Abstract
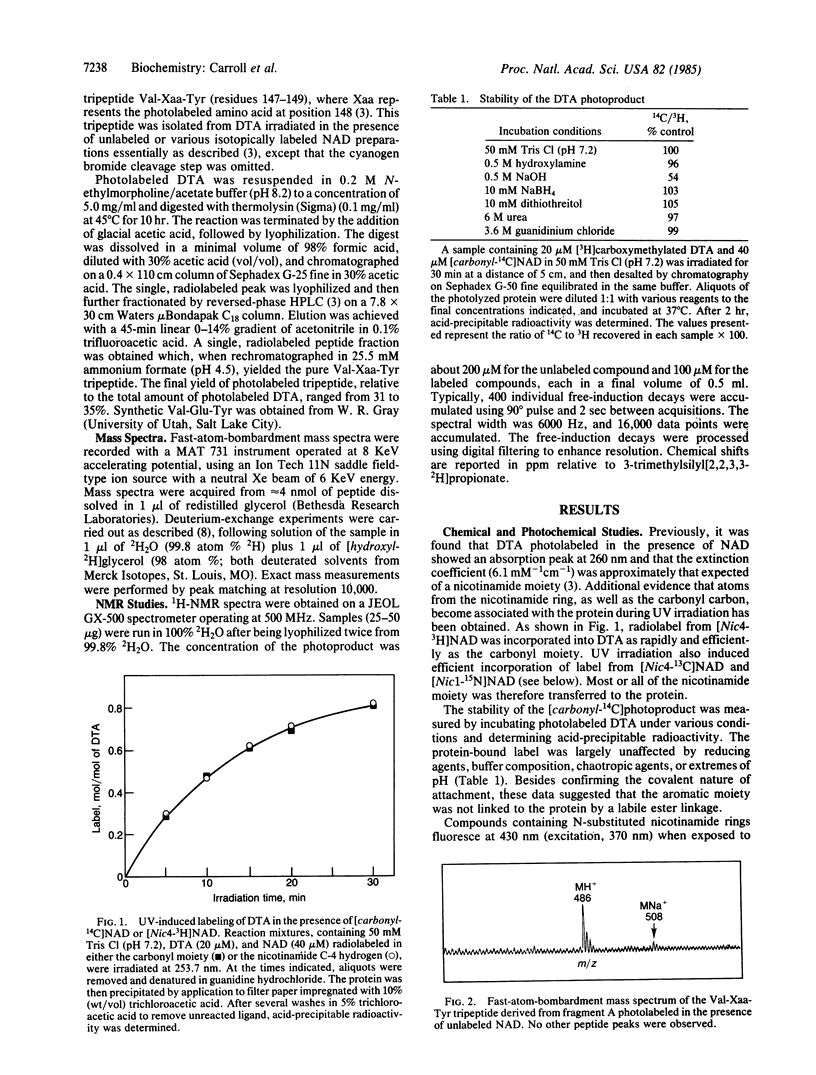
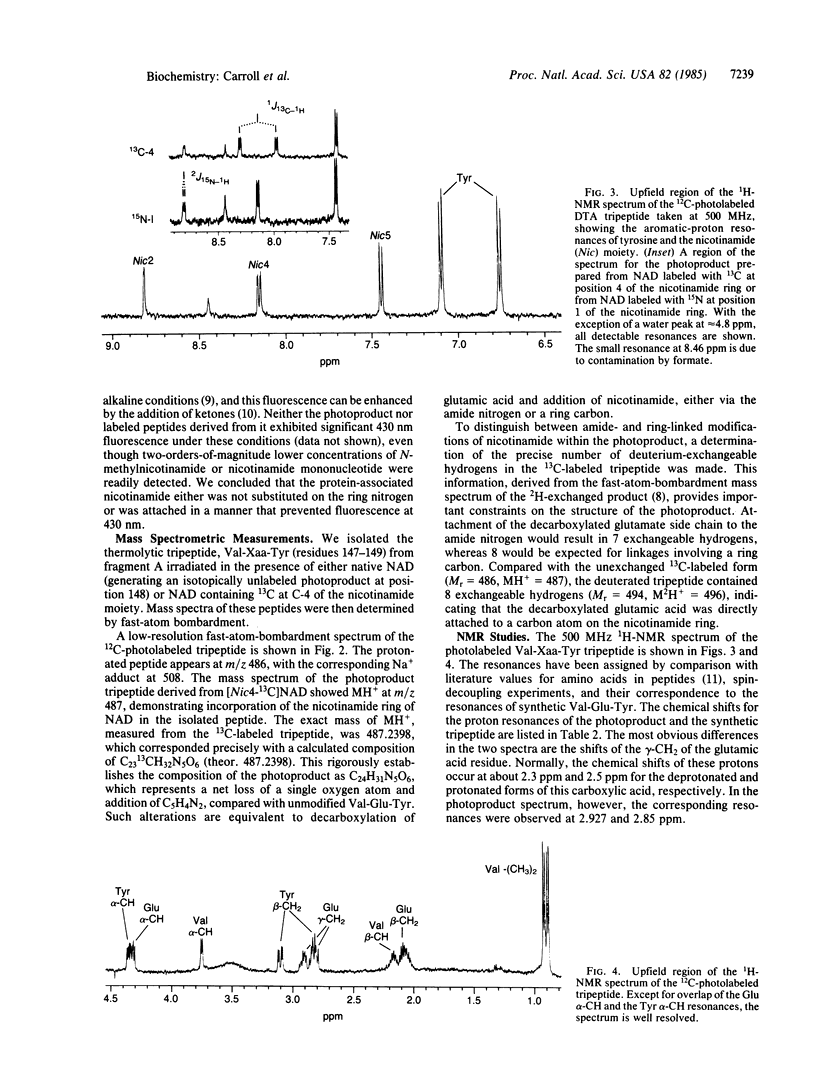
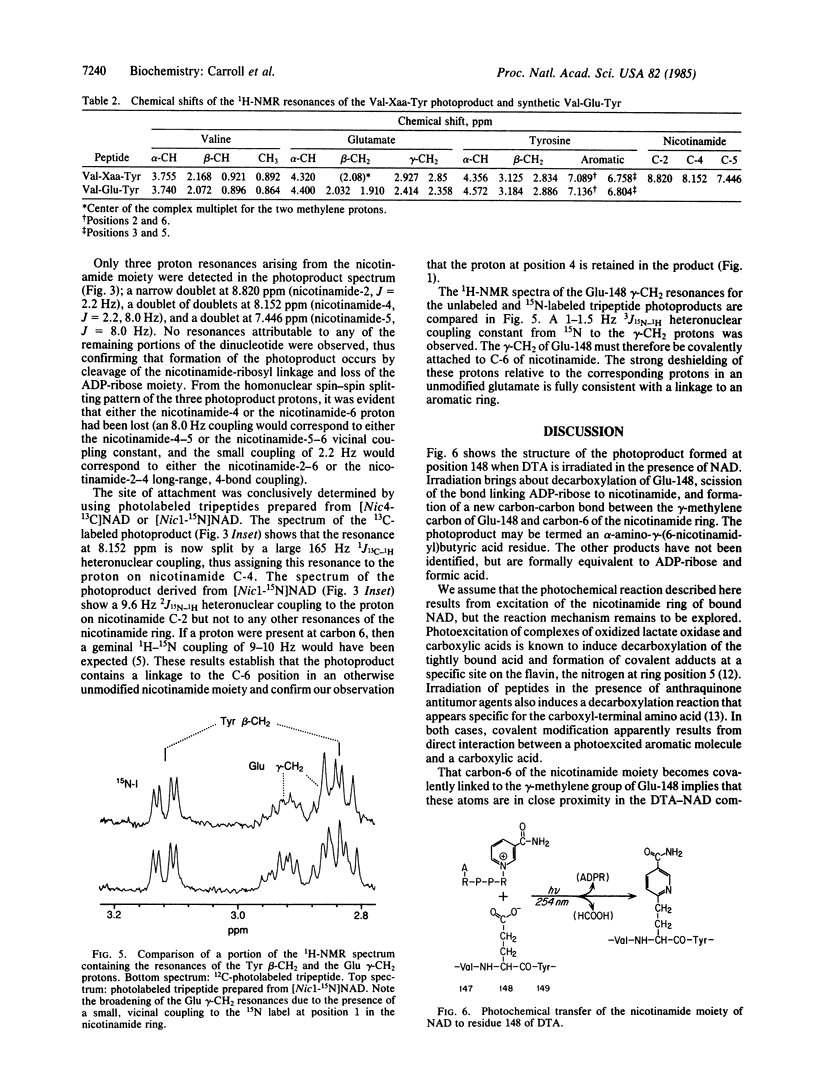
Irradiation of mixtures of diphtheria toxin fragment A and [carbonyl-14C]NAD with UV light (253.7 nm) is known to induce efficient transfer of the radiolabel to position 148, corresponding to glutamic acid in the unmodified protein. Here we report the structure of the photoproduct at position 148, as determined by chemical and photochemical methods, fast-atom-bombardment mass spectrometry, and nuclear magnetic resonance. The photoproduct [an alpha-amino-gamma-(6-nicotin-amidyl)butyric acid residue] contains the entire nicotinamide moiety of NAD linked via its number 6 carbon to the decarboxylated gamma-methylene carbon of Glu-148. No portion of the ADP-ribosyl group of NAD is present. These findings are consistent with the idea that Glu-148 lies at or near the catalytic center of diphtheria toxin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carmichael A. J., Riesz P. Photoinduced reactions of anthraquinone antitumor agents with peptides and nucleic acid bases: an electron spin resonance and spin trapping study. Arch Biochem Biophys. 1985 Mar;237(2):433–444. doi: 10.1016/0003-9861(85)90297-8. [DOI] [PubMed] [Google Scholar]
- Carroll S. F., Collier R. J. NAD binding site of diphtheria toxin: identification of a residue within the nicotinamide subsite by photochemical modification with NAD. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3307–3311. doi: 10.1073/pnas.81.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B. R., Halpern R. M., Smith R. A. A fluorimetric method for quantitation in the picomole range of N1-methylnicotinamide and nicotinamide in serum. Anal Biochem. 1975 Sep;68(1):54–61. doi: 10.1016/0003-2697(75)90678-8. [DOI] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J., Westbrook E. M., McKay D. B., Eisenberg D. X-ray grade crystals of diphtheria toxin. J Biol Chem. 1982 May 10;257(9):5283–5285. [PubMed] [Google Scholar]
- Ghisla S., Massey V., Choong Y. S. Covalent adducts of lactate oxidase. Photochemical formation and structure identification. J Biol Chem. 1979 Nov 10;254(21):10662–10669. [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., BARNES C. C. Effect of alkali on diphosphopyridine nucleotide. J Biol Chem. 1951 Aug;191(2):461–472. [PubMed] [Google Scholar]
- Kandel J., Collier R. J., Chung D. W. Interaction of fragment A from diphtheria toxin with nicotinamide adenine dinucleotide. J Biol Chem. 1974 Apr 10;249(7):2088–2097. [PubMed] [Google Scholar]
- McKeever B., Sarma R. Preliminary crystallographic investigation of the protein toxin from corynebacterium diphtheriae. J Biol Chem. 1982 Jun 25;257(12):6923–6925. [PubMed] [Google Scholar]
- Sethi S. K., Smith D. L., McCloskey J. A. Determination of active hydrogen content by fast atom bombardment mass spectrometry following hydrogen-deuterium exchange. Biochem Biophys Res Commun. 1983 Apr 15;112(1):126–131. doi: 10.1016/0006-291x(83)91806-5. [DOI] [PubMed] [Google Scholar]
- Tweten R. K., Barbieri J. T., Collier R. J. Diphtheria toxin. Effect of substituting aspartic acid for glutamic acid 148 on ADP-ribosyltransferase activity. J Biol Chem. 1985 Sep 5;260(19):10392–10394. [PubMed] [Google Scholar]
- Williams T. J., Zens A. P., Wisowaty J. C., Fisher R. R., Dunlap R. B., Bryson T. A., Ellis P. D. Nuclear magnetic resonance studies on pyridine dinucleotides. The pH dependence of the carbon-13 nuclear magnetic resonance of NAD+ analogs. Arch Biochem Biophys. 1976 Feb;172(2):490–501. doi: 10.1016/0003-9861(76)90102-8. [DOI] [PubMed] [Google Scholar]


