubMed] [Google Scholar]
- Bathurst I. C., Travis J., George P. M., Carrell R. W. Structural and functional characterization of the abnormal Z alpha 1-antitrypsin isolated from human liver. FEBS Lett. 1984 Nov 19;177(2):179–183. doi: 10.1016/0014-5793(84)81279-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Larsson C. Purification and partial characterization of pas-positive inclusion bodies from the liver in alpha 1-antitrypsin deficiency. N Engl J Med. 1975 Jan 23;292(4):176–180. doi: 10.1056/NEJM197501232920403. [DOI] [PubMed] [Google Scholar]
- Foreman R. C., Judah J. D., Colman A. Xenopus oocytes can synthesise but do not secrete the Z variant of human alpha 1-antitrypsin. FEBS Lett. 1984 Mar 12;168(1):84–88. doi: 10.1016/0014-5793(84)80211-2. [DOI] [PubMed] [Google Scholar]
- Hercz A., Katona E., Cutz E., Wilson J. R., Barton M. alpha1-Antitrypsin: the presence of excess mannose in the Z variant isolated from liver. Science. 1978 Sep 29;201(4362):1229–1232. doi: 10.1126/science.308696. [DOI] [PubMed] [Google Scholar]
- Jeppsson J. O. Amino acid substitution Glu leads to Lys alpha1-antitrypsin PiZ. FEBS Lett. 1976 Jun 1;65(2):195–197. doi: 10.1016/0014-5793(76)80478-4. [DOI] [PubMed] [Google Scholar]
- Jeppsson J. O., Larsson C., Eriksson S. Characterization of alpha1-antitrypsin in the inclusion bodies from the liver in alpha 1-antitrypsin deficiency. N Engl J Med. 1975 Sep 18;293(12):576–579. doi: 10.1056/NEJM197509182931203. [DOI] [PubMed] [Google Scholar]
- Jeppsson J. O., Laurell C. B., Nosslin B., Cox D. W. Catabolic rate of alpha1-antitrypsin of of Pi types S, and MMalton and of asialylated M-protein in man. Clin Sci Mol Med. 1978 Jul;55(1):103–107. doi: 10.1042/cs0550103. [DOI] [PubMed] [Google Scholar]
- Kidd V. J., Wallace R. B., Itakura K., Woo S. L. alpha 1-antitrypsin deficiency detection by direct analysis of the mutation in the gene. Nature. 1983 Jul 21;304(5923):230–234. doi: 10.1038/304230a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane C. D., Marbaix G., Gurdon J. B. Rabbit haemoglobin synthesis in frog cells: the translation of reticulocyte 9 s RNA in frog oocytes. J Mol Biol. 1971 Oct 14;61(1):73–91. doi: 10.1016/0022-2836(71)90207-5. [DOI] [PubMed] [Google Scholar]
- Laurell C. B., Nosslin B., Jeppsson J. O. Catabolic rate of alpha1-antitrypsin of Pi type M and Z in man. Clin Sci Mol Med. 1977 May;52(5):457–461. doi: 10.1042/cs0520457. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Kong N. Glucose removal from N-linked oligosaccharides is required for efficient maturation of certain secretory glycoproteins from the rough endoplasmic reticulum to the Golgi complex. J Cell Biol. 1984 May;98(5):1720–1729. doi: 10.1083/jcb.98.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebermann H., Tokuoka R., Deisenhofer J., Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984 Aug 15;177(3):531–557. [PubMed] [Google Scholar]
- Makino S., Reed C. E. Distribution and elimination of exogenous alpha1-antitrypsin. J Lab Clin Med. 1970 May;75(5):742–746. [PubMed] [Google Scholar]
- Morii M., Odani S., Ikenaka T. Characterization of a peptide released during the reaction of human alpha 1-antitrypsin and bovine alpha-chymotrypsin. J Biochem. 1979 Oct;86(4):915–921. doi: 10.1093/oxfordjournals.jbchem.a132623. [DOI] [PubMed] [Google Scholar]
- Owen M. C., Carrell R. W. alpha-1-Antitrypsin: sequence of the Z variant tryptic peptide. FEBS Lett. 1977 Jul 15;79(2):245–247. doi: 10.1016/0014-5793(77)80796-5. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Cole F. S., Kilbridge P., Rossing T. H., Colten H. R. Expression of the alpha 1-proteinase inhibitor gene in human monocytes and macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(3):795–799. doi: 10.1073/pnas.82.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Kalsheker N., Wallis S., Speer A., Coutelle C. H., Woods D., Humphries S. E. The isolation of a clone for human alpha 1-antitrypsin and the detection of alpha 1-antitrypsin in mRNA from liver and leukocytes. Biochem Biophys Res Commun. 1983 Oct 31;116(2):375–382. doi: 10.1016/0006-291x(83)90532-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Whitehead A. S., Goldberger G., Woods D. E., Markham A. F., Colten H. R. Use of a cDNA clone for the fourth component of human complement (C4) for analysis of a genetic deficiency of C4 in guinea pig. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5387–5391. doi: 10.1073/pnas.80.17.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Edge M. D., Colten H. R. Isolation of a complementary DNA clone for the human complement protein C2 and its use in the identification of a restriction fragment length polymorphism. J Clin Invest. 1984 Aug;74(2):634–638. doi: 10.1172/JCI111461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Markham A. F., Ricker A. T., Goldberger G., Colten H. R. Isolation of cDNA clones for the human complement protein factor B, a class III major histocompatibility complex gene product. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5661–5665. doi: 10.1073/pnas.79.18.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo T. K., Yeo K. T., Parent J. B., Olden K. Swainsonine treatment accelerates intracellular transport and secretion of glycoproteins in human hepatoma cells. J Biol Chem. 1985 Feb 25;260(4):2565–2569. [PubMed] [Google Scholar]
- Yoshida A., Lieberman J., Gaidulis L., Ewing C. Molecular abnormality of human alpha1-antitrypsin variant (Pi-ZZ) associated with plasma activity deficiency. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1324–1328. doi: 10.1073/pnas.73.4.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Kramps J. A., Diesselhof-den Dulk M. M. Synthesis of alpha 1-anti-trypsin by human monocytes. Clin Exp Immunol. 1983 Mar;51(3):551–557. [PMC free article] [PubMed] [Google Scholar]