Abstract
Aim: To confirm the hypothesis of the presence of a possible endometriosis inducing factor(s) (EIF) in the blood of women with endometriosis.
Patients and Methods: Forty infertile women were studied. The study group compromised of fifteen women of each three different degrees of endometriosis and fifteen women without endometriosis as a control group. Stem cells are characterized by being spindle shaped and proliferate in appropriate culture indefinitely. The women sera were co-cultured with mesenchymal stem cells (MSCs) which were followed up weekly to look for morphological changes and to detect Annexin 1 marker and ß-actin gene by reverse transcriptase polymerase chain reaction.
Results: MSCs cultured with sera of cases with, mild, moderate and severe endometriosis, showed morphological changes to be columnar and cuboidal shaped cells -resembling endometrial cells and glands- by the 4th week in 60%, 60% & 100% respectively. These cells were detected from as early as the first week in women with moderate and severe types (20% for each group). The percentage of the change into endometrial like cells increased among the three groups where it was 30±25.8%, 45±29.9% and 75±37.9% respectively. Moreover, increasing number of endometrial like cells are detected weekly, the more severe the disease is. None of the cultures of serum of the control group had made such changes all over the study. Furthermore, with more differentiation there was a considerable decrease in number of stem cells. These differentiated cells expressed the Annexin-1 marker.
Conclusion: It was evident that serum of women with endometriosis posses a factor(s) that enables the MSCs to be transformed into endometrial like cells and glands in vitro. This finding supports a new theory for the etiology of endometriosis. This observation may have a tremendous effect on the therapeutic implications of this debilitating condition.
Keywords: mesenchymal stem cells (MSCs), endometriosis
Introduction
Endometriosis is a common condition that results from the presence of endometrial glands and stroma outside the uterus. The exact incidence of endometriosis is uncertain because the disease process exists in several stages, from microscopic lesions to macroscopic disease, some of which are not apparent during evaluation. Although, the current prevalence of endometriosis is estimated to be up to 10%1, endometriosis is found in 40–60% of women with pelvic pain2 and in 20–30% of women suffering from infertility3. Moreover, women with more advanced disease have a higher rate of infertility4. Endometriosis can be found in different sites, however, it has been estimated that endometriosis is predominately found in ovaries (44%), Pouch of Douglas (38%) and vesico-uterine space (34%), uterosacral ligaments and surrounding pelvic peritoneum (22%)5. Additional sites include laparotomy 6, and episiotomy scars7, Appendix8, cervix9, pleura10, abdominal wall11-12, lungs13, nose14and rarely the brain15. The consequences of endometriosis include pelvic pain, dysparunia, pelvic adhesions and infertility16.
Endometriosis is a disease of theories, where the metaplasia theory suggests that under diverse influences, coelomic tissue could be transformed into endometrium17. Unfortunately, no direct evidence showing the formation of endometrial stroma has been reported at the end of the metaplastic process. Moreover, according to this theory, ectopic endometrium develops in situ from local tissues, including germinal epithelium of the ovary and remnants of the Müllerian and Wolffian ducts. In a broader context, this theory also implies that peritoneal endometriosis results from in situ metaplasia of totipotent mesothelial serosal cells18. The fact that endometriosis mostly occurs when endometrium is present, and that males are spared from this disease, weakens the power of the concept of metaplasia to explain endometriosis. Another theory proposes that the physiological phenomenon of endometrial reflux in the fallopian tubes during menstruation may, in certain conditions, overcome local defense mechanisms, implant, and proliferate19. However, the occurrence of endometriosis in sites very remote from pelvic organs directed the research towards other theories such as genetic background20, embryonic rest theory21 and stem cell dysfunction22.
Stem cells are primitive cells which are known for their capacity to self renew as well as to differentiate into one or more mature cell types23. A growing body of evidence has implicated stem cells as possible endometrial progenitors. In one study, bone marrow-derived stem cells have been identified in the endometrium of women who were bone marrow transplant recipients; these cells appear histologically indistinguishable from endogenous endometrial cells and express markers of glandular and stromal differentiation24. The examination of a sexually dimorphic organ such as the uterus demonstrates the ability of male bone marrow, which cannot harbor circulating endometrial cells, to generate endometrium de novo and proves their mesenchymal stem cell origin. In addition, finding Y chromosome bearing endometrial cells demonstrates the potential to recapitulate embryonic developmental pathways that were never activated in males22.
The aim of this study was to confirm the hypothesis of the presence of a possible endometriosis inducing factor(s) (EIF) in the blood of women with endometriosis that transforms allogenic stem cells into endometrial like cells.
Study Design
This was prospective case control experimental study that acquired the approval of Bioethical Committee of the National Research Center under number 09-079.
Setting
The study was carried out in collaboration between the Obstetrics and Gynecology Department, Cairo University;
Unit of Biochemistry and Molecular Biology, Medical Biochemistry Department, Cairo University; Reproductive Health Research Department, National Research Centre; and Biomedical Technology Department, National Research Center.
Patients and Methods
The study included thirty infertile women undergoing diagnostic laparoscopy for infertility work out. Fifteen cases served as a control group where the diagnostic laparoscopy showed no endometriotic implants. The other fifteen cases had endometriosis of variable degrees based on the modified American Fertility Society (mAFS) classification25. The study group was subdivided into 3 sub-groups of 5 women each, with mild, moderate and severe endometriosis. No women had history of immunological diseases or received any type of hormonal therapy in last six months before the diagnostic laparoscopy procedure. Venous blood samples were obtained and left to clot at 37°C, centrifuged and serum was separated and kept in sterile tubes at -20°C till later use.
Stem cell preparation
Human umbilical cord blood (UCB) was collected from mothers delivering at full term. Informed consent was obtained from all mothers in accordance with the Ethical Committee of National Research Centre guidelines, Research Ethical Committee of Kasr El-Aini teaching hospital and Islamic conference guidelines on stem cell research. In each sample, UCB was harvested in sterile tubes containing 100 mM EDTA as anticoagulant at 22°C and the low-density mononuclear cells were isolated using Ficoll-Plaque Plus (Amersham Biosciences, Sweden). Then, the cells were co-cultured in growth medium (Dulbecco’s Modified Eagle medium-low glucose) with the addition of 10% fetal bovine serum with 2 mmol/l L-glutamine and 0.3% penicillin-streptomycin (Gibco- BRL, USA). Co-cultures were incubated at 37°C and 5% CO2 concentration. The mesenchymal stem cells (MSCs) derived from UCB were propagated and characterized.
Follow-up
Ten μL serum samples from the different subgroups were added to the stem cell culture medium at 37°C26 and followed up weekly for 4 weeks for possible differentiation of stem cells into endometrial like cells. Thereafter, Annexin-1 gene expression in the cells was carried out using RT-PCR.
PCR detection of Annexin-1 gene expression
Total RNA was extracted from co-cultured cells using RNeasy Purification kit (Qiagen, Valencia, CA), and then 1 μg RNA was reverse transcribed with AMV reverse transcriptase for 30 min at 42 °C in the presence of oligo-dT primer. PCR amplification was performed using the following primer; forward 5’ GCAGGCCTGGTTTATTGAAA-3’, and reverse primer 5’-GCTGTGCATTGTTTCGCTTA-3’. For PCR reaction, 4 μl cDNA was added to 30.5 μ l water, 4 μl 25 mM MgCl2,I μ I dNTPs (10 mM) , 5 μl 10x PCR buffer, 0.5 μ l (2.5 U) Taq polymerase and 2.5 μl of each primer containing 10 pmol. The amplification profile was, denaturation at 95 °C for 30 s, annealing at 55°C for 30 s, and elongation at 72 °C for 1 min; for 35 cycles with final extension for 10 min at 72 °C. To exclude the possibility of contaminating genomic DNA, PCRs were also run without RT. The PCR product was separated by electrophoresis using 1% agarose gel. The presence of RNA in all tissues was assessed by analysis of the “house-keeping” gene -actin according to Chun-yan L, et al 27. The β- Actin gene expression was carried out to the sera of the control and study groups to confirm the integrity of RNA.
Results
There was no statistical significant difference between the two groups as regards the mean age (29.2 ± 4.5 vs. 31.7 ±3.8; P=0.2), duration of infertility (3 ± 0.8 vs. 2.5 ± 0.6; P=0.1) or their hormonal profile levels as FSH, LH and Progesterone (Table 1).
Table 1: Demographic characteristics of women included in the study
| Study group (n=15) | Control group (n=15) | P | |
| Age (years) | 29.2 ± 4.5 | 31.7 ±3.8 | 0.2 |
| Duration of infertility (years) | 3 ± 0.8 | 2.5 ± 0.6 | 0.1 |
| Serum FSH (mIU/mL) | 7.1 ± 0.8 | 7.8 ± 1.2 | 0.3 |
| Serum LH (mIU/mL) | 3.9 ± 0.7 | 5.8 ± 1.3 | 0.1 |
| Serum Progesterone (ng/Ml) | 15.3 ± 2.9 | 13.8 ± 1.4 | 0.1 |
Stem cells after co-culturing with serum of women with mild endometriosis. (a) After the 1st week MSCs showed no differentiation, (b) At the 2nd week the MSCs proliferation decreased and they start to differentiate into columnar- shaped cells (col). (c) On the 3rd week the MSCs were transformed into cuboidal shaped cells (cu). (d) After the 4th week some cells were found to be cuboidal cells while others acquired a columnar shape.
MSCs are spindle shaped cell, however, after co-culture with serum of women with varying degrees of endometriosis they showed morphological changes and were transformed into columnar and cuboidal shaped cells after variable period of time based on the disease severity. Furthermore, with more differentiation there was a considerable decrease in the number of stem cells with time and, even disappeared after 4 weeks in some cases. By co-culturing the sera of women with endometriosis and stem cells, it was shown that 60%, 60% & 100% of cultures of sera of cases with mild, moderate and severe endometriosis respectively have been transformed into cells resembling endometrial cells and glands by the 4th week (Table 2). These cells were detected from as early as the first week in women with moderate and severe types (20% for each group). The percentage of the change into endometrial like cells did increase among the three groups where it was 30±25.8%, 45±29.9% and 75±37.9%. Moreover, increasing number of transformed cells were detected weekly, the more severe the disease is, where no changes were seen in cultures of mild endometriosis by the first week then 20%, 40% and 60% by the second, third and fourth weeks respectively (Fig 1). Twenty percent of he serum cultures of women with moderate endometriosis showed the transformation from the first week and this trend was 40% by the subsequent week whereas, 60% of cultures were endometrial like by the third and fourth week (Fig 2). All cultures from women with severe endometriosis were endometrial like as early as the third week (Fig 3). None of the cultures of serum of the control group had made such changes all over the entire duration of the study (Fig 4).
Table 2: Incidence of morphological changes of cultured stem cells within each group
| Weeks | Mild endomet (n = 5) | Moderate endomet (n = 5) | severe endomet (n = 5) | Control Group (n = ′15) |
| 1 st week | 0/5 (0%) | 1/5 (20%) | 3/5 (60%) | 0/20 (0%) |
| 2nd week | 1/5 (20%) | 2/5 (40%) | 4/5 (80%) | 0/20 (0%) |
| 3rd week | 2/5 (40%) | 3/5 (60%) | 5/5 (100%) | 0/20 (0%) |
| 4th week | 3/5 (60%) | 3/5 (60%) | 5/5 (100%) | 0/20 (0%) |
| Percentage of Transfor | 30±25.8% | 45±29.9% | 75±37.9% | 0 % |
Figure 1.

Figure 2.
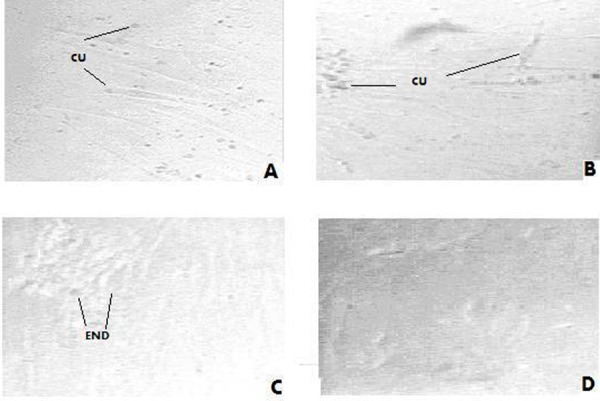
Figure 3.
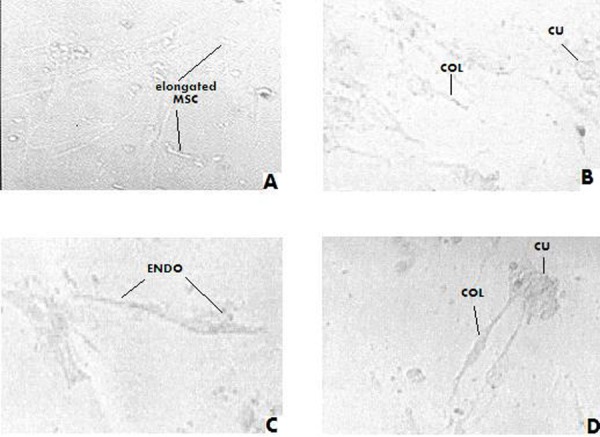
Figure 4.
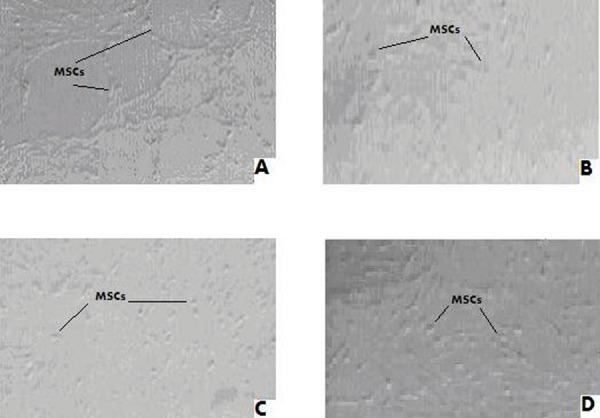
Stem cells after co-culturing with serum of women with moderate endometriosis. (a) After the 1st week MSCs were differentiate into cuboidal cells (cu), (b) After the 2nd week they were more cuboidal cells. (c) MSCs decreased in number with more differentiation after the 3rd week. (d) MSCs were totally disappeared from the culture dish after four weeks and they were replaced by endometrial like cells (end).
Stem cells after co- culturing with serum of women with severe endometriosis. (a) After 1st week MSCs became elongated. (b) After the 2nd week MSCs decreased in number and some cells were transformed into cuboidal cells (cu) and others acquired a columnar shape (col). (c) After the 3rd week MSCs became elongated with morphological changes consistent with endometrial like cells(end), the MSCs almost disappeared from the culture. (d) These changes were even marked on the 4th week.
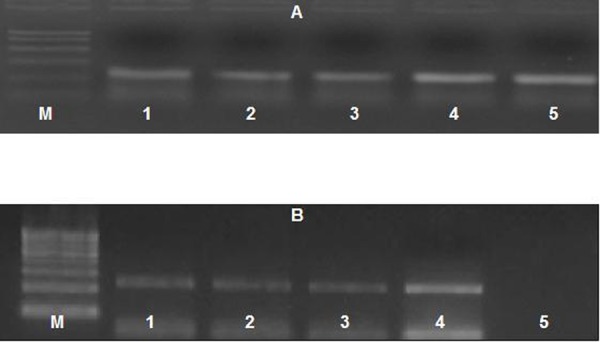
β-actin gene was expressed in all conditions confirming the integrality of RNA. While Annexin-1 gene was expressed only in MSCs cultured with serum of women with variable degrees of endometriosis, whereas MSCs cultured with serum of the control group didn’t show any expression. Furthermore, expression of Annexin-1 gene was also detected in serum of women with endometriosis, even more than the expression detected in MSCs (Fig 5).
Figure 5.
Stem cells after co-culturing with serum of women with no endometriosis (control group). (a) After 1st week, MSCs showed no differentiation. (b) MSCs were still undifferentiated after the second week of culture. (c) MSCs were also undifferentiated but their proliferation increased on the 3rd week).(d) The same observation was again noted by the 4th week of follow up. .
Ultra-violet trans-illuminated agarose gel showing PCR products of (A) β-actin gene expression (B) Annexin-1 gene, in the differentiated stem cells and serum of study group and control group. M lane: PCR marker, lane 1-3: gene expression in stem cells in mild, moderate, and severe cases respectively, lane 4: gene expression in serum of patient and lane 5: gene expression in control group.
Discussion
The stem cell theory explains how endometriosis can be found remote from the peritoneal cavity, resists some treatments, and occasionally occurs even after hysterectomy. Recently, it was demonstrated that bone marrow–derived (mesenchymal) stem cells could lead to expression of endometriosis in a mouse model. The work of Du and Taylor22 demonstrated how endometriosis was initiated from stem cells, where they removed the uterus of a mouse model so that endometriosis could not arise from endometrial cells (either through retrograde menstruation, or hematogenous or lymphatic dissemination). In their animal model, stem cells populated endometriotic implants, leading to disease progression28. Stem cells are present in nearly all organs of the body including the peritoneal cavity and uterus29-30.
Our results had convincingly proved that; by adding the serum of women with varying degrees of endometriosis to MSC for 4 weeks, the stem cells acquired endometrial morphology after cultivation. Whereas adding the serum of women without endometriosis could not lead to these morphological changes. The observed morphological changes included change from the spindle shape of mesenchymal stem cells31 into the columnar epithelium that looks similar to the lining of the endometrium32. In some cases, MSCs transformed into cuboidal cells that looks similar to the histological appearance of glandular cells. Also these changes were more evident, the more advanced the disease is (75%±37.9% of cases of severe endometriosis, versus 30%±25.8% of cases of mild endometriosis) but all the co-cultures showed these changes with variable intensity. In addition, according to the differentiation ability, stem cells culture from the study group was associated with less proliferation of cells hence these cells continued to decrease with time and even disappeared from some co-culture dishes. This phenomenon did not appear with co-cultures from the control group, where the proliferative potential of MSCs increased with time. Furthermore, the change of the stem cells status into endometrial like cells were confirmed by Annexin-1 expression. Annexin-1 protein had been shown to be a sensitive marker of endometrial cells27. We postulate a triggering substance spark for that endometrotic transformation and we named this substance as “Endometriosis Inducing Factor, EIF ”.
We observed that the more severe the endometriosis is, the earlier the transformation of the stem cells into endometrial like cells (0% & 60% in the 1st & 4th week of culturing cases with mild endometriosis versus 20% & 100% in same weeks of culturing cases with severe endometriosis. Therefore, we believe that the concentration of this EIF has a proportional relationship with the severity of endometriosis. To the best of our knowledge this is the first time that such a factor is postulated to explain the pathogenesis of endometriosis. This factor may be the signaling molecule that regulates primarily the survival, proliferation, and/or neoangiogenesis of allogenic stem cells into endometrial like implants. Furthermore, it may play a part in disturbing the normal regulation of cAMP, endometrial proliferation, survival, and embryonic receptivity in the endometrium. The nature and chemical composition of this factor had not been looked at during this study but further research is urgently needed to address this issue.
Conclusion
This study has proved that an endometriosis inducing factor (EIF) does exit in the serum of women suffering from endometriosis. This may have a tremendous effect on the therapeutic implications of this debilitating condition and opens up a new era in its management and therapy. This EIF supports a new theory for the etiology of endometriosis. We endorse the need for further research with large randomized control studies to confirm this finding and clarify the nature of this substance and its molecular composition.
References
- 1.Vigano P, Parazzini F, Somigliana E, Vercellini P.Endometriosis: epidemiology and etiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):177–200 [DOI] [PubMed] [Google Scholar]
- 2.Ballard K, Lowton K, Wright J.What’s the delay? A qualitative study of women’s experience of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296-1301 [DOI] [PubMed] [Google Scholar]
- 3.Berube S, Marcoux S, Maheux R.Characteristics related to the prevalence of minimal or mild endometriosis in infertile women. Canadian Collaborative Group on Endometriosis. Epidemiology. 1998;9:504-510 [DOI] [PubMed] [Google Scholar]
- 4.< Sinaii N, Plumb K, Cotton L, Lambert A, Kennedy S, Zondervan K, Stratton P.Differences in characteristics among 1,000 women with endometriosis based on extent of disease. Fertil Steril. 2008;89(3):538-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arruda M, Petta C, Abrao M, Benetti-Pinto C.Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003; 18(4):756–759 [DOI] [PubMed] [Google Scholar]
- 6.Picod G, Boulanger L, Bounoua F, Leduc F, Duval G.Abdominal wall endometriosis after caesarean section: report of fifteen cases. Gynecol Obstet Fertil. 2006;34(1):8-13 [DOI] [PubMed] [Google Scholar]
- 7.Gunes M, Kayikcioglu F, Ozturkoglu E, Haberal A.Incisional endometriosis after cesarean section, episiotomy and other gynecologic procedures. J Obstet Gynaecol Res. 2005;31(5):471-475 [DOI] [PubMed] [Google Scholar]
- 8.Vercellini P, Chapron C, Fedele L, Gattei U, Daguati R, Crosignani PG.Evidence for asymmetric distribution of lower intestinal tract. endometriosis. BJOG 2004; 111(11):12l3–1217 [DOI] [PubMed] [Google Scholar]
- 9.Félix A, Nogales FF, Arias-Stella J.Polypoid endometriosis of the uterine cervix with Arias-Stella reaction in a patient taking phytoestrogens. Int J Gynecol Pathol. 2010; 29(2):l85-l88 [DOI] [PubMed] [Google Scholar]
- 10.Guedj N, Côte JF, Lepimpec-Barthes F, Badoual C, Carnot F, Riquet M, Danel C.Immuno-histochemical study contribution in thoracic endometriosis: about an analysis of eight cases. Ann Pathol. 2009; 29(6):475-480 [DOI] [PubMed] [Google Scholar]
- 11.Medeiros FD, Cavalcante DI, Medeiros MA, Eleutério J., JrFine-needle aspiration cytology of scar endometriosis: Study of seven cases and literature review. Diagn Cytopathol. 2010; 7 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Omranipour R, Najafi M.Papillary serous carcinoma arising in abdominal wall endometriosis treated with neoadjuvant chemotherapy and surgery. Fertil Steril. 2010: 93(4): 1347.el7-8 Epub 2010 Jan l5 [DOI] [PubMed] [Google Scholar]
- 13.Lee CH, Huang YC, Huang SF, Wu YK, Kuo KT.Thoracic endometriosis: rare presentation as a solitary pulmonary nodule with eccentric cavitations. Thorax. 2009; 64(10):919-920 [DOI] [PubMed] [Google Scholar]
- 14.Laghzaoui O, Laghzaoui M.Nasal endometriosis: apropos of l case. J Gynecol Obstet Biol Reprod. 2001; 30(8):786-788 [PubMed] [Google Scholar]
- 15.Ichida M, Gomi A, Hiranouchi N, Fujimoto K, Suzuki K, Yoshida M, Nokubi M, Masuzawa T.A case of cerebral endometriosis causing catamenial epilepsy. Neurology. l993; 43(12):2708-2709 [DOI] [PubMed] [Google Scholar]
- 16.Buchweitz O, Poel T, Diedrich K, Malik E.The diagnostic dilemma of minimal and mild endometriosis under routine conditions. J Am Assoc Gynecol Laparosc 2003; 10(1):85–89 [DOI] [PubMed] [Google Scholar]
- 17.Nap AW, Groothuis PG, Demir AY, Evers JL, Dunselman GA.Pathogenesis of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004; 18(2):233-244 [DOI] [PubMed] [Google Scholar]
- 18.Fujii S.Secondary Müwho d~llerian system and endometriosis. Am J Obstet Gynecol, 1991; 165(1): 219–225 [DOI] [PubMed] [Google Scholar]
- 19.Giudice LC and, Kao LC.Endometriosis. Lancet 2004; 364: 1789–l799 [DOI] [PubMed] [Google Scholar]
- 20.Stefansson H, Geirsson RT, Steinthorsdottir V, Jonsson H, Manolescu A, Kong A, Ingadottir G, Gulcher J, Stefansson K.Genetic factors contribute to the risk of developing endometriosis. Hum Reprod. 2002:17(3):555-559 [DOI] [PubMed] [Google Scholar]
- 21.Marsh E, Laufer M.Endrometriosis in premenarcheal girls who do not have an associated obstructive anomaly. I.cil Steril 2005; 83(3): 758–760 [DOI] [PubMed] [Google Scholar]
- 22.Du H, Taylor H.Contribution of bone marrow derived stem cells to endometrium and endometriosis. Stem cells 2007; 25:2082-2086 [DOI] [PubMed] [Google Scholar]
- 23.Barry F, Murphy J.Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 2004;36(4):568-584 [DOI] [PubMed] [Google Scholar]
- 24.Taylor HS.Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 2004; 292(1):81-85 [DOI] [PubMed] [Google Scholar]
- 25.Buttram VC., JrEvolution of the revised American Fertility Society classification of endometriosis. Fertil Steril 1985; 43: 347–350 [DOI] [PubMed] [Google Scholar]
- 26.Mizuno N, Shiba H, Ozeki Y, Mouri Y.Human autologous serum obtained using a completely closed bag system as a substitute for fetal calf serum in human mesenchymal stem cell cultures. Cell Bio Int.2006; 30(6): 52l-524 [DOI] [PubMed] [Google Scholar]
- 27.Li CY, Lang JH, Liu HY, Zhou HM.Expression of Annexin-1 in patients with endometriosis. Chin Med. J.2008; 12l(10):927-931 [PubMed] [Google Scholar]
- 28.Du H, Taylor HS.Stem cells and female reproduction. Reprod Sci. 2009;16(2):126-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gargett C, Schwab K, Zillwood R, Nguyen H, Wu D.Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80(6):1136-1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Revel A.Multitasking human endometrium: a review of endometrial biopsy as a diagnostic tool, therapeutic applications, and a source of adult stem cells. Obstet Gynecol Surv. 2009; 64(4):249-257 [DOI] [PubMed] [Google Scholar]
- 31.Wan C, He Q, McCaigue M, Marsh D, Li G.Nonadherent cell population of human marrow culture is a complementary source of mesenchymal stem cells (MSCs). J.of Orthop Res. 2006; 24(1): 21–28 [DOI] [PubMed] [Google Scholar]
- 32.Niklaus A, Aubuchon M, Zapantis G, Li P, Qian H, Isaac B, Kim M, Adel G, Pollard J, Santoro N.Assessment of the proliferative status of epithelial cell types in the endometrium of young and menopausal transition women. Hum. Reprod. 2007; 22(6): 1778 – 1788 [DOI] [PubMed] [Google Scholar]


