Abstract
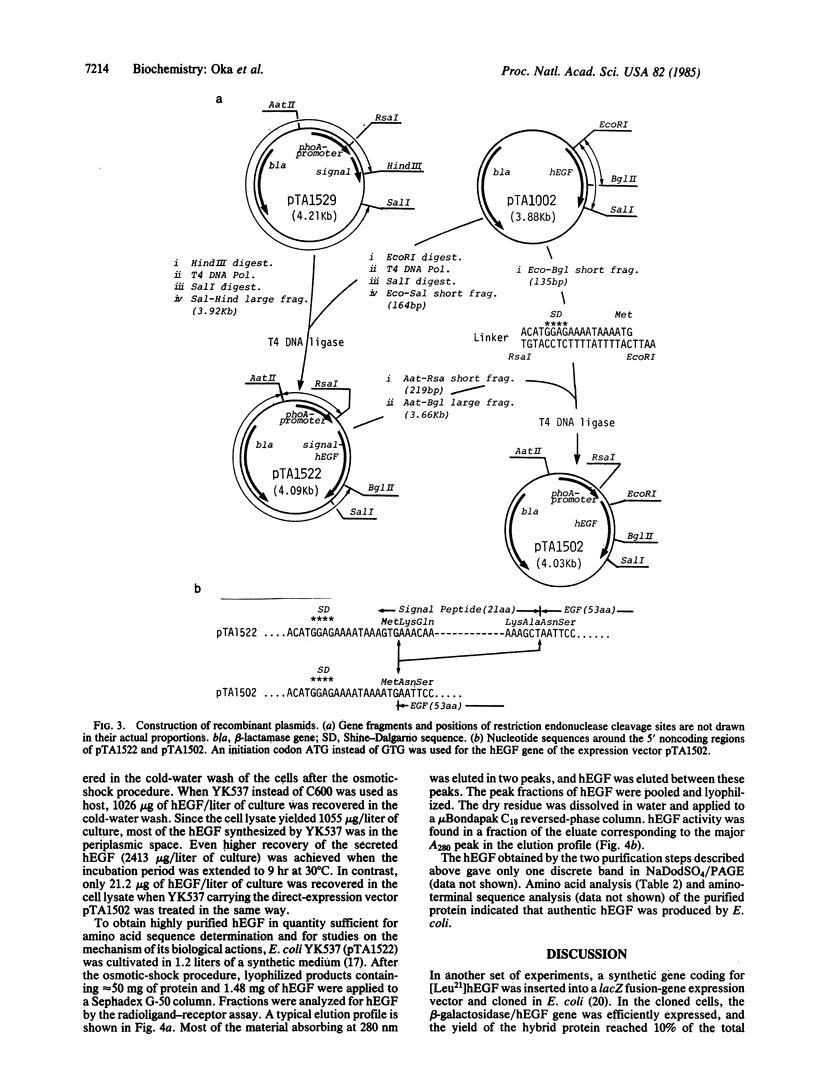
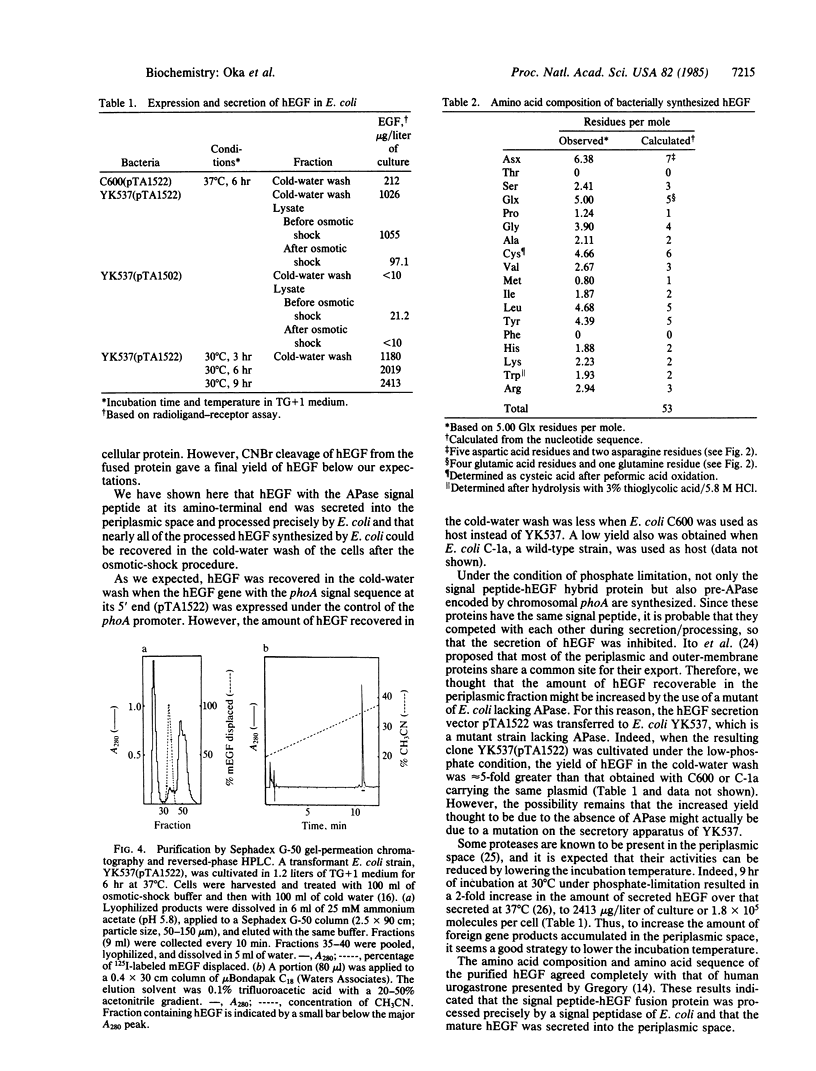
A synthetic gene for human epidermal growth factor (hEGF) was joined to a sequence encoding the signal peptide of Escherichia coli alkaline phosphatase. This hybrid gene was placed under the control of the alkaline phosphatase gene (phoA) promoter in a recombinant plasmid, which was used to transfect E. coli. The hybrid protein that was expressed in host cells under conditions of phosphate limitation was processed accurately during the secretion process, and mature hEGF was recovered in the periplasmic fraction. On the other hand, no EGF was detected in the periplasmic space when the synthetic hEGF gene was not accompanied by the phoA signal sequence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Model P. A prokaryotic membrane anchor sequence: carboxyl terminus of bacteriophage f1 gene III protein retains it in the membrane. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5200–5204. doi: 10.1073/pnas.79.17.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., King L., Jr, Cohen S. Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro. Nature. 1978 Nov 23;276(5686):409–410. doi: 10.1038/276409a0. [DOI] [PubMed] [Google Scholar]
- Derynck R., Roberts A. B., Winkler M. E., Chen E. Y., Goeddel D. V. Human transforming growth factor-alpha: precursor structure and expression in E. coli. Cell. 1984 Aug;38(1):287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- Elder J. B., Ganguli P. C., Gillespie I. E., Gerring E. L., Gregory H. Effect of urogastrone on gastric secretion and plasma gastrin levels in normal subjects. Gut. 1975 Nov;16(11):887–893. doi: 10.1136/gut.16.11.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. B., Gillespie I. E., Ganguli P. C., Delamore W. I., Gregory H. Effect of urogastrone in the Zollinger-Ellison syndrome. Lancet. 1975 Sep 6;2(7932):424–427. doi: 10.1016/s0140-6736(75)90842-9. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Ghrayeb J., Kimura H., Takahara M., Hsiung H., Masui Y., Inouye M. Secretion cloning vectors in Escherichia coli. EMBO J. 1984 Oct;3(10):2437–2442. doi: 10.1002/j.1460-2075.1984.tb02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Swamy K. H., Chung C. H., Larimore F. S. Proteases in Escherichia coli. Methods Enzymol. 1981;80(Pt 100):680–702. doi: 10.1016/s0076-6879(81)80052-3. [DOI] [PubMed] [Google Scholar]
- Gray A., Dull T. J., Ullrich A. Nucleotide sequence of epidermal growth factor cDNA predicts a 128,000-molecular weight protein precursor. Nature. 1983 Jun 23;303(5919):722–725. doi: 10.1038/303722a0. [DOI] [PubMed] [Google Scholar]
- Gregory H. Isolation and structure of urogastrone and its relationship to epidermal growth factor. Nature. 1975 Sep 25;257(5524):325–327. doi: 10.1038/257325a0. [DOI] [PubMed] [Google Scholar]
- Hitzeman R. A., Leung D. W., Perry L. J., Kohr W. J., Levine H. L., Goeddel D. V. Secretion of human interferons by yeast. Science. 1983 Feb 11;219(4585):620–625. doi: 10.1126/science.6186023. [DOI] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura K., Hirose T., Crea R., Riggs A. D., Heyneker H. L., Bolivar F., Boyer H. W. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977 Dec 9;198(4321):1056–1063. doi: 10.1126/science.412251. [DOI] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Gautier A. E., Straus D. R., Charles A. D., Edge M. D., Knowles J. R. The role of the beta-lactamase signal sequence in the secretion of proteins by Escherichia coli. J Biol Chem. 1984 Feb 25;259(4):2149–2154. [PubMed] [Google Scholar]
- Kikuchi Y., Yoda K., Yamasaki M., Tamura G. The nucleotide sequence of the promoter and the amino-terminal region of alkaline phosphatase structural gene (phoA) of Escherichia coli. Nucleic Acids Res. 1981 Nov 11;9(21):5671–5678. doi: 10.1093/nar/9.21.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miyake T., Oka T., Nishizawa T., Misoka F., Fuwa T., Yoda K., Yamasaki M., Tamura G. Secretion of human interferon-alpha induced by using secretion vectors containing a promoter and signal sequence of alkaline phosphatase gene of Escherichia coli. J Biochem. 1985 May;97(5):1429–1436. doi: 10.1093/oxfordjournals.jbchem.a135197. [DOI] [PubMed] [Google Scholar]
- Miyoshi K., Miyake T., Hozumi T., Itakura K. Solid-phase synthesis of polynucleotides. II. Synthesis of polythymidylic acids by the block coupling phosphotriester method. Nucleic Acids Res. 1980 Nov 25;8(22):5473–5489. doi: 10.1093/nar/8.22.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Ohsuye K., Nomura M., Tanaka S., Kubota I., Nakazato H., Shinagawa H., Nakata A., Noguchi T. Expression of chemically synthesized alpha-neo-endorphin gene fused to E. coli alkaline phosphatase. Nucleic Acids Res. 1983 Mar 11;11(5):1283–1294. doi: 10.1093/nar/11.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Talmadge K., Brosius J., Gilbert W. An 'internal' signal sequence directs secretion and processing or proinsulin in bacteria. Nature. 1981 Nov 12;294(5837):176–178. doi: 10.1038/294176a0. [DOI] [PubMed] [Google Scholar]
- Talmadge K., Gilbert W. Cellular location affects protein stability in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1830–1833. doi: 10.1073/pnas.79.6.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K., Kaufman J., Gilbert W. Bacteria mature preproinsulin to proinsulin. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3988–3992. doi: 10.1073/pnas.77.7.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Blomberg C. Trans-membrane translocation of proteins. The direct transfer model. Eur J Biochem. 1979 Jun;97(1):175–181. doi: 10.1111/j.1432-1033.1979.tb13100.x. [DOI] [PubMed] [Google Scholar]



