Abstract
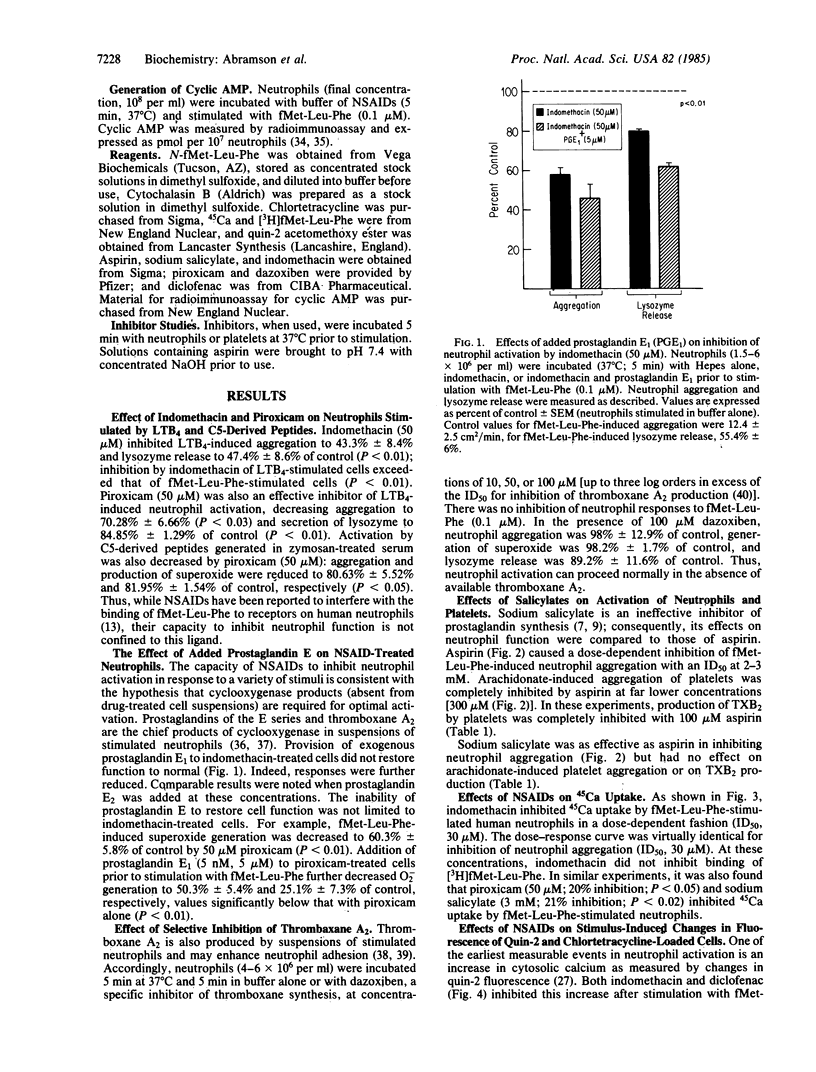
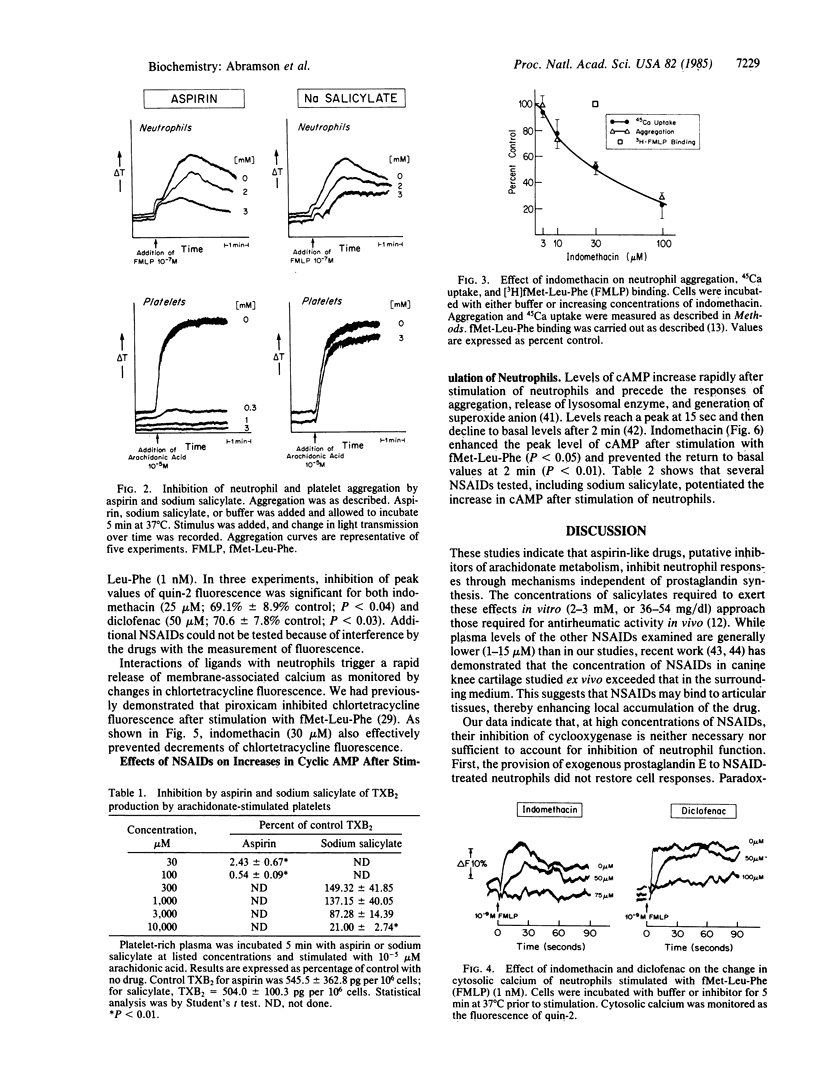
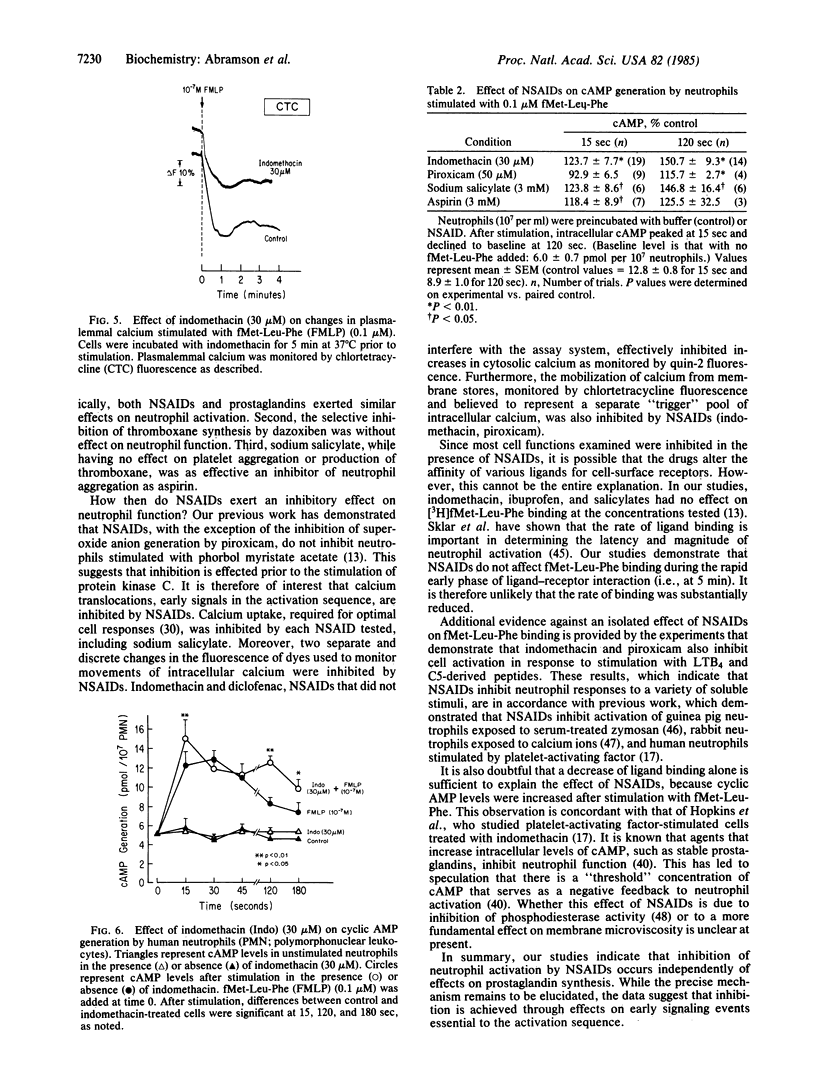
Current dogma holds that nonsteroidal anti-inflammatory drugs (NSAIDs) act by inhibition of the synthesis and release of prostaglandins. However, NSAIDs also inhibit the activation of neutrophils, which provoke inflammation by releasing products other than prostaglandins. We now report that NSAIDs (e.g., indomethacin, piroxicam) inhibit activation of neutrophils by inflammatory stimuli, such as C5-derived peptides and leukotriene B4, even when cyclooxygenase products generated in suspensions of stimulated neutrophils (prostaglandin E and thromboxanes) are present. Sodium salicylate (3 mM) greatly inhibited aggregation of neutrophils but had no effect on aggregation of platelets or production of thromboxane induced by arachidonate. Sodium salicylate and other NSAIDs also inhibit calcium movements (45Ca uptake, changes in fluorescence of chlortetracycline and quin-2). Aspirin, sodium salicylate, indomethacin, and piroxicam also enhanced the poststimulation increase in intracellular cyclic AMP. NSAIDs therefore inhibit early steps in neutrophil activation as reflected by their capacity to inhibit movements of Ca and to enhance intracellular levels of cyclic AMP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Edelson H. S., Kaplan H. B., Korchak H. M., Smolen J. E., Weissmann G. Dissociation by piroxicam of degranulation and superoxide anion generation from decrements in chlortetracycline fluorescence of activated human neutrophils. Biochem Biophys Res Commun. 1982 Jan 15;104(1):247–253. doi: 10.1016/0006-291x(82)91966-0. [DOI] [PubMed] [Google Scholar]
- Flower R. J. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974 Mar;26(1):33–67. [PubMed] [Google Scholar]
- Ford-Hutchinson A. W. Neutrophil aggregating properties of PAF-acether and leukotriene B4. Int J Immunopharmacol. 1983;5(1):17–21. doi: 10.1016/0192-0561(83)90067-x. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Brindley L. L., Goldman D. W. Enhancement of human neutrophil adherence by synthetic leukotriene constituents of the slow-reacting substance of anaphylaxis. Immunology. 1983 Sep;50(1):35–41. [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Brai M., Osler A. G., Weissmann G. Lysosomal enzyme release from human leukocytes: mediation by the alternate pathway of complement activation. J Immunol. 1973 Jul;111(1):33–37. [PubMed] [Google Scholar]
- Goldstein I. M., Cerqueira M., Lind S., Kaplan H. B. Evidence that the superoxide-generating system of human leukocytes is associated with the cell surface. J Clin Invest. 1977 Feb;59(2):249–254. doi: 10.1172/JCI108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Malmsten C. L., Kindahl H., Kaplan H. B., Rådmark O., Samuelsson B., Weissmann G. Thromboxane generation by human peripheral blood polymorphonuclear leukocytes. J Exp Med. 1978 Sep 1;148(3):787–792. doi: 10.1084/jem.148.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Herlin T., Petersen C. S., Esmann V. The role of calcium and cyclic adenosine 3',5'-monophosphate in the regulation of glycogen metabolism in phagocytozing human polymorphonuclear leukocytes. Biochim Biophys Acta. 1978 Aug 3;542(1):63–76. doi: 10.1016/0304-4165(78)90233-7. [DOI] [PubMed] [Google Scholar]
- Higgs G. A., Harvey E. A., Ferreira S. H., Vane J. R. The effects of antiinflammatory drugs on the production of prostaglandins in vivo. Adv Prostaglandin Thromboxane Res. 1976;1:105–110. [PubMed] [Google Scholar]
- Hopkins N. K., Lin A. H., Gorman R. R. Evidence for mediation of acetyl glyceryl ether phosphorylcholine stimulation of adenosine 3',5'-(cyclic)monophosphate levels in human polymorphonuclear leukocytes by leukotriene B4. Biochim Biophys Acta. 1983 Oct 25;763(3):276–283. doi: 10.1016/0167-4889(83)90135-0. [DOI] [PubMed] [Google Scholar]
- Humes J. L., Winter C. A., Sadowski S. J., Kuehl F. A., Jr Multiple sites on prostaglandin cyclooxygenase are determinants in the action of nonsteroidal antiinflammatory agents. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2053–2056. doi: 10.1073/pnas.78.4.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. B., Edelson H. S., Korchak H. M., Given W. P., Abramson S., Weissmann G. Effects of non-steroidal anti-inflammatory agents on human neutrophil functions in vitro and in vivo. Biochem Pharmacol. 1984 Feb 1;33(3):371–378. doi: 10.1016/0006-2952(84)90228-4. [DOI] [PubMed] [Google Scholar]
- Korchak H. M., Rutherford L. E., Weissmann G. Stimulus response coupling in the human neutrophil. I. Kinetic analysis of changes in calcium permeability. J Biol Chem. 1984 Apr 10;259(7):4070–4075. [PubMed] [Google Scholar]
- Korchak H. M., Vienne K., Rutherford L. E., Wilkenfeld C., Finkelstein M. C., Weissmann G. Stimulus response coupling in the human neutrophil. II. Temporal analysis of changes in cytosolic calcium and calcium efflux. J Biol Chem. 1984 Apr 10;259(7):4076–4082. [PubMed] [Google Scholar]
- Korchak H. M., Wilkenfeld C., Rich A. M., Radin A. R., Vienne K., Rutherford L. E. Stimulus response coupling in the human neutrophil. Differential requirements for receptor occupancy in neutrophil responses to a chemoattractant. J Biol Chem. 1984 Jun 25;259(12):7439–7445. [PubMed] [Google Scholar]
- Levin R. I., Jaffe E. A., Weksler B. B., Tack-Goldman K. Nitroglycerin stimulates synthesis of prostacyclin by cultured human endothelial cells. J Clin Invest. 1981 Mar;67(3):762–769. doi: 10.1172/JCI110093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Volpi M., Showell H. J., Becker E. L., Sha'afi R. I. Chemotactic factor-induced release of membrane calcium in rabbit neutrophils. Science. 1979 Feb 2;203(4379):461–463. doi: 10.1126/science.760200. [DOI] [PubMed] [Google Scholar]
- Palmoski M. J., Brandt K. D. Correction of data on salicylate and indomethacin concentrations in cartilage. Arthritis Rheum. 1985 Feb;28(2):237–237. doi: 10.1002/art.1780280226. [DOI] [PubMed] [Google Scholar]
- Palmoski M. J., Brandt K. D. Effects of salicylate and indomethacin on glycosaminoglycan and prostaglandin E2 synthesis in intact canine knee cartilage ex vivo. Arthritis Rheum. 1984 Apr;27(4):398–403. doi: 10.1002/art.1780270406. [DOI] [PubMed] [Google Scholar]
- Pedersen A. K., FitzGerald G. A. Dose-related kinetics of aspirin. Presystemic acetylation of platelet cyclooxygenase. N Engl J Med. 1984 Nov 8;311(19):1206–1211. doi: 10.1056/NEJM198411083111902. [DOI] [PubMed] [Google Scholar]
- Perianin A., Roch-Arveiller M., Giroud J. P., Hakim J. In vivo interaction of nonsteroidal anti-inflammatory drugs on the locomotion of neutrophils elicited by acute non-specific inflammations in the rat--effect of indomethacin, ibuprofen and flurbiprofen. Biochem Pharmacol. 1984 Jul 15;33(14):2239–2243. doi: 10.1016/0006-2952(84)90661-0. [DOI] [PubMed] [Google Scholar]
- Perianin A., Torres M., Labro M. T., Hakim J. The different inhibitory effects of phenylbutazone on soluble and particle stimulation of human neutrophil oxidative burst. Biochem Pharmacol. 1983 Sep 15;32(18):2819–2822. doi: 10.1016/0006-2952(83)90098-9. [DOI] [PubMed] [Google Scholar]
- Randall M. J., Parry M. J., Hawkeswood E., Cross P. E., Dickinson R. P. UK-37, 248, a novel, selective thromboxane synthetase inhibitor with platelet anti-aggregatory and anti-thrombotic activity. Thromb Res. 1981 Jul 1;23(1-2):145–162. doi: 10.1016/0049-3848(81)90247-4. [DOI] [PubMed] [Google Scholar]
- Rich A. M., Weissmann G., Anderson C., Vosshall L., Haines K. A., Humphreys T., Dunham P. Calcium dependent aggregation of marine sponge cells is provoked by leukotriene B4 and inhibited by inhibitors of arachidonic acid oxidation. Biochem Biophys Res Commun. 1984 Jun 29;121(3):863–870. doi: 10.1016/0006-291x(84)90757-5. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Fischbein L. C., Spilberg I., Atkinson J. P. Induction of a transient elevation in intracellular levels of adenosine-3',5'-cyclic monophosphate by chemotactic factors: an early event in human neutrophil activation. J Immunol. 1980 Mar;124(3):1482–1491. [PubMed] [Google Scholar]
- Simchowitz L., Mehta J., Spilberg I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: effect of metabolic inhibitors and antiinflammatory drugs. Arthritis Rheum. 1979 Jul;22(7):755–763. doi: 10.1002/art.1780220711. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Jesaitis A. J., Painter R. G., Cochrane C. G. The kinetics of neutrophil activation. The response to chemotactic peptides depends upon whether ligand-receptor interaction is rate-limiting. J Biol Chem. 1981 Oct 10;256(19):9909–9914. [PubMed] [Google Scholar]
- Smith R. J. Nonsteroid anti-inflammatory agents: regulators of the phagocytic secretion of lysosomal enzymes from guinea-pig neutrophils. J Pharmacol Exp Ther. 1978 Nov;207(2):618–629. [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. Increased levels of cyclic adenosine-3',5'-monophosphate in human polymorphonuclear leukocytes after surface stimulation. J Clin Invest. 1980 May;65(5):1077–1085. doi: 10.1172/JCI109760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnuolo P. J., Ellner J. J., Hassid A., Dunn M. J. Thromboxane A2 mediates augmented polymorphonuclear leukocyte adhesiveness. J Clin Invest. 1980 Sep;66(3):406–414. doi: 10.1172/JCI109870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovich V. Inhibition of 3',5'-cyclic AMP phosphodiesterase with anti-inflammatory agents. Res Commun Chem Pathol Pharmacol. 1974 Mar;7(3):573–582. [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L., WACKER W. E. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956 Sep 6;255(10):450–456. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J. Acute hypotension due to carrageenan, arachidonic acid and slow reacting substance C in the rabbit: role of platelets and nature of pharmacological antagonism. Eur J Pharmacol. 1977 May 15;43(2):125–141. doi: 10.1016/0014-2999(77)90125-x. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Smith M. J., Ford-Hutchinson A. W. Anti-inflammatory drugs, prostaglandins and leucocyte migration. Agents Actions. 1976 Sep;6(5):602–606. doi: 10.1007/BF01971577. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Quagliata F. Effect of prostaglandin E 1 on adjuvant arthritis. Nature. 1971 Dec 3;234(5327):304–305. doi: 10.1038/234304a0. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]


