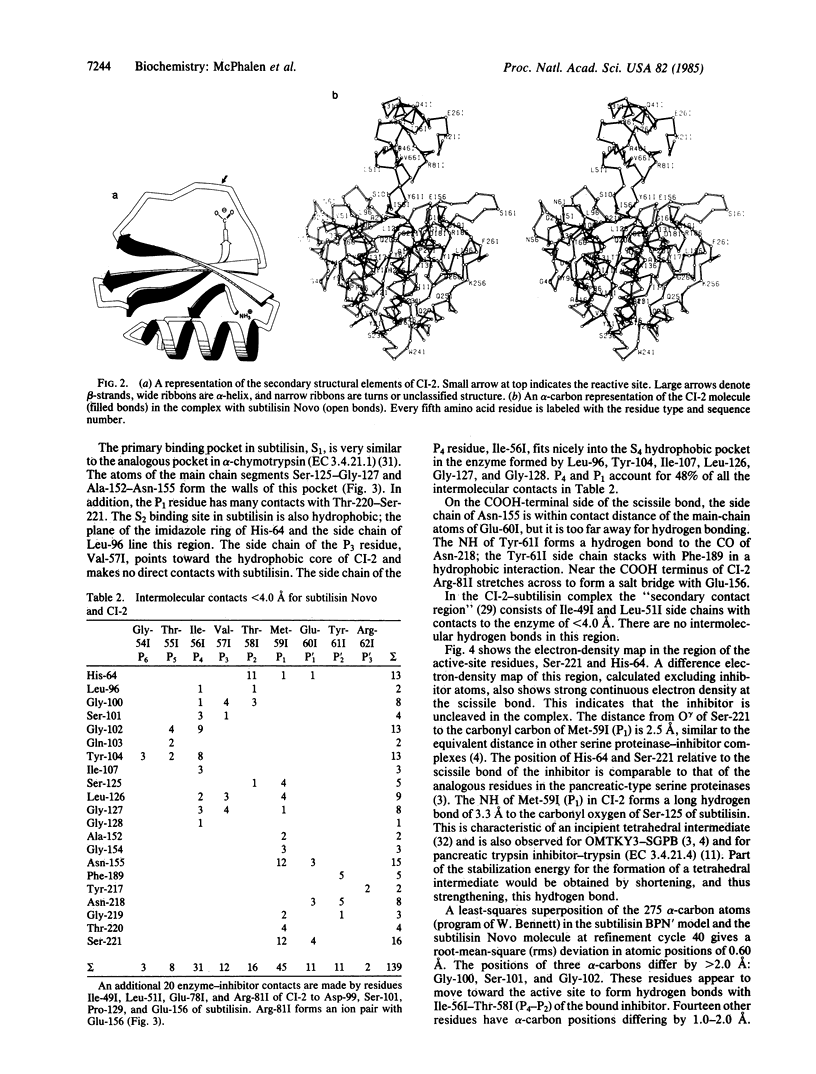
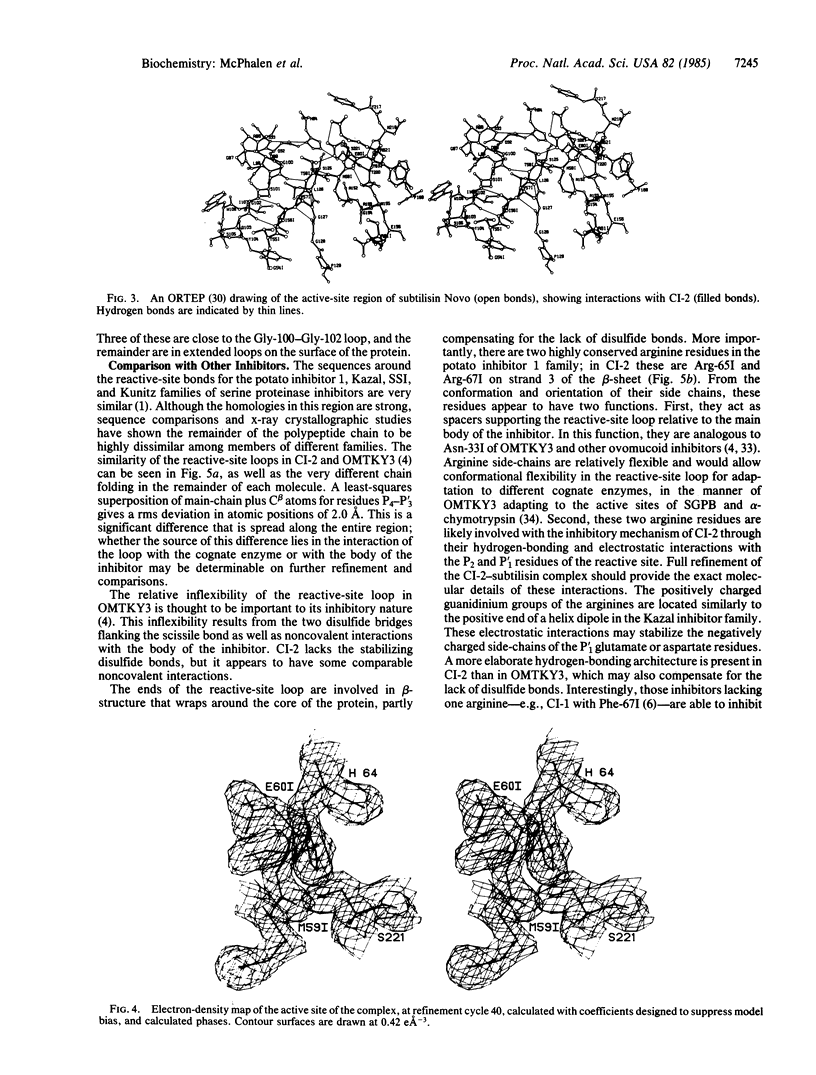
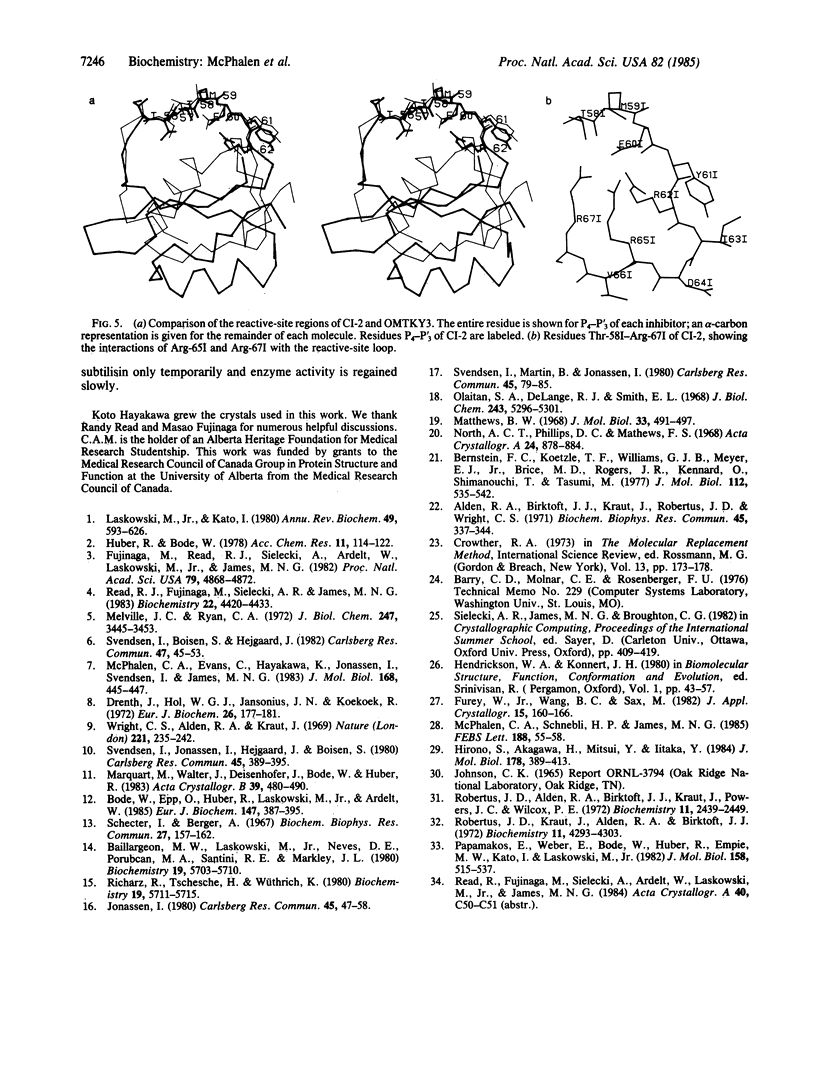
Abstract
The serine proteinase inhibitor from barley seeds, chymotrypsin inhibitor 2(CI-2), has been crystallized in a molecular complex with subtilisin Novo (EC 3.4.21.14). The crystal structure of this complex has been determined at 2.1-Å resolution by the molecular replacement method and partially refined by restrained-parameter least-squares methods. The present crystallographic R factor (ΣǁFo[unk] - [unk]Fcǁ/Σ[unk]Fo[unk]) is 0.193. CI-2 is a member of the potato inhibitor 1 family; it is a serine proteinase inhibitor lacking disulfide bonds. Comparison of the subtilisin molecule in this complex with the native subtilisin shows that the two molecules are very similar in structure. The inhibitor binds in a mode presumably resembling that of a true substrate, but it is not cleaved. This is in accord with the reported structures of other serine proteinase-inhibitor complexes. CI-2 consists of a four-stranded mixed parallel and antiparallel β-sheet against which an α-helix packs to form a hydrophobic core. A wide loop crossover connection between parallel strands 2 and 3 of the β-sheet contains the reactive-site bond. The conformation of the four residues to either side of the reactive-site bond is similar to that of the analogous residues in the third domain of the turkey ovomucoid inhibitor (Kazal family); the overall polypeptide chain fold of these inhibitors and the location of the reactive site in the respective chains are different.
Keywords: serine proteinase, potato inhibitor 1, molecular replacement method, crystallography
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alden R. A., Birktoft J. J., Kraut J., Robertus J. D., Wright C. S. Atomic coordinates for subtilisin BPN' (or Novo). Biochem Biophys Res Commun. 1971 Oct 15;45(2):337–344. doi: 10.1016/0006-291x(71)90823-0. [DOI] [PubMed] [Google Scholar]
- Baillargeon M. W., Laskowski M., Jr, Neves D. E., Porubcan M. A., Santini R. E., Markely J. L. Soybean trypsin inhibitor (Kunitz) and its complex with trypsin. Carbon-13 nuclear magnetic resonance studies of the reactive site arginine. Biochemistry. 1980 Dec 9;19(25):5703–5703. doi: 10.1021/bi00566a006. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Bode W., Epp O., Huber R., Laskowski M., Jr, Ardelt W. The crystal and molecular structure of the third domain of silver pheasant ovomucoid (OMSVP3). Eur J Biochem. 1985 Mar 1;147(2):387–395. doi: 10.1111/j.1432-1033.1985.tb08762.x. [DOI] [PubMed] [Google Scholar]
- Drenth J., Hol W. G., Jansonius J. N., Koekoek R. Subtilisin Novo. The three-dimensional structure and its comparison with subtilisin BPN'. Eur J Biochem. 1972 Mar 27;26(2):177–181. doi: 10.1111/j.1432-1033.1972.tb01754.x. [DOI] [PubMed] [Google Scholar]
- Fujinaga M., Read R. J., Sielecki A., Ardelt W., Laskowski M., Jr, James M. N. Refined crystal structure of the molecular complex of Streptomyces griseus protease B, a serine protease, with the third domain of the ovomucoid inhibitor from turkey. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4868–4872. doi: 10.1073/pnas.79.16.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono S., Akagawa H., Mitsui Y., Iitaka Y. Crystal structure at 2.6 A resolution of the complex of subtilisin BPN' with streptomyces subtilisin inhibitor. J Mol Biol. 1984 Sep 15;178(2):389–414. doi: 10.1016/0022-2836(84)90150-5. [DOI] [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- McPhalen C. A., Evans C., Hayakawa K., Jonassen I., Svendsen I., James M. N. Preliminary crystallographic data for the serine protease inhibitor CI-2 from barley seeds. J Mol Biol. 1983 Aug 5;168(2):445–447. doi: 10.1016/s0022-2836(83)80028-x. [DOI] [PubMed] [Google Scholar]
- McPhalen C. A., Schnebli H. P., James M. N. Crystal and molecular structure of the inhibitor eglin from leeches in complex with subtilisin Carlsberg. FEBS Lett. 1985 Aug 19;188(1):55–58. doi: 10.1016/0014-5793(85)80873-5. [DOI] [PubMed] [Google Scholar]
- Melville J. C., Ryan C. A. Chymotrypsin inhibitor I from potatoes. Large scale preparation and characterization of its subunit components. J Biol Chem. 1972 Jun 10;247(11):3445–3453. [PubMed] [Google Scholar]
- Olaitan S. A., DeLange R. J., Smith E. L. The structure of subtilisin Novo. J Biol Chem. 1968 Oct 25;243(20):5296–5301. [PubMed] [Google Scholar]
- Papamokos E., Weber E., Bode W., Huber R., Empie M. W., Kato I., Laskowski M., Jr Crystallographic refinement of Japanese quail ovomucoid, a Kazal-type inhibitor, and model building studies of complexes with serine proteases. J Mol Biol. 1982 Jul 5;158(3):515–537. doi: 10.1016/0022-2836(82)90212-1. [DOI] [PubMed] [Google Scholar]
- Read R. J., Fujinaga M., Sielecki A. R., James M. N. Structure of the complex of Streptomyces griseus protease B and the third domain of the turkey ovomucoid inhibitor at 1.8-A resolution. Biochemistry. 1983 Sep 13;22(19):4420–4433. doi: 10.1021/bi00288a012. [DOI] [PubMed] [Google Scholar]
- Richarz R., Tschesche H., Wüthrich K. Carbon-13 nuclear magnetic resonance studies of the selectively isotope-labeled reactive site peptide bond of the basic pancreatic trypsin inhibitor in the complexes with trypsin, trypsinogen, and anhydrotrypsin. Biochemistry. 1980 Dec 9;19(25):5711–5715. doi: 10.1021/bi00566a007. [DOI] [PubMed] [Google Scholar]
- Robertus J. D., Alden R. A., Birktoft J. J., Kraut J., Powers J. C., Wilcox P. E. An x-ray crystallographic study of the binding of peptide chloromethyl ketone inhibitors to subtilisin BPN'. Biochemistry. 1972 Jun 20;11(13):2439–2449. doi: 10.1021/bi00763a009. [DOI] [PubMed] [Google Scholar]
- Robertus J. D., Kraut J., Alden R. A., Birktoft J. J. Subtilisin; a stereochemical mechanism involving transition-state stabilization. Biochemistry. 1972 Nov 7;11(23):4293–4303. doi: 10.1021/bi00773a016. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Wright C. S., Alden R. A., Kraut J. Structure of subtilisin BPN' at 2.5 angström resolution. Nature. 1969 Jan 18;221(5177):235–242. doi: 10.1038/221235a0. [DOI] [PubMed] [Google Scholar]


