Abstract
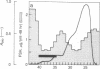
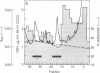
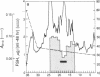
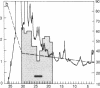
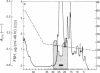
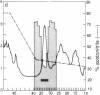
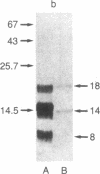
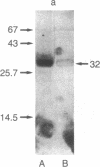
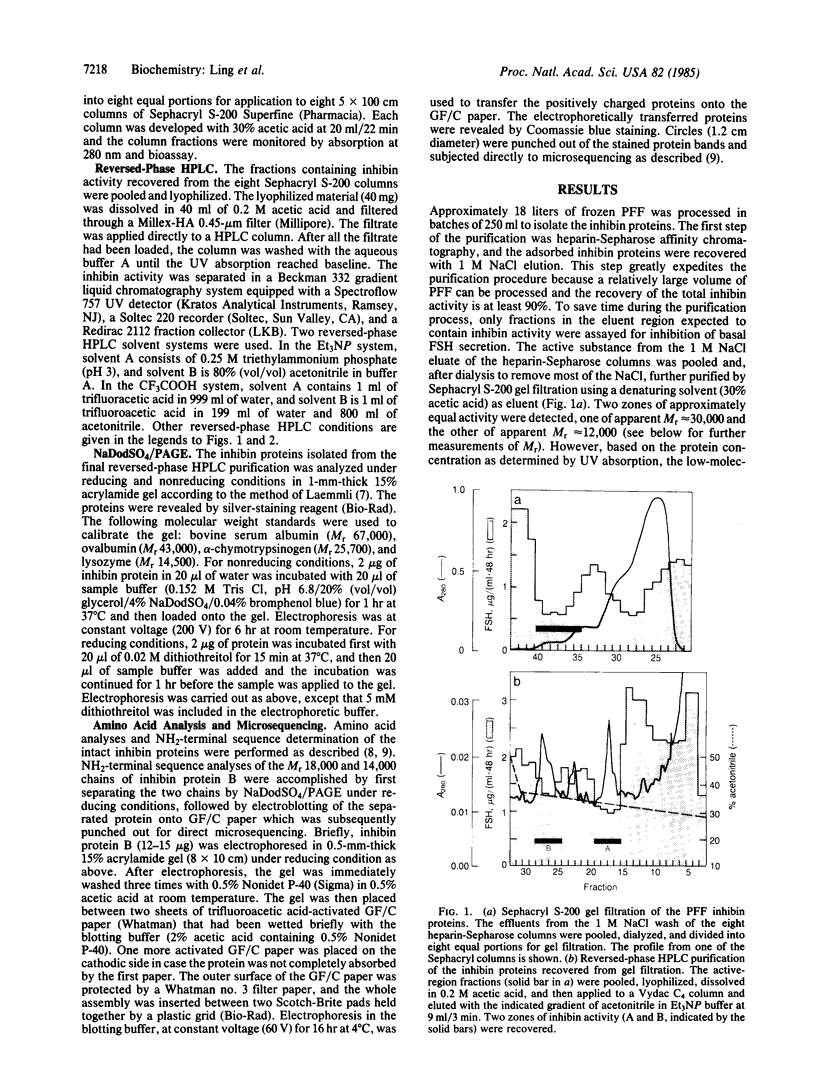
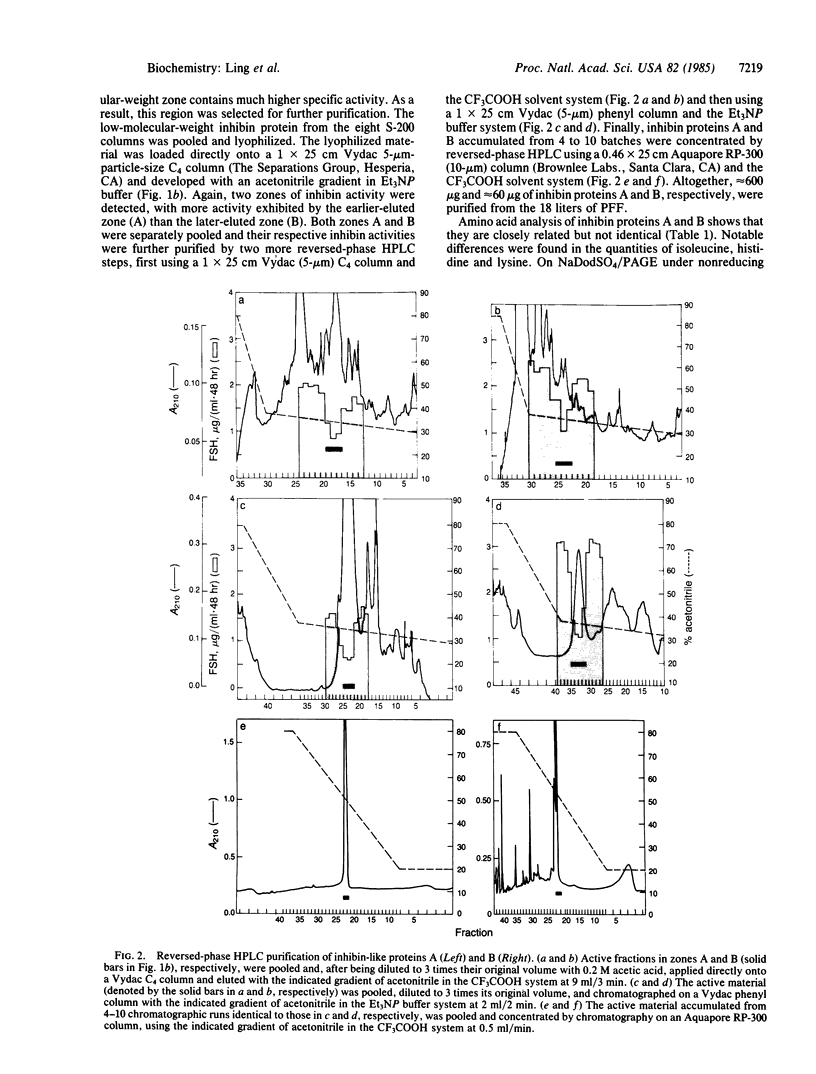
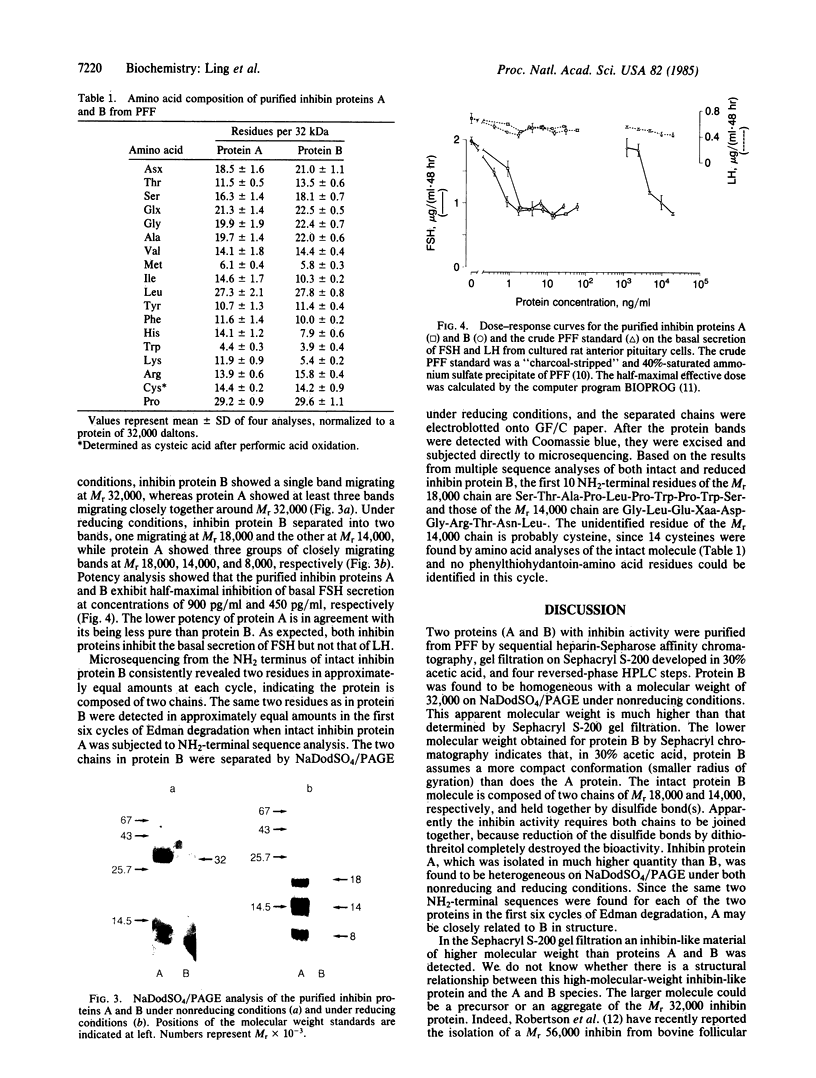
A Mr 32,000 protein with inhibin activity was isolated from porcine follicular fluid by heparin-Sepharose affinity chromatography, gel filtration on Sephacryl S-200, and four reversed-phase HPLC steps. The isolated molecule is composed of two chains having molecular weights of 18,000 and 14,000, respectively, and bound together by disulfide bonds. Amino acid sequence analysis revealed the 10 NH2-terminal residues of the Mr 18,000 chain to be Ser-Thr-Ala-Pro-Leu-Pro-Trp-Pro-Trp-Ser- and those of the Mr 14,000 chain to be Gly-Leu-Glu-Xaa-Asp-Gly-Arg-Thr-Asn-Leu-. This Mr 32,000 protein specifically inhibits the basal secretion of FSH, but not that of LH, in the rat anterior pituitary monolayer culture system, with a half-maximal effective dose of 450 pg/ml.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbatti N. J., Seidah N. G., Rochemont J., Escher E., Sheth A. R., Chrétien M. beta 2-Inhibin contains the active core of human seminal plasma beta-inhibin: synthesis and bioactivity. FEBS Lett. 1985 Feb 11;181(1):57–63. doi: 10.1016/0014-5793(85)81113-3. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Schroeder R. High-sensitivity amino acid analysis: methodology for the determination of amino acid compositions with less than 100 picomoles of peptides. Anal Biochem. 1982 Oct;126(1):144–152. doi: 10.1016/0003-2697(82)90120-8. [DOI] [PubMed] [Google Scholar]
- Esch F. S. Polypeptide microsequence analysis with the commercially available gas-phase sequencer. Anal Biochem. 1984 Jan;136(1):39–47. doi: 10.1016/0003-2697(84)90305-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li C. H., Hammonds R. G., Jr, Ramasharma K., Chung D. Human seminal alpha inhibins: isolation, characterization, and structure. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4041–4044. doi: 10.1073/pnas.82.12.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja H., Jeppsson J. O. Amino acid sequence of the predominant basic protein in human seminal plasma. FEBS Lett. 1985 Mar 11;182(1):181–184. doi: 10.1016/0014-5793(85)81179-0. [DOI] [PubMed] [Google Scholar]
- McCullagh D. R. DUAL ENDOCRINE ACTIVITY OF THE TESTES. Science. 1932 Jul 1;76(1957):19–20. doi: 10.1126/science.76.1957.19. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Hasegawa Y., Fukuda M., Nomura M., Igarashi M., Kangawa K., Matsuo H. Isolation of porcine follicular fluid inhibin of 32K daltons. Biochem Biophys Res Commun. 1985 Jun 14;129(2):396–403. doi: 10.1016/0006-291x(85)90164-0. [DOI] [PubMed] [Google Scholar]
- Ramasharma K., Sairam M. R., Seidah N. G., Chrétien M., Manjunath P., Schiller P. W., Yamashiro D., Li C. H. Isolation, structure, and synthesis of a human seminal plasma peptide with inhibin-like activity. Science. 1984 Mar 16;223(4641):1199–1202. doi: 10.1126/science.6422553. [DOI] [PubMed] [Google Scholar]
- Robertson D. M., Foulds L. M., Leversha L., Morgan F. J., Hearn M. T., Burger H. G., Wettenhall R. E., de Kretser D. M. Isolation of inhibin from bovine follicular fluid. Biochem Biophys Res Commun. 1985 Jan 16;126(1):220–226. doi: 10.1016/0006-291x(85)90594-7. [DOI] [PubMed] [Google Scholar]
- Rodbard D. Statistical quality control and routine data processing for radioimmunoassays and immunoradiometric assays. Clin Chem. 1974 Oct;20(10):1255–1270. [PubMed] [Google Scholar]
- Schwartz N. B., Channing C. P. Evidence for ovarian "inhibin": suppression of the secondary rise in serum follicle stimulating hormone levels in proestrous rats by injection of porcine follicular fluid. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5721–5724. doi: 10.1073/pnas.74.12.5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah N. G., Arbatti N. J., Rochemont J., Sheth A. R., Chrétien M. Complete amino acid sequence of human seminal plasma beta-inhibin. Prediction of post Gln-Arg cleavage as a maturation site. FEBS Lett. 1984 Oct 1;175(2):349–355. doi: 10.1016/0014-5793(84)80766-8. [DOI] [PubMed] [Google Scholar]
- Vale W., Grant G., Amoss M., Blackwell R., Guillemin R. Culture of enzymatically dispersed pituitary cells: functional validation of a method. Endocrinology. 1972 Aug;91(2):562–572. doi: 10.1210/endo-91-2-562. [DOI] [PubMed] [Google Scholar]
- Yamashiro D., Li C. H., Ramasharma K., Sairam M. R. Synthesis and biological activity of human inhibin-like peptide-(1-31). Proc Natl Acad Sci U S A. 1984 Sep;81(17):5399–5402. doi: 10.1073/pnas.81.17.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong F. H. Inhibin--fact or artifact. Mol Cell Endocrinol. 1979 Jan;13(1):1–10. doi: 10.1016/0303-7207(79)90071-6. [DOI] [PubMed] [Google Scholar]