Abstract
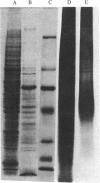
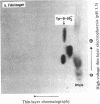
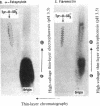
[35S]Sulfate labeling of the human hepatoma cell line HepG2 showed it to contain many sulfated proteins of diverse molecular weight range. The isolation of tyrosine O-sulfate indicated the supernatant fraction to contain a 5- to 7-fold higher level than the cellular fraction at the end of a 24-hr incubation. The proteins in the supernatant fraction were immunoprecipitated and examined for sulfation. Of 15 proteins tested, 7 were found to be sulfated as indicated by [35S]sulfate incorporation into proteins separated by NaDodSO4/PAGE and detected by autoradiography. The 35S-labeled bands were excised from the dried gel and subjected to extensive Pronase hydrolysis and the hydrolysates were analyzed for tyrosine [35S]sulfate by a two-dimensional procedure combining high-voltage electrophoresis and thin-layer chromatography [Liu, M. C. & Lipmann, F. (1984) Proc. Natl. Acad. Sci. USA 81, 3695-3698]. Of the sulfated proteins, three--fibrinogen, alpha-fetoprotein, and fibronectin--were found to contain tyrosine O-sulfate. The simultaneous presence of carbohydrate-bound sulfate, however, could not be exactly determined, but the other four [35S]sulfate-containing proteins--alpha 1-antitrypsin, alpha 1-antichymotrypsin, alpha 2-macroglobulin, and transferrin--did not reveal any tyrosine O-sulfate and might be sulfated on their carbohydrate moieties.
Full text
PDF
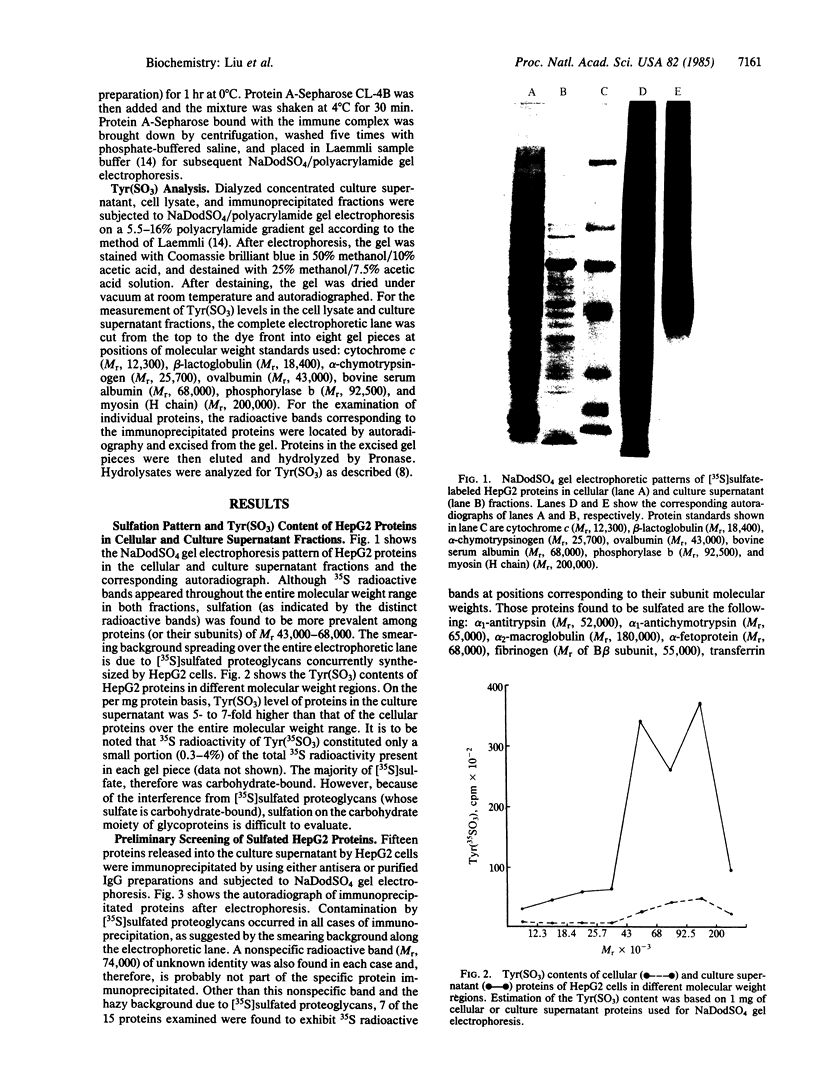



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert E., Drysdale J. W., Isselbacher K. J., Schur P. H. Human -fetoprotein. Isolation, characterization, and demonstration of microheterogeneity. J Biol Chem. 1972 Jun 25;247(12):3792–3798. [PubMed] [Google Scholar]
- BERGSTRAND C. G., CZAR B. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest. 1956;8(2):174–174. doi: 10.3109/00365515609049266. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Huttner W. B. Inhibition of N-glycosylation induces tyrosine sulphation of hybridoma immunoglobulin G. EMBO J. 1984 Oct;3(10):2209–2215. doi: 10.1002/j.1460-2075.1984.tb02118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Pinter A. Incorporation of sulfate into influenza virus glycoproteins. Virology. 1975 Jul;66(1):151–160. doi: 10.1016/0042-6822(75)90186-5. [DOI] [PubMed] [Google Scholar]
- Foidart J. M., Berman J. J., Paglia L., Rennard S., Abe S., Perantoni A., Martin G. R. Synthesis of fibronectin, laminin, and several collagens by a liver-derived epithelial line. Lab Invest. 1980 May;42(5):525–532. [PubMed] [Google Scholar]
- Heifetz A., Lennarz W. J. Biosynthesis of N-glycosidically linked glycoproteins during gastrulation of sea urchin embryos. J Biol Chem. 1979 Jul 10;254(13):6119–6127. [PubMed] [Google Scholar]
- Huttner W. B. Sulphation of tyrosine residues-a widespread modification of proteins. Nature. 1982 Sep 16;299(5880):273–276. doi: 10.1038/299273a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEVONS F. R. TYROSINE O-SULPHATE IN FIBRINOGEN AND FIBRIN. Biochem J. 1963 Dec;89:621–624. doi: 10.1042/bj0890621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp D. R. Post-translational modification of the fourth component of complement. Sulfation of the alpha-chain. J Biol Chem. 1983 Nov 10;258(21):12745–12748. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee R. W., Huttner W. B. Tyrosine-O-sulfated proteins of PC12 pheochromocytoma cells and their sulfation by a tyrosylprotein sulfotransferase. J Biol Chem. 1983 Sep 25;258(18):11326–11334. [PubMed] [Google Scholar]
- Liu M. C., Lipmann F. Decrease of tyrosine-O-sulfate-containing proteins found in rat fibroblasts infected with Rous sarcoma virus or Fujinami sarcoma virus. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3695–3698. doi: 10.1073/pnas.81.12.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. C., Lipmann F. Isolation of tyrosine-O-sulfate by Pronase hydrolysis from fibronectin secreted by Fujinami sarcoma virus-infected rat fibroblasts. Proc Natl Acad Sci U S A. 1985 Jan;82(1):34–37. doi: 10.1073/pnas.82.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. The cellular site of sulfation of influenza viral glycoproteins. Virology. 1977 Jun 15;79(2):381–392. doi: 10.1016/0042-6822(77)90365-8. [DOI] [PubMed] [Google Scholar]
- Nishi S. Isolation and characterization of a human fetal-alpha-globulin from the sera of fetuses and a hepatoma patient. Cancer Res. 1970 Oct;30(10):2507–2513. [PubMed] [Google Scholar]
- Silver H. K., Deneault J., Gold P., Thompson W. G., Shuster J., Freedman S. O. The detection of alpha 1-fetoprotein in patients with viral hepatitis. Cancer Res. 1974 Jan;34(1):244–247. [PubMed] [Google Scholar]
- Smith C. J., Kelleher P. C. Alpha-fetoprotein molecular heterogeneity. Physiologic correlations with normal growth, carcinogenesis and tumor growth. Biochim Biophys Acta. 1980 Mar 12;605(1):1–32. doi: 10.1016/0304-419x(80)90020-7. [DOI] [PubMed] [Google Scholar]
- Sorkin B. C., Hoffman S., Edelman G. M., Cunningham B. A. Sulfation and phosphorylation of the neural cell adhesion molecule, N-CAM. Science. 1984 Sep 28;225(4669):1476–1478. doi: 10.1126/science.6474186. [DOI] [PubMed] [Google Scholar]
- Stadler J., Gerisch G., Bauer G., Suchanek C., Huttner W. B. In vivo sulfation of the contact site A glycoprotein of Dictyostelium discoideum. EMBO J. 1983;2(7):1137–1143. doi: 10.1002/j.1460-2075.1983.tb01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]