Abstract
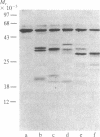
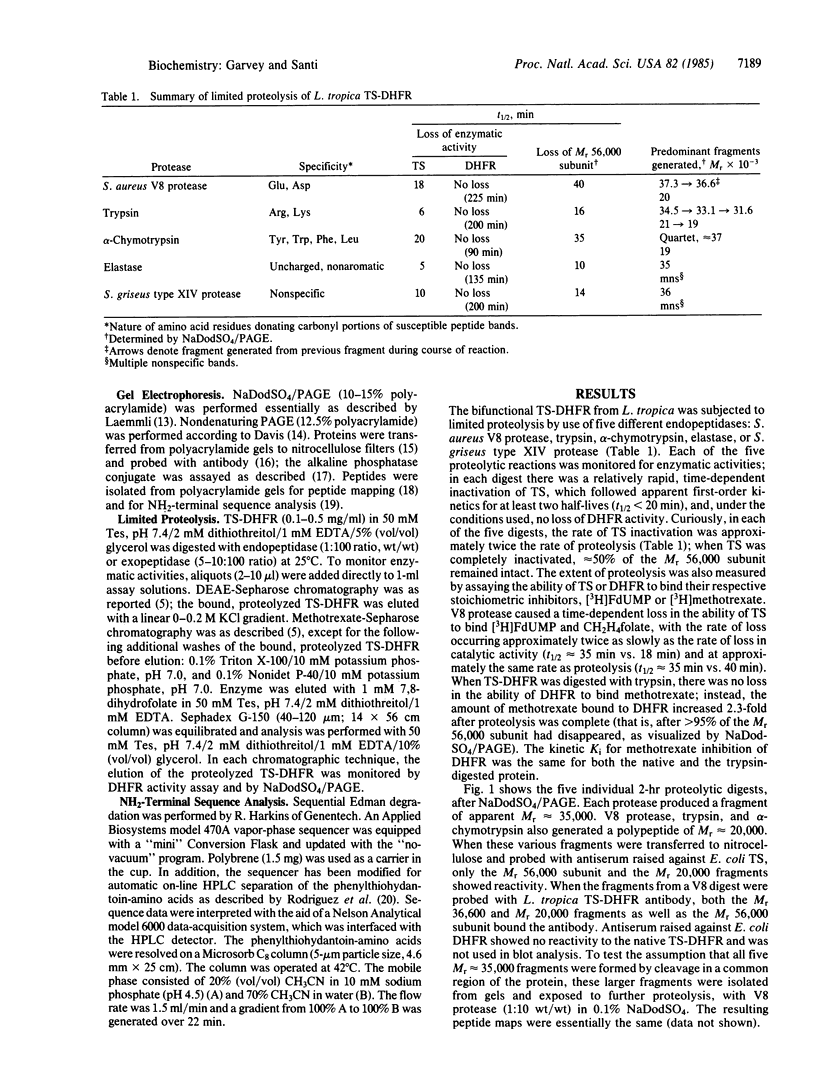
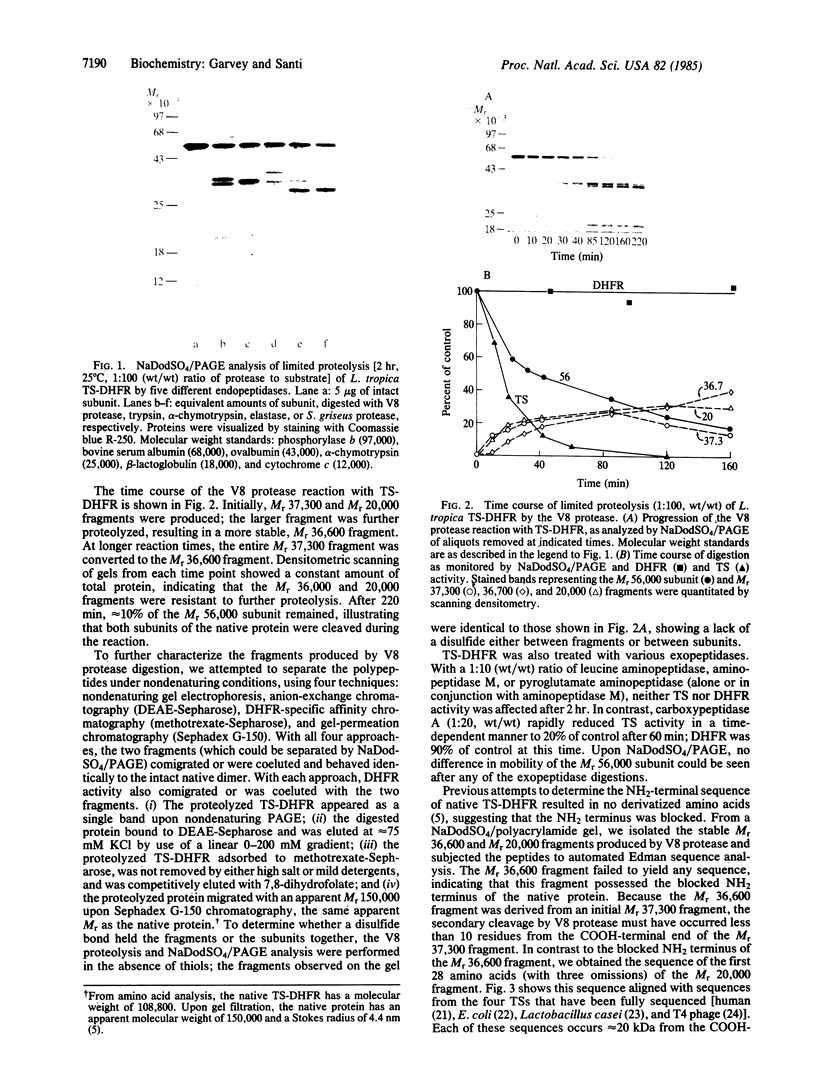
The structure and activity of the bifunctional thymidylate synthase-dihydrofolate reductase (TS-DHFR) from the protozoan parasite Leishmania tropica were examined by limited proteolysis with five different endopeptidases. Each reaction resulted in a rapid, time-dependent loss of TS activity and no effect on DHFR activity. The proteolytic products were examined by NaDodSO4/PAGE; each digestion produced a fragment of apparent Mr approximately 35,000, and three of the five digestions generated a fragment of Mr approximately 20,000. Attempts to separate the fragments under nondenaturing conditions failed, suggesting that the proteolyzed protein remains a dimer with the gross structure of the subunits more or less undisturbed. In contrast, kinetic data indicate that some aspects of higher-order structure in the native protein are affected by proteolysis. The fragments (Mr 36,600 and 20,000) generated by Staphylococcus aureus V8 protease were subjected to sequence analysis. Whereas neither the native protein nor the Mr 36,600 fragment yielded an NH2-terminal amino acid, we obtained the sequence of the first 28 amino acids of the Mr 20,000 fragment. This sequence bore strong homology with sequences situated within TS of human, Lactobacillus casei, Escherichia coli, and bacteriophage T4. These and other data indicate that the TS-DHFR polypeptide consists of a DHFR sequence at the blocked NH2-terminal and a TS sequence at the COOH-terminal end of the protein. The region that is the target of the five proteases corresponds to a highly variable region within the sequences of the other four TSs. We suggest that an insertion occurs within the TS-DHFR sequence, positioned on the surface of the protein and quite vulnerable to the action of endopeptidases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. R., Reddy G. P., Lasser G. W., Mathews C. K. T4 ribonucleotide reductase. Physical and kinetic linkage to other enzymes of deoxyribonucleotide biosynthesis. J Biol Chem. 1980 Aug 25;255(16):7583–7588. [PubMed] [Google Scholar]
- Aull J. L., Loeble R. B., Dunlap R. B. The carboxypeptidase-dependent inactivation of thymidylate synthetase. J Biol Chem. 1974 Feb 25;249(4):1167–1172. [PubMed] [Google Scholar]
- Belfort M., Maley G., Pedersen-Lane J., Maley F. Primary structure of the Escherichia coli thyA gene and its thymidylate synthase product. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4914–4918. doi: 10.1073/pnas.80.16.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Breeze A. S., Sims P., Stacey K. A. Trimethoprim-resistant mutants of E. coli K12: preliminary genetic mapping. Genet Res. 1975 Jun;25(3):207–214. doi: 10.1017/s0016672300015640. [DOI] [PubMed] [Google Scholar]
- Chu F. K., Maley G. F., Maley F., Belfort M. Intervening sequence in the thymidylate synthase gene of bacteriophage T4. Proc Natl Acad Sci U S A. 1984 May;81(10):3049–3053. doi: 10.1073/pnas.81.10.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Ferone R., Roland S. Dihydrofolate reductase: thymidylate synthase, a bifunctional polypeptide from Crithidia fasciculata. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5802–5806. doi: 10.1073/pnas.77.10.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S. G. Peptide mapping in gels. Methods Enzymol. 1983;100:424–430. doi: 10.1016/0076-6879(83)00071-3. [DOI] [PubMed] [Google Scholar]
- Fisher P. A., Berrios M., Blobel G. Isolation and characterization of a proteinaceous subnuclear fraction composed of nuclear matrix, peripheral lamina, and nuclear pore complexes from embryos of Drosophila melanogaster. J Cell Biol. 1982 Mar;92(3):674–686. doi: 10.1083/jcb.92.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett C. E., Coderre J. A., Meek T. D., Garvey E. P., Claman D. M., Beverley S. M., Santi D. V. A bifunctional thymidylate synthetase-dihydrofolate reductase in protozoa. Mol Biochem Parasitol. 1984 Apr;11:257–265. doi: 10.1016/0166-6851(84)90070-7. [DOI] [PubMed] [Google Scholar]
- Grayson D. R., Evans D. R. The isolation and characterization of the aspartate transcarbamylase domain of the multifunctional protein, CAD. J Biol Chem. 1983 Apr 10;258(7):4123–4129. [PubMed] [Google Scholar]
- Hillcoat B. L., Nixon P. F., Blakley R. L. Effect of substrate decomposition on the spectrophotometric assay of dihydrofolate reductase. Anal Biochem. 1967 Nov;21(2):178–189. doi: 10.1016/0003-2697(67)90179-0. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maley G. F., Bellisario R. L., Guarino D. U., Maley F. The primary structure of Lactobacillus casei thymidylate synthetase. III. The use of 2-(2-nitrophenylsulfenyl)-3-methyl-3-bromoindolenine and limited tryptic peptides to establish the complete amino acid sequence of the enzyme. J Biol Chem. 1979 Feb 25;254(4):1301–1304. [PubMed] [Google Scholar]
- Matthews R. G., Vanoni M. A., Hainfeld J. F., Wall J. Methylenetetrahydrofolate reductase. Evidence for spatially distinct subunit domains obtained by scanning transmission electron microscopy and limited proteolysis. J Biol Chem. 1984 Oct 10;259(19):11647–11650. [PubMed] [Google Scholar]
- Mattick J. S., Tsukamoto Y., Nickless J., Wakil S. J. The architecture of the animal fatty acid synthetase. I. Proteolytic dissection and peptide mapping. J Biol Chem. 1983 Dec 25;258(24):15291–15299. [PubMed] [Google Scholar]
- Meek T. D., Garvey E. P., Santi D. V. Purification and characterization of the bifunctional thymidylate synthetase-dihydrofolate reductase from methotrexate-resistant Leishmania tropica. Biochemistry. 1985 Jan 29;24(3):678–686. doi: 10.1021/bi00324a021. [DOI] [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit S., Mathews C. K. Nucleotide sequence reveals overlap between T4 phage genes encoding dihydrofolate reductase and thymidylate synthase. J Biol Chem. 1984 May 25;259(10):6261–6266. [PubMed] [Google Scholar]
- Rodriguez H., Kohr W. J., Harkins R. N. Design and operation of a completely automated Beckman microsequencer. Anal Biochem. 1984 Aug 1;140(2):538–547. doi: 10.1016/0003-2697(84)90205-7. [DOI] [PubMed] [Google Scholar]
- Santi D. V., McHenry C. S., Sommer H. Mechanism of interaction of thymidylate synthetase with 5-fluorodeoxyuridylate. Biochemistry. 1974 Jan 29;13(3):471–481. doi: 10.1021/bi00700a012. [DOI] [PubMed] [Google Scholar]
- Takeishi K., Kaneda S., Ayusawa D., Shimizu K., Gotoh O., Seno T. Nucleotide sequence of a functional cDNA for human thymidylate synthase. Nucleic Acids Res. 1985 Mar 25;13(6):2035–2043. doi: 10.1093/nar/13.6.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. S., DeMoss J. A. Purification and characterization of the trifunctional beta-subunit of anthranilate synthase from Neurospora crassa. J Biol Chem. 1983 Mar 25;258(6):3571–3575. [PubMed] [Google Scholar]